Abstract
Early life stress (ELS) is associated with altered stress responsivity, structural and functional brain changes and an increased risk for the development of psychopathological conditions in later life. Due to its behavioral and physiological effects, the neuropeptide oxytocin (OXT) is a useful tool to investigate stress responsivity, even though the neurobiological underpinnings of its effects are still unknown. Here we investigate the effects of OXT on cortisol stress response and neural activity during psychosocial stress. Using functional magnetic resonance imaging in healthy subjects with and without a history of ELS, we found attenuated hormonal reactivity and significantly reduced limbic deactivation after OXT administration in subjects without a history of ELS. Subjects who experienced ELS showed both blunted stress reactivity and limbic deactivation during stress. Furthermore, in these subjects OXT had opposite effects with increased hormonal reactivity and increased limbic deactivation. Our results might implicate that reduced limbic deactivation and hypothalamic–pituitary–adrenal axis responsivity during psychosocial stress are markers for biological resilience after ELS. Effects of OXT in subjects with a history of maltreatment could therefore be considered detrimental and suggest careful consideration of OXT administration in such individuals.
Keywords: early life stress, oxytocin, stress
INTRODUCTION
Early life stress (ELS) is associated with a considerable increase in the risk to develop psychiatric disorders such as post-traumatic stress disorder (PTSD) and major depressive disorder (MDD) in later life (Kendler et al., 2004; Burke et al., 2005). Converging evidence from animal models and human studies indicates that ELS causes persisting changes to hypothalamic–pituitary–adrenal axis (HPA) reactivity with altered cortisol responses to psychosocial stress (Sanchez et al., 2001; Heim et al., 2001, 2008; Pryce et al., 2005; Carpenter et al., 2007, 2009; Elzinga et al., 2008; Klaasens et al., 2009).
It has been suggested that the inconsistent findings of either increased or decreased stress responsiveness after ELS might be explained by a trajectory of initial hyperactivation of the HPA system progressing to a state of chronic stress hyporeactivity (Fries et al., 2005; Pryce et al., 2005) as a type of counterregulatory adaptation after sustained exposure to stress during development (Miller et al., 2007). This proposed mechanism mirrors findings of blunted stress responsiveness in animals exposed to chronic stress (Saltzman et al., 2006; Sterlemann et al., 2008). Over time, experimentally stressed animals persistently show lower levels of hippocampal mineralocorticoid receptor expression, suggesting long-term alterations in gene expression regulation. Along this line, ELS is associated with structural and functional changes in brain regions implicated in neuroendocrine control and emotional regulation (Plotsky et al., 2005; Kaffman and Meaney, 2007; Lupien et al., 2009; Heim and Binder 2012). Findings include reduced gray matter volumes in medial prefrontal cortex, anterior cingulate gyrus (ACC), hippocampus, insula, amygdala and caudate (Vythilingam et al., 2002; Buss et al., 2007; van Harmelen et al., 2010; Edmiston et al., 2011; Dannlowski et al., 2012) as well as decreased functional connectivity between amygdala and medial prefrontal cortex (Burghy et al., 2012).
Previous studies in healthy subjects without ELS reported widespread deactivation in limbic and associated regions, including the hippocampus, amygdala and ACC (Pruessner et al., 2008; Dedovic et al., 2009a,b; Dagher et al., 2009; Khalili et al., 2010; Lederbogen et al., 2011; Soliman et al., 2011) during psychosocial stress. These regions are all HPA modulators (Herman et al., 2005), and the deactivations are thought to be stress-related as specifically the intensity of hippocampus deactivation correlates with the salivary cortisol response (Pruessner et al., 2008; Lederbogen et al., 2011). In line with animal studies that identified the hippocampus (HC) as a structure that can have an inhibitory effect on HPA-axis activity (Herman et al., 2003) these findings suggest that a persistent active state of the HC exerts a tonic inhibition of HPA-axis activity. In response to a stressor, the activity of the HC might be curtailed, which then disinhibits the HPA axis and initiates stress hormone release (Herman et al., 2005; Jacobson, 2005; Pruessner et al., 2008).
The neuropeptide oxytocin (OXT) is a potent modulator of HPA activity in both animals and humans. Evidence from rodent models suggests that both acute and chronic administration of OXT reduces physiological and behavioral stress responsivity (Windle et al., 2004, 2006; Slattery and Neumann, 2010; Lukas et al., 2011). In humans, intranasal OXT attenuated cortisol and behavioral responses to psychosocial stress (Heinrichs et al., 2003; Ditzen et al., 2009; Quirin et al., 2011; Linnen et al., 2012). OXT receptors in the hippocampus are subject to modulation by stress and glucocorticoids (Liberzon and Young, 1997) and alterations of the OXT system as a consequence of early experience may contribute to individual vulnerability to the pathologic effects of stress in humans. Decreased urinary concentrations of OXT have been measured in maltreated children (Fries et al., 2005) and Heim et al. (2009) demonstrated decreased cerebrospinal fluid (CSF) OXT concentrations in women with ELS. Also, experiencing early parental separation stress has been shown to reduce the suppressing effect of OXT on cortisol levels, suggesting altered OXT sensitivity (Meinlschmidt and Heim, 2007). These findings are in accordance with animal studies showing that early nurturing experiences induce persistent alterations in OXT receptor levels in rats and decreased CSF OXT concentrations in nursery-reared rhesus monkeys compared with mother-reared controls (Francis et al., 2000; Winslow et al., 2003).
