Abstract
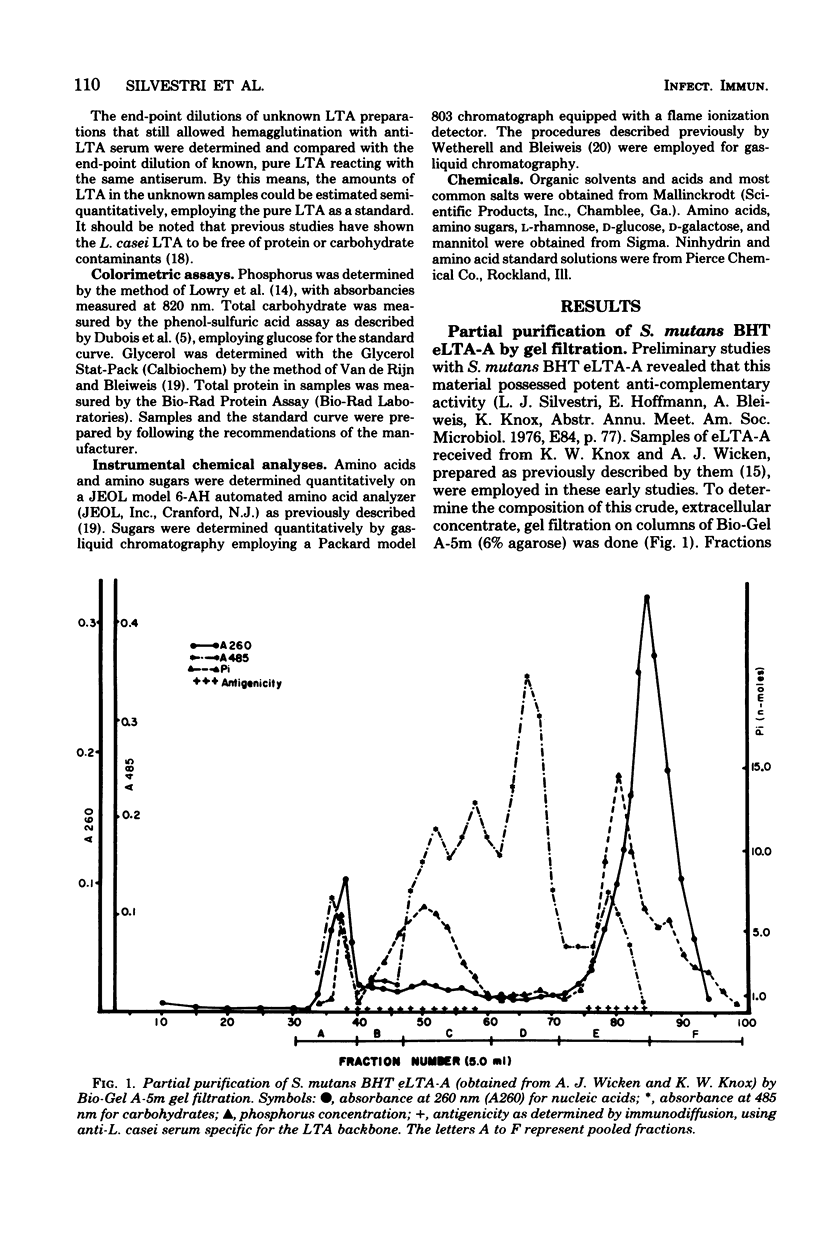
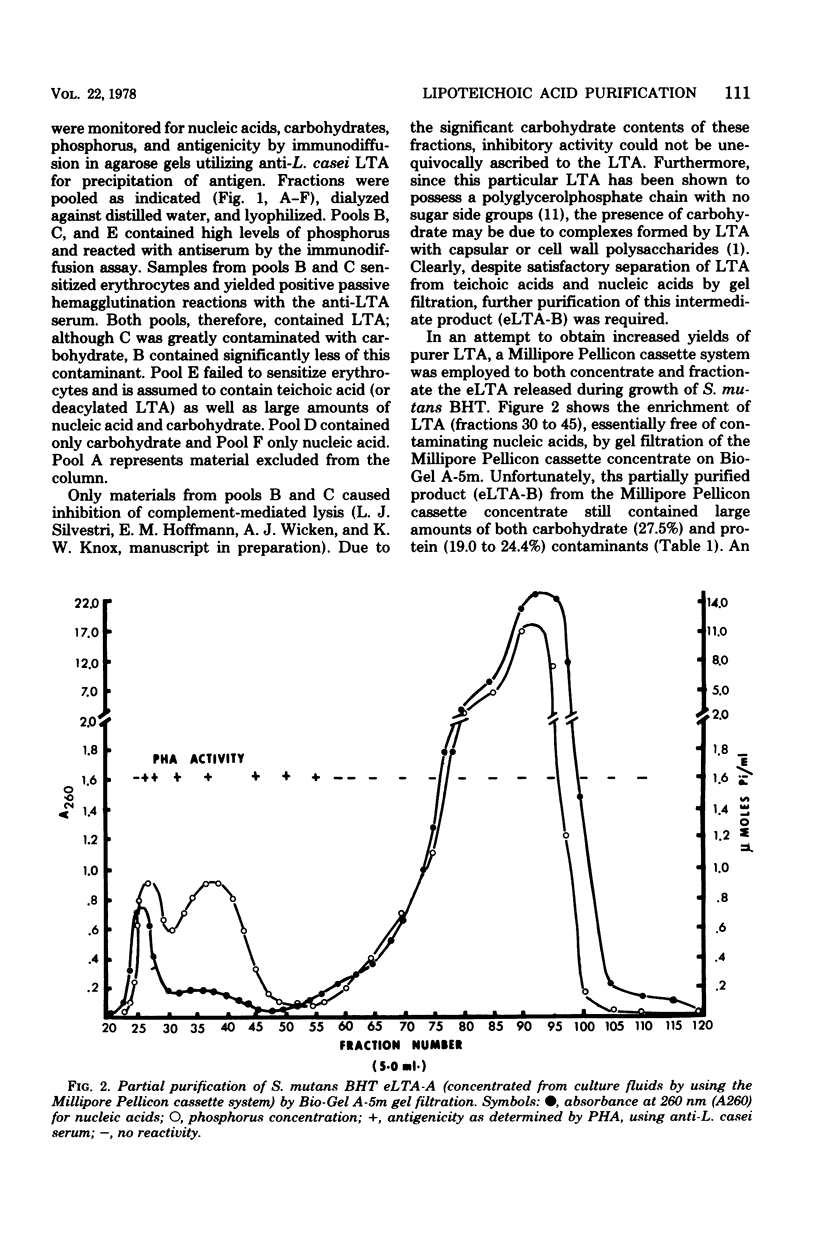
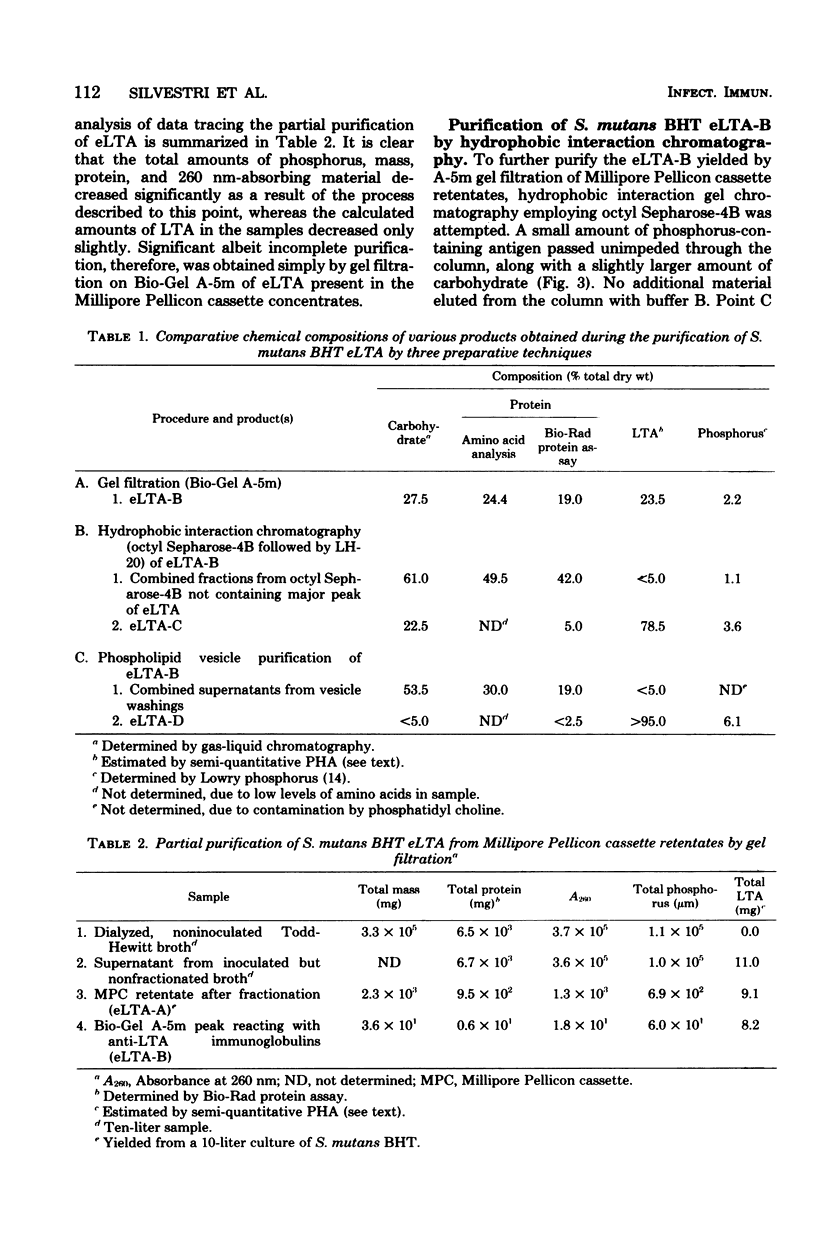
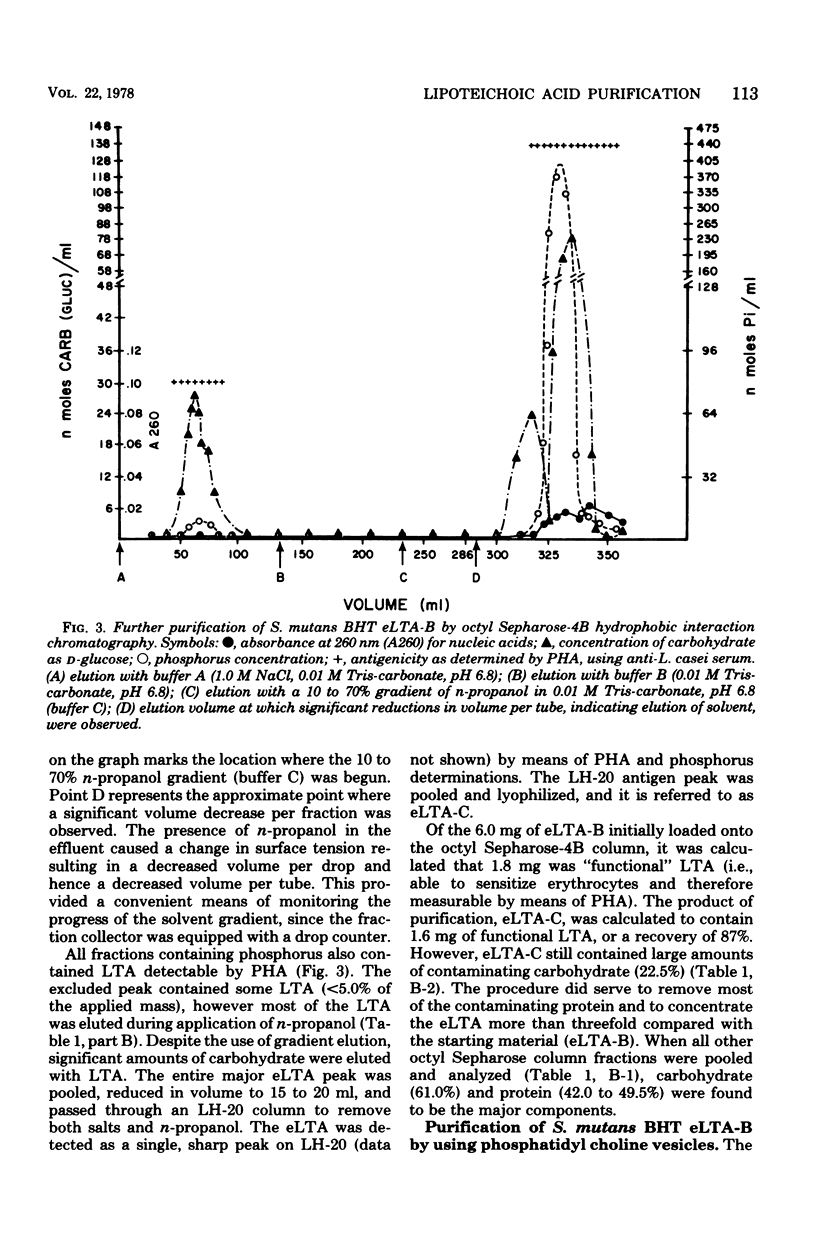
Lipoteichoic acid (LTA) is a component of nearly all gram-positive membranes and recently has been found to be excreted into growth media by certain lactic acid bacteria. Cell-free extracts of LTA are usually contaminated with proteins, polysaccharides, and nucleic acids, thus causing problems to investigators studying the true biological function(s) of LTA. This report describes the preparation of purified extracellular LTA of Streptococcus mutans BHT and intracellular LTA of S. mutans AHT by three techniques: gel filtration, hydrophobic interaction chromatography, and adsorption to phospholipid vesicles. Gel filtration, the most commonly employed method for LTA purification, was found to remove nucleic acids, teichoic acids, and much polysaccharide while greatly concentrating LTA. But gross amounts of antigenic carbohydrate and protein remained associated with the LTA preparation. Hydrophobic interaction chromatography