Despite the established effects of OXT on stress response, altered stress responsivity and OXT sensitivity in ELS subjects, so far, there have neither been neuroimaging studies investigating OXT effects on stress-induced changes in neural activity nor studies investigating neural activity in response to stress and OXT in ELS subjects. Furthermore, the majority of previous imaging studies described functional and structural changes in MDD or PTSD patients with a history of ELS. Thus, it is still unclear whether these changes only occur in subjects who develop psychiatric disorders or if they are an ELS consequence also detectable in healthy subjects without any history of psychiatric disorders and might therefore constitute vulnerability markers. In the present study, we sought to clarify the impact of ELS on stress responsivity and associated neural activity as well as to determine OXT effects on these parameters in healthy subjects with and without a history of ELS. We therefore, firstly, aimed to investigate the effects of OXT on deactivation of limbic regions implicated in psychosocial stress response. Secondly, we aimed to examine whether these OXT effects might differ in subjects with/without ELS.
METHODS
Subjects
Thirty-two healthy male subjects (age 28.4 ± 4.5; range 21–37 years; IQ 112 ± 15) were recruited out of a preexisting psychologically and somatically healthy community-dwelling sample (N = 541). All subjects were screened for psychiatric disorders using the short version of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, SCID). Subjects with any history of psychiatric or neurological disease were excluded from the study. Participants were recruited depending on whether or not they had experienced ELS as assessed with the Childhood Trauma Questionnaire (CTQ; Bernstein and Fink, 1998). The CTQ consists of 28 items that are assigned to the following 5 subscales: emotional neglect, emotional abuse, physical neglect, physical abuse and sexual abuse. Scores range from 5 to 25 for each subscale with high scores indicating a strong exposure to early life stressors. Since there are no clearly defined cut-off scores to distinguish between subjects with or without childhood trauma, participants were recruited for the ELS group, if their CTQ-score was no lower than 3 points below the 90th percentile, whereas control subjects had scores no higher than 3 points above the 50th percentile (Scher et al., 2001). All subjects were right-handed as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). The study was carried out in accordance with the latest version of the Declaration of Helsinki and approved by the Institutional Review Board of the German Psychological Society. All subjects gave written informed consent before screening and were reimbursed for participation.
Study design
In a double-blind, placebo-controlled, within-subjects design, participants were administered either OXT (Syntocinon Spray; Novartis, Basel, Switzerland) or a placebo intranasally. Consistent with previous studies (Hurlemann et al., 2010; Labuschagne et al., 2010; Marsh et al., 2010; Gamer and Buchel, 2012), participants self-administered 3 puffs per nostril with a dose of 24 international units (IU). Substances were randomly administered 45 min prior to each of the two scanning sessions (Born et al., 2002).
Stress task
Psychological stress was induced using the Montreal Imaging Stress Task (MIST). The MIST uses a block design, and consists of mental arithmetic challenges that must be answered under time pressure (see Dedovic et al., 2005, for full description). It induces psychosocial stress using elements of uncontrollability and social evaluative threat. The MIST algorithm continuously varies task difficulty based on user performance by adjusting the time constraints per question and the complexity of the arithmetic problems, to yield a 45–50% correct performance for all subjects. Subjects receive correct or incorrect feedback from the computer after each math question and a performance bar shows their cumulative performance as well as the expected performance of the ‘average subject’, which is artificially set to 80% success. This task has been shown to induce behavioral and physiological stress and anxiety responses (Pruessner et al., 1999) and limbic deactivation (Pruessner et al., 2008). Each scanning session consisted of three 7 min runs containing three stimulus conditions presented in block format: the static arithmetic interface screen (0.5 min), control arithmetic questions presented without feedback, progress bar or time constraint (1 min) and stressful arithmetic questions with a time limit and a visible progress bar (2 min), always in this order. Each condition was presented twice in each run. After each 7 min scanning run, a confederate delivered the scripted negative verbal feedback and emphasized the need to improve performance via headphones for ∼1 min. Stimuli were presented via video goggles (Visual Stim Digital for fMRI, Resonance Technology Inc., Northridge, CA, USA). Participants responded by pushing a fiber-optic light sensitive key press. After completing the session and a short break, subjects were told that their performance was insufficient for the data to be used and were therefore asked to repeat the task in a second session, where a parallel version of the task was used. In this way, each participant completed two scanning sessions (one placebo and one OXT session, respectively, in a randomnized order) using the stress task. After the sessions, subjects were debriefed, told that the task was designed to be impossible to accomplish and that it did not assess their ability to perform mental arithmetic.
Psychological and physiological measures
Subjects completed the Spielberger State–Trait Anxiety inventory (Spielberger et al., 1983), the NEO five-factor inventory (NEO-FFI, German version, Costa and McCrae, 1992; Borkenau and Ostendorf, 1993) and the Rosenberg Self-Esteem scale (Rosenberg, 1965). Perception of stress was assessed immediately before and after the imaging sessions by asking subjects to complete the State Anxiety questionnaire (Spielberger et al., 1983) to assess feelings of negativity and uncontrollability. Nine saliva samples were collected with the Salivette sampling device (Sarstedt Inc.) throughout the experiment at the following time-points: Baseline (saliva 1 = T0), 45 min after OXT/placebo administration (saliva 2 / 6 = T1), immediately before (saliva 3 / 7 = T2 (20 min after T1)) and after the MIST (saliva 4 / 8 = T3 (20 min after T2)) as well as 20 min after the completion of the MIST (saliva 5 / 9 = T4). To ensure relatively stable endogenous cortisol levels, all subjects arrived between 1 and 3 p.m. in our laboratory. Saliva-derived cortisol was analysed using a time-resolved fluorescence immunoassay (Dressendorfer et al., 1992).
Functional imaging
Functional data were acquired on a Siemens Trio 3T scanner using a standard echo planar imaging sequence with 37 oblique axial slices of 3 mm (field of view 192 mm, 3 × 3 mm in-plane, repetition time 2 s, echo time 30 ms, flip angle 70°). Two sessions, each with 3 runs of 220 volumes were acquired, as well as a T1-weighted high-resolution MP-Rage scan.
Statistical analysis
Psychological and physiological data
Behavioral data were analysed using t-tests for independent samples. Physiological data were analysed using repeated measures analyses of variance (ANOVAs) with the within-subjects factor substance (OXT, placebo) and the between-subjects factor ELS. Greenhouse–Geisser corrections were applied where appropriate. Further statistical analysis was conducted using t-test comparisons. All tests were two-tailed and the significant threshold was set at a probability of P < 0.05. Statistical analyses were carried out using PASW (Predictive Analysis SoftWare, version 18.0; SPSS Inc., Chicago, IL, USA).
fMRI data
fMRI data were analysed using MATLAB 2009 (The Mathworks Inc., Natick, MA, USA) and SPM8 (Statistical parametric mapping software, SPM; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk). Functional data were realigned to the first volume, corrected for motion artifacts, mean-adjusted by proportional scaling, normalized into standard stereotactic space (template provided by the Montreal Neurological Institute) and spatially smoothed using a 6 mm FWHM Gaussian kernel. The time series were high-pass filtered to eliminate low-frequency components (filter width 128 s). Statistical analysis was performed by modeling the different conditions convolved with a hemodynamic response function as explanatory variables within the context of the general linear model on a voxel-by-voxel basis. Realignment parameters were included as additional regressors in the statistical model. A fixed-effect model at a single-subject level was performed to create images of parameter estimates, which were then entered into a second-level random-effects analysis. For the fMRI data group analysis the contrast images from the analysis of the individual participants were analysed using one-sample t-tests, paired t-tests and ANOVAs. ROI analyses were performed for further investigation of signal changes during psychosocial stress, for comparison between ELS and control subjects, and for elucidating the relationship with physiological stress measures as well as with CTQ-scores. For the ROI analyses of peak voxels, coordinates which were obtained in contrasts of the group analyses (Table 3) were selected. Regions of interest were functionally defined by centering spheres on the respective peak voxels with a radius of 5 mm. For the ROI analyses, % signal changes for the different conditions were extracted for each subject separately using Marsbar (http://marsbar.sourceforge.net/). For each event % signal changes were calculated relative to the mean signal intensity of this ROI across the whole experiment. Data were further analysed using repeated measures ANOVAs with the within-subjects factor substance (OXT, placebo) and the between-subjects factor ELS. Greenhouse–Geisser corrections were applied where appropriate. Further statistical analysis was conducted using t-test comparisons. All tests were two-tailed and the significant threshold was set at a probability of P < 0.05. To detect the association of signal changes in response to psychosocial stress with cortisol concentrations and childhood trauma we performed Pearson correlation analyses. Statistical analyses were carried out using PASW (Predictive Analysis SoftWare, version 18.0; SPSS Inc., Chicago, IL, USA).
Table 3.