employing octyl Sepharose-4B allowed the separation of protein but not polysaccharide from partially purified BHT LTA preparations. By means of a new technique described in this paper, synthetic membranes (vesicles) were found to effectively separate all contaminants from the intracellular (AHT) and extracellular (BHT) LTA of S. mutans. This rapid method, on a comparative basis, proved to be the most effective approach for the purification of LTA from two widely differing sources.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Chatterjee A. N., Streips U. N., Young F. E. Soluble macromolecular complexes involving bacterial teichoic acids. J Bacteriol. 1975 Oct;124(1):341–347. doi: 10.1128/jb.124.1.341-347.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. W. The effect of anaesthetic-like molecules on the phase transition in smectic mesophases of dipalmitoyllecithin. I. The normal alcohol up to C equals 9 and three inhalation anaesthetics. Biochim Biophys Acta. 1974 Jul 12;356(1):117–124. doi: 10.1016/0005-2736(74)90299-5. [DOI] [PubMed] [Google Scholar]
- Hoffman E. M. Inhibition of complement by a substance isolated from human erythrocytes. I. Extraction from human erythrocyte stromata. Immunochemistry. 1969 May;6(3):391–403. doi: 10.1016/0019-2791(69)90296-1. [DOI] [PubMed] [Google Scholar]
- Hughes A. H., Hancock I. C., Baddiley J. The function of teichoic acids in cation control in bacterial membranes. Biochem J. 1973 Jan;132(1):83–93. doi: 10.1042/bj1320083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Markham J. L., Wicken A. J. Formation of cross-reacting antibodies against cellular and extracellular lipoteichoic acid of Streptococcus mutans BHT. Infect Immun. 1976 Mar;13(3):647–652. doi: 10.1128/iai.13.3.647-652.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. The interaction of magnesium ions with teichoic acid. Biochem J. 1975 Sep;149(3):519–524. doi: 10.1042/bj1490519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvaer K. L., Helgeland K., Rölla G. A charged component in purified polysaccharide preparations from Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1974 Jul;19(7):589–595. doi: 10.1016/0003-9969(74)90077-6. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Extraction, purification, and chemical and immunological properties of the Streptococcus mutans group "a" polysaccharide cell wall antigen. Infect Immun. 1973 Aug;8(2):190–198. doi: 10.1128/iai.8.2.190-198.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M. E., Brock J. H., Knox K. W., Wicken A. J. Glycerol teichoic acid as a common antigenic factor in lactobacilli and some other gram-positive organisms. J Gen Microbiol. 1973 Jan;74(1):119–126. doi: 10.1099/00221287-74-1-119. [DOI] [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S. Antigens of Streptococcus mutans. I. Characterization of a serotype-specific determinant from Streptococcus mutans. Infect Immun. 1973 May;7(5):795–804. doi: 10.1128/iai.7.5.795-804.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell J. R., Jr, Bleiweis A. S. Antigens of Streptococcus mutans: characterization of a polysaccharide antigen from walls of strain GS-5. Infect Immun. 1975 Dec;12(6):1341–1348. doi: 10.1128/iai.12.6.1341-1348.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]