Effect of ELS and OXT
| Region | OXT effect | Interaction ELS × OXT |
|---|---|---|
| pgACC | −3 41 7 | |
| z = 3.96 | ||
| Left hippocampus | −36 −13 −17 | |
| z = 3.24 | ||
| Left amygdala | −18 2 −14 | |
| z = 3.36 | ||
| Left PHG | −30 2 −14 | |
| z = 3.89 | ||
| Left insula | −36 20 4 | |
| z = 3.79 | ||
| Left caudate | −9 5 4 | |
| z = 3.71 | ||
| Right caudate | 15 8 7 | |
| z = 3.64 | ||
| Left putamen | −18 14 −5 | |
| z = 4.35 | ||
| Right putamen | 21 14 −2 | |
| z = 3.49 | ||
| Middle temporal gyrus | 51 2 −17 | |
| z = 2.99 | ||
| Premotor cortex | 6 −22 67 | |
| z = 4.51 | ||
| Left superior temporal gyrus | −39 2 −14 | |
| z = 3.46 | ||
| Left occipital cortex | −21 −97 10 | |
| z = 4.25 | ||
| Precuneus | 9 −46 55 | |
| z = 4.11 | ||
| Thalamus | −3 −10 −2 | −12 −7 10 |
| z = 2.76 | z = 4.17 |
ELS, early life stress; OXT, oxytocin; pgACC, pregenual anterior cingulate cortex; PHG, parahippocampal gyrus. P < 0.001 uncorrected, k ≥ 20.
RESULTS
Behavioral measures
Data from one subject had to be excluded from the analyses due to technical difficulties during the scanning session. There were no significant differences between groups with respect to age and IQ. Subjects of the ELS group had significantly higher CTQ- and trait-anxiety scores and scored significantly higher on the neuroticism subscale of the NEO-FFI. Furthermore, they showed significantly lower self-esteem, extraversion and agreeableness. State anxiety scores did not differ between groups (Table 1).
Table 1.
Sociodemographic and questionnaire data of study participants
| ELS (N = 14) | Controls (N = 17) | |
|---|---|---|
| M ± SD | M ± SD | |
| Age | 29.5 (4.5) | 29.0 (5.1) |
| IQ | 109.2 (14.3) | 115.4 (16.1) |
| CTQ | 55.3 (20.4) | 28.2 (2.2)** |
| Emotional neglect | 16.0 (4.4) | 6.9 (1.5)** |
| Emotional abuse | 13.2 (5.8) | 5.5 (0.7)** |
| Physical neglect | 10.8 (4.4) | 5.3 (0.4)** |
| Physical abuse | 8.8 (5.6) | 5.3 (0.8)* |
| Sexual abuse | 6.4 (4.2) | 5.0 (0.0) |
| Rosenberg self-esteem scale | 36.5 (6.5) | 42.2 (4.2)* |
| NEO-FFI | ||
| Extraversion | 25.8 (6.4) | 31.7 (5.7)* |
| Neuroticism | 26.2 (10.5) | 15.0 (5.3)** |
| Agreeableness | 26.9 (6.4) | 33.6 (4.6)** |
| Openness | 34.0 (7.0) | 32.0 (10.5) |
| Conscientiousness | 30.5 (7.4) | 29.1 (8.2) |
| STAI-trait | 39.4 (6.6) | 34.3 (6.7)* |
| STAI-state (prior to scanning) | 35.9 (6.1) | 34.1 (4.2) |
| STAI-state (post-session 1) | 45.2 (10.8) | 39.9 (9.4) |
| STAI-state (post-session 2) | 41.7 (8.9) | 36.5 (7.5) |
ELS, early life stress; M, mean; SD, standard deviation; IQ, intelligence quotient; CTQ, Childhood Trauma Questionnaire; NEO-FFI, NEO five-factor inventory; STAI, state-trait anxiety inventory. *P < 0.05, **P < 0.01.
Cortisol stress response
Three subjects did not provide enough saliva to be analysed. There was a main effect of ELS (F(1,27) = 6.24, P = 0.021) on cortisol concentrations at T3 (immediately after the MIST) and of substance on cortisol concentrations at T2 (immediately before the MIST; (F(1,27) = 4.89, P = 0.039) with lower concentrations in the ELS group and the placebo condition, respectively. Hormonal reactivity during the MIST as indicated by the difference in cortisol concentrations between T3 and T1 was significantly lower in the ELS group (P < 0.05) during the placebo condition. When looking at cortisol concentrations regardless of group, we observed a significant increase induced by the MIST (from T1 to T3 (P < 0.01)) in the placebo, but not in the OXT condition (Figure 1a). When analysing each group separately, we found that cortisol concentrations increased during the MIST (from T1 to T3 (P < 0.05)) in the control group during the placebo, but not in the OXT condition. In the ELS group cortisol concentrations decreased from T0 to T1, T0 to T2 and T0 to T3 (P < 0.05) in the placebo condition and from T0 to T2 and T3 to T4 during the OXT condition (P < 0.05). Hormonal reactivity (difference between T3 and T1) was attenuated by OXT in the control group (P = 0.05) and increased in the ELS group (P = 0.08) (Figure 1b and c). There was a significant correlation between the CTQ subscore for emotional neglect and the cortisol concentration at baseline (r = 0.37, P < 0.05). Hormonal reactivity during the MIST as indicated by the difference in cortisol concentrations between T3 and T1 was negatively correlated with the CTQ subscores for emotional abuse (r = −0.54, P < 0.01) as well as emotional neglect (r = −0.47, P < 0.01) during the placebo, but not the OXT condition.
Fig. 1.
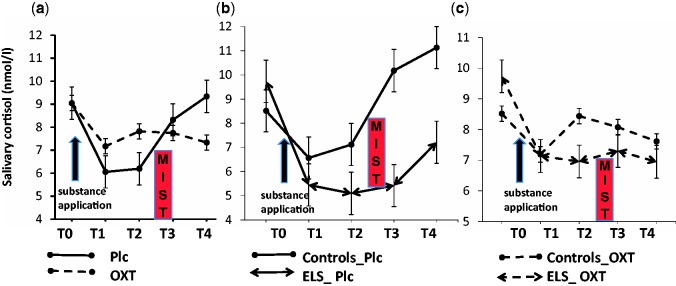
Salivary cortisol concentrations (a) before and after intranasal administration of OXT or placebo in all subjects; (b) before and after intranasal administration of placebo in subjects with ELS and controls; and (c) before and after intranasal administration of OXT in subjects with ELS and controls. Abbreviations: ELS, early life stress; OXT, oxytocin; Plc, placebo; MIST, Montreal Imaging Stress Task; T0, baseline; T1, 45 min after OXT/placebo administration; T2, immediately before the MIST; T3, immediately after the MIST; T4, 20 min after the completion of the MIST. Error bars denote SEM.
fMRI results
Effect of stress during OXT and placebo conditions
Compared to the non-stressful control task, stress yielded BOLD-signal changes in hippocampus/parahippocampal gyrus, insula, medial temporal gyrus and precuneus (Table 2) during the OXT and placebo condition in all subjects. When contrasting these two conditions in a paired t-test, stronger BOLD-signal changes during the placebo condition where observed in the left hippocampus (−33 −40 −5, z = 2.76) and the dorsomedial thalamus (3 −13 −2, z = 2.91). During the OXT condition, stronger BOLD-signal changes were observed in the right insula (36 20 −2, z = 2.90), the anterior cingulate cortex (3 32 10, z = 2.80), the posterior cingulate cortex (3 −43 19, z = 2.71) and the left parahippocampal gyrus (−21 −28 −14, z = 2.79).
Table 2.
Effect of stress during OXT and placebo condition
| Region | OXT condition | Placebo condition |
|---|---|---|
| Right hippocampus / PHG | 27 −43 −8 | |
| z = 5.21 | ||
| Left hippocampus / PHG | −39 −19 −17 | |
| z = 5.21 | ||
| Right insula | 39 26 −5 | |
| z = 5.85 | ||
| Left insula | −45 2 −8 | |
| z = 5.71 | ||
| Precuneus | 3 −61 52 | |
| z = 5.94 | ||
| Premotor cortex | 3 11 46 | |
| z = 5.30 | ||
| Medial temporal gyrus | 45 −67 10 | |
| z = 6.02 | ||
| Right occipital cortex | 15 −88 19 | 27 −85 16 |
| z = 5.90 | z = 4.97 | |
| Left occipital cortex | −27 −91 22 | −27 −85 16 |
| z = 5.48 | z = 5.17 | |
| Cerebellum | 21 −67 −11 | |
| z = 5.91 |
PHG, parahippocampal gyrus; OXT, oxytocin. FWE-corrected. P < 0.05.
Effect of ELS
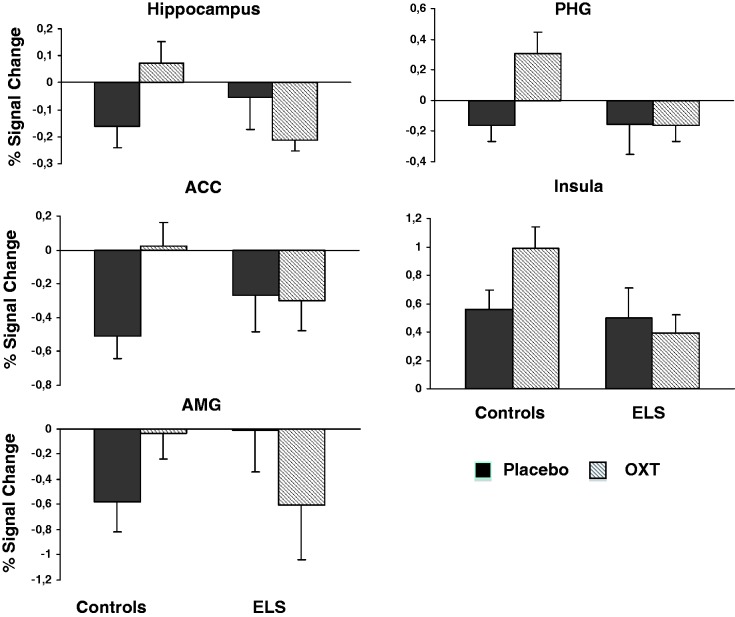
An ANOVA was performed to assess the effects and interaction of ELS and substance (OXT/placebo) on BOLD-signal changes. There were no significant ELS-effects, but OXT had significant effects on signal changes in left hippocampus, thalamus and middle temporal gyrus. A significant ELS × OXT interaction was observed in pregenual anterior cingulate cortex (pgACC), left amygdala, left parahippocampal gyrus, left insula, bilateral putamen and bilateral caudate (Table 3). ROI analyses revealed that deactivations during stress were attenuated in ELS subjects. Although OXT reduced deactivations in controls, it yielded stronger deactivations in ELS subjects. A different pattern was observed in the left insula, though, with an OXT-associated increase in activity in the control subjects, but a decreased activation in ELS subjects (Figure 2).
Fig. 2.
Effect of psychosocial stress on BOLD-signal changes during OXT and placebo conditions in subjects with ELS and controls. Abbreviations: ELS, early life stress; OXT, oxytocin; ACC, anterior cingulate cortex; PHG, parahippocampal gyrus; AMG, amygdala. Error bars denote SEM.
Association of cortisol stress response and BOLD-signal changes
During the placebo condition, a significant negative correlation between cortisol concentration at T3 (immediately after the MIST) and signal changes in left hippocampus was observed (r = −0.54, P < 0.01).
Association of ELS measures and BOLD-signal changes
CTQ subscores for emotional neglect (EN) and emotional abuse (EA) were significantly and inversely correlated with signal changes in left hippocampus during the placebo condition (EN: r = 0.47, P < 0.01; EA: r = 0.49, P < 0.01) and in left insula during the OXT condition (EN: r = −0.39, P < 0.05; EA: r = −0.42, P < 0.05).
DISCUSSION
Our findings demonstrate that healthy subjects who experienced moderate to severe emotional neglect and abuse in their childhood not only show attenuated hormonal and limbic reactivity during psychosocial stress, but might also have an altered OXT sensitivity, since the previously reported stress-buffering effect of OXT (Heinrichs et al., 2003; Quirin et al., 2011; Linnen et al., 2012) was only found in subjects who did not experience ELS. In subjects who experienced ELS we found an increased hormonal and limbic reactivity after OXT administration. Emotional abuse and neglect along with inadequate social support during childhood might modulate sensitivity to interpersonal rejection and negative social evaluation (Ditzen et al., 2008; Quirin et al., 2008), thereby altering the impact of the stress task. Alterations of the OXT system as a consequence of early experience may contribute to individual vulnerability to the pathologic effects of stress. ELS and particularly emotional abuse is associated with decreased CSF and urinary OXT concentrations (Fries et al., 2005; Heim et al., 2009) and it has been suggested that early adverse social experiences might interfere with the developing neuropeptide system and alter receptor binding of OXT. ELS-associated alterations in OXT brain systems implicated in social attachment and stress protection might explain why exogenous OXT effects on cooperative behavior, emotional response to stress and social reward are strongly predicted by individual differences in attachment style, personality traits such as agreeableness and history of childhood trauma (Bartz et al., 2011).
As in previous studies with the MIST, we observed a significant deactivation during stress in limbic regions such as the hippocampus, anterior cingulate cortex, amygdala and parahippocampal gyrus and also a correlation between hippocampus deactivation with the salivary cortisol response (Pruessner et al., 2008; Dedovic et al., 2009a,b). Since these limbic regions are all HPA modulators our data thereby further support the idea that their persistent active state exerts a tonic inhibition of HPA-axis activity. In response to a stressor, the limbic activity might be curtailed, which then disinhibits the HPA axis and initiates stress hormone release (Pruessner et al., 2008; Dedovic et al., 2009a,b). Considering the negative feedback inhibitory influence of the hippocampus on HPA-axis activity (Herman and Mueller, 2006), the hippocampal deactivation in response to experimental stress is hypothesized to trigger the initial HPA-axis activation. Stress-induced limbic deactivation is attenuated by OXT in controls and might result in a stronger inhibition of HPA-axis. This notion is supported by reduced cortisol response and the association between increased hippocampal activation and decreased cortisol concentration. ELS subjects showed significantly reduced limbic deactivation during stress and, contrary to controls, increased deactivation during the OXT condition.
Consistent with previous studies, we found prominent stress-related signal changes in pgACC (Pruessner et al., 2008), which is a major part of the limbic stress regulation system and exhibits high neuronal glucocorticoid receptor expression, modulates HPA-axis activation during stress and is implicated in processing chronic social stressors such as social defeat (Diorio et al., 1993). In the context of the MIST, the pgACC might be implicated in the appraisal process and error monitoring, detection of social evaluative threat and stress perception (Pruessner et al., 2008). Data by King et al. (2009) suggest that deactivation of the pgACC may be involved in HPA-axis activation, while its activation may be involved in regulation of HPA-axis responses. Thus, our present data of OXT- induced pgACC activation are consistent with modulation or ‘gating’ of HPA-axis responses via top-down inhibitory control.
The insula as part of the so-called salience network (Seeley et al., 2007) is implicated in mediating autonomic arousal, aversive emotional processing and estimation of uncertainty and risk (Craig, 2004; Ernst et al., 2013). Increased insula activation during the OXT condition might reflect an increased perceptual salience of social cues and enhanced feelings of uncertainty and risk (Bartz et al., 2011). This hypothesis is supported by studies demonstrating increased eye contact (Guastella et al., 2008), attention-shift to socially relevant cues (Gamer et al., 2010), improved mind-reading from facial expressions (Domes et al., 2007) and increased impact of aversive social simuli (Striepens et al., 2012) as a result of OXT treatment. The social salience hypothesis also sheds further light on ELS-specific effects of OXT. If OXT increases subjects’ attention to social cues it might be detrimental to individuals who are generally hypersensitive to social cues and have a bias toward interpreting them negatively as might be the case in our ELS subjects, who differ in personality traits such as self-esteem, neuroticism, extraversion and agreeableness. Accordingly, recent findings by Bartz et al. (2011) showed individual differences in OXT effects with enhanced cooperative behavior for anxiously attached but low avoidant (i.e., intimacy seekers) individuals but impeded cooperative behavior for anxiously attached, intimacy-avoidant individuals. The underlying mechanism might be that alterations of the OXT system as a consequence of early experience may contribute to a differential response to exogenous OXT (Meinlschmidt and Heim, 2007).
Converging evidence from animal models and human studies suggests that depending on the severity and type of ELS, it might be associated with increased or decreased stress responsiveness and vulnerability or resilience to psychiatric disorders (Elzinga et al., 2008; Heim et al., 2008; Carpenter et al., 2009; Klaasens et al., 2009). Stress responsiveness and HPA function seem to be shaped by the nature of the threat, emotions elicited by the stress, controllability of the stress and individual response to the situation (Miller et al., 2007). Different types of ELS might therefore translate into distinct functional alterations. There is evidence that the hippocampus might act as a mediator for the relation between ELS, MDD and PTSD (Gilbertson et al., 2002; Rao et al., 2010). The hippocampus is a highly susceptible structure for the detrimental effects of stress, considering the high expression of glucocorticoid receptors and maternal deprivation was shown to be associated with decreased glucocorticoid receptor expression in the HC and decreased glucocorticoid negative feedback (Ladd et al., 2000; Plotsky et al., 2005). The previously reported association of a history of severe physical or sexual abuse in MDD or PTSD patients with increased stress responsiveness and changes in hippocampal volume (Heim et al., 2008; Rao et al., 2010) might either be a result of the psychopathological condition or an ELS consequence constituting a vulnerability marker. Subjects in our sample were healthy young males without a previous history of psychiatric disorders, who had experienced moderate to severe emotional abuse and neglect (Bernstein and Fink, 1998), which might have resulted into functional changes such as reduced limbic deactivation and blunted HPA-axis responsivity that might serve as markers for biological resilience. According to the ‘stress inoculation’ hypothesis and some evidence from studies in rodents and non-human primates, exposure to moderately stressful events early in life may lead to decreased cortisol reactivity and resilience, while severe stress increases HPA-axis reactivity (Parker et al., 2006; Del Giudice et al., 2011). Another explanation might be that a state of chronic stress hyporeactivity (Fries et al., 2005; Heim et al., 2009) is a type of counterregulatory adaptation after sustained exposure to stress during development (Miller et al., 2007). If reduced limbic deactivation and HPA-axis responsivity during psychosocial stress are indeed markers for biological resilience in ELS subjects, the OXT-induced changes might be considered a detrimental effect of the treatment. On the other hand, one might also interpret these changes as a ‘normalization’ of neural activity patterns and hormonal reactivity since they correspond to those seen in control subjects during the placebo condition.
It might be considered a limitation of our study that we investigated only male subjects. However, we decided against an inclusion of female subjects since several previous studies suggested greater acute HPA and autonomic responses in men (Kudielka and Kirschbaum, 2005) as well as sex- specific OXT effects (Domes et al., 2010). Also, we cannot exclude the possibility that ELS-exposed subjects investigated in this study might still develop a psychiatric disorder later in life, therefore, future studies should consider a longitudinal approach. Furthermore, the CTQ assesses ELS retrospectively, so that recall biases and reconstructive memories might pose a problem (Hyman and Loftus, 1998). Our subjects suffered from emotional abuse and neglect, however, so that medical records would have been of limited use. In addition, many subjects were not in contact with their families, so that family interviews would have been difficult to arrange.
In summary, our data suggest that OXT attenuates stress responsivity by modulating limbic deactivations. In subjects who experienced emotional abuse or neglect in their childhood we not only found blunted limbic deactivations and hormonal reactivity to psychosocial stress, but also differential effects of OXT on hormonal and neuronal signatures of stress. Our results might implicate that reduced limbic deactivation and HPA-axis responsivity during psychosocial stress are markers for biological resilience after ELS. Effects of OXT in subjects with a history of maltreatment could therefore be considered detrimental and suggest careful consideration of OXT administration in such individuals.
Acknowledgments
Funding was provided by Deutsche Forschungsgemeinschaft (DFG) to the Cluster of Excellence “Languages of Emotion” at Freie Universitaet Berlin (NG 102 “Emotional Modulation”).
REFERENCES
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. San Antonia: TX: The Psychological Corporation; 1998. [Google Scholar]
- Borkenau P, Ostendorf F. NEO-Fünf-Faktoren Inventar (NEO-FFI) nach Costa und McCrae, Handanweisung. Göttingen: Hogrefe; 1993. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5(6):514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–56. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. Journal of Neuroscience. 2007;27(10):2592–5. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biological Psychiatry. 2009;66(1):69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory and NEO Five-Factor Inventory (Professional Manual) Odessa: Psychological Assessment Resources; 1992. [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8(6):239–41. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Research. 2009;1293:40–8. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009a;47(3):864–71. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, et al. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30(5):319–25. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, et al. Neural correlates of processing stressful information: an event-related fMRI study. Brain Research. 2009b;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35(7):1562–92. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13(9):3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schmidt S, Strauss B, Nater UM, Ehlert U, Heinrichs M. Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. Journal of Psychosomatic Research. 2008;64(5):479–86. doi: 10.1016/j.jpsychores.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61(6):731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43(7):683–92. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics and Adolescent Medicine. 2011;165(12):1069–77. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–37. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Human Brain Mapping. 2013;34(7):1615–24. doi: 10.1002/hbm.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12(12):1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–6. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gamer M, Buchel C. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology. 2012;37(1):87–93. doi: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9400–5. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology. 2012;233(1):102–11. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller. AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry. 2009;14(10):954–8. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24(3):151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behavioural Brain Research. 2006;174(2):215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuropsychopharmacology and Biological Psychiatry. 2005;29(8):1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman IE, Loftus EF. Errors in autobiographical memory. Clinical Psychology Review. 1998;18(8):933–47. doi: 10.1016/s0272-7358(98)00041-5. [DOI] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinology Metabolism Clinics of North America. 2005;34(2):271–92, vii. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. Journal of Child Psychology and Psychiatry. 2007;48(3–4):224–44. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychological Medicine. 2004;34(8):1475–82. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Dedovic K, Engert V, Pruessner M, Pruessner JC. Hippocampal activation during a cognitive task is associated with subsequent neuroendocrine and cognitive responses to psychological stress. Hippocampus. 2010;20(2):323–34. doi: 10.1002/hipo.20623. [DOI] [PubMed] [Google Scholar]
- King AP, Abelson JL, Britton JC, Phan KL, Taylor SF, Liberzon I. Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage. 2009;47(3):872–80. doi: 10.1016/j.neuroimage.2009.05.088. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33(5):889–94. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69(1):113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–13. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Young EA. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology. 1997;22(6):411–22. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Linnen AM, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15(4):393–402. doi: 10.3109/10253890.2011.631154. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159–68. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209(3):225–32. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological Psychiatry. 2007;61(9):1109–11. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):3000–5. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63(2):234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicinei. 1999;61(2):197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, et al. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):649–74. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36(6):898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Quirin M, Pruessner JC, Kuhl J. HPA system regulation and adult attachment anxiety: individual differences in reactive and awakening cortisol. Psychoneuroendocrinology. 2008;33(5):581–90. doi: 10.1016/j.psyneuen.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological Psychiatry. 2010;67(4):357–64. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biological Psychiatry. 2006;60(8):843–9. doi: 10.1016/j.biopsych.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13(3):419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The Childhood Trauma Questionnaire in a community sample: psychometric properties and normative data. Journal of Traumatic Stress. 2001;14(4):843–57. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58(1):56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Soliman A, O'Driscoll GA, Pruessner J, et al. Limbic response to psychosocial stress in schizotypy: a functional magnetic resonance imaging study. Schizophrenia Research. 2011;131(1–3):184–91. doi: 10.1016/j.schres.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Hormones and Behavior. 2008;53(2):386–94. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Striepensa N, Scheelea D, Kendrick KM, et al. Oxytocin facilitates protective responses to aversive social stimuli in males. Proceedings of the National Academy of Sciences of the USA. 2012;109(44):18144–9. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010;68(9):832–8. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry. 2002;159(12):2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology. 2006;147(5):2423–31. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience. 2004;24(12):2974–82. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–8. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]