Summary
The p53 tumor suppressor coordinates a series of anti-proliferative responses that restrict the expansion of malignant cells and, as a consequence, p53 is lost or mutated in the majority of human cancers. Here, we show that p53 restricts expression of the stem and progenitor cell-associated protein nestin in an Sp1/3 transcription factor-dependent manner and that nestin is required for tumor initiation in vivo. Moreover, loss of p53 facilitates dedifferentiation of mature hepatocytes into nestin-positive progenitor-like cells, which are poised to differentiate into hepatocellular carcinomas (HCCs) or cholangiocarcinomas (CCs) in response to lineage-specific mutations that target Wnt and Notch signaling, respectively. Many human HCCs and CCs show elevated nestin expression, which correlates with p53 loss of function and is associated with decreased patient survival. Therefore, transcriptional repression of Nestin by p53 restricts cellular plasticity and tumorigenesis in liver cancer.
Introduction
p53 mutations occur in a wide range of human cancers and are often associated with aggressive tumor behavior and poor patient prognosis (Spike and Wahl, 2011). Wild-type p53 is activated by DNA damage and various forms of oncogenic stress, where it induces genes that promote cell-cycle blockade, apoptosis, senescence, differentiation and/or autophagy, various aspects of cell metabolism (Vousden and Lane, 2007), and can even suppress epigenetic reprogramming of differentiated cells into induced pluripotent stem (IPS) cells (Hong et al., 2009; Kawamura et al., 2009; Marion et al., 2009). In addition to its cell autonomous activities, p53 can promote the secretion of a variety of factors that influence the tissue microenvironment in a non-cell autonomous manner (Lujambio et al., 2013). Which of these p53 activities is most relevant for its tumor suppressor role has been widely debated and is likely context dependent (Kenzelmann Broz and Attardi, 2010).
p53 promotes transcriptional activation through the recruitment of chromatin modifying proteins to the promoters of genes with p53 response elements and, indeed, key p53 target genes contribute to specific effector functions (Vousden and Prives, 2009). p53 can also repress gene expression through mechanisms that are less well-understood. p53 can directly repress transcription by binding p53 response elements in, for example, the Nanog or CD44 promoters (Godar et al., 2008; Lin et al., 2005), or indirectly, either by inducing genes such as p21, E2F7 and miR-34 that act through transcriptional or post-transcriptional mechanisms or by antagonizing the basal transcription machinery and/or transcriptional activators such as Sp1, ETS1 (Ho and Benchimol, 2003). Regardless, the contribution of this p53 property to tumor suppression is not clear.
p53 mutations are common in primary liver cancers, which represent the 5th most frequent tumor type worldwide (Hussain et al., 2007). These tumors present as either hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (CC), and can easily be distinguished histologically and by assessing expression of lineage specific markers. HCC typically consists of polygonal cells growing in a solid-trabecular growth pattern while CC often displays a ductal morphology with a substantial stromal reaction. While the mutational profiles of HCC and CC are distinct, p53 mutations occur in both tumor types and are associated with a particularly poor prognosis (Hussain et al., 2007; Nault and Zucman-Rossi, 2011). Studies in mouse models indicate that p53 inactivation is required for the maintenance of murine liver carcinomas in vivo (Xue et al., 2007). Still, how p53 acts to limit the development of primary liver cancers remains poorly understood.
While it is commonly assumed that HCC and CC arise through malignant transformation of resident hepatocytes and cholangiocytes, respectively, the cell of origin of each disease is controversial. For example, some studies suggest cholangiocarcinoma can arise through transdifferentiation of adult hepatocytes to cholangiocytes (Fan et al., 2012; Sekiya and Suzuki, 2012), whereas others imply that each tumor type can arise from bi-potential progenitor cells residing in the adult liver (Roskams, 2006). Consistent with the latter view, rare liver tumors show a mixed HCC/CC histopathology.
The class IV intermediate filament protein nestin has been identified as a marker of bi-potential liver progenitor cells (oval cells) that reside in the adult liver and expand upon chronic liver damage (Gleiberman et al., 2005). Nestin is highly expressed in the mammalian brain and frequently used as a marker of neuronal stem cells (Mignone et al., 2004). In glioma, nestin-positive cells are crucial for tumor initiation and maintenance, and mark a stem-cell like population that is necessary to propagate disease (Chen et al., 2012). Here we show that p53 can repress Nestin through an indirect mechanism that restricts tumorigenesis by limiting cellular plasticity and the expansion of progenitor-like populations in response to oncogenic stress. Consequently, p53 loss, together with lineage specific lesions, enables the emergence of either HCC or CC with progenitor like properties that, in patients, is associated with reduced survival. Our results suggest that the ability of p53 to restrict the “reprogramming” of differentiated cells into a more pluripotent state contributes to its tumor suppressive role.
Results
p53 deletion leads to mixed lineage tumors with high nestin expression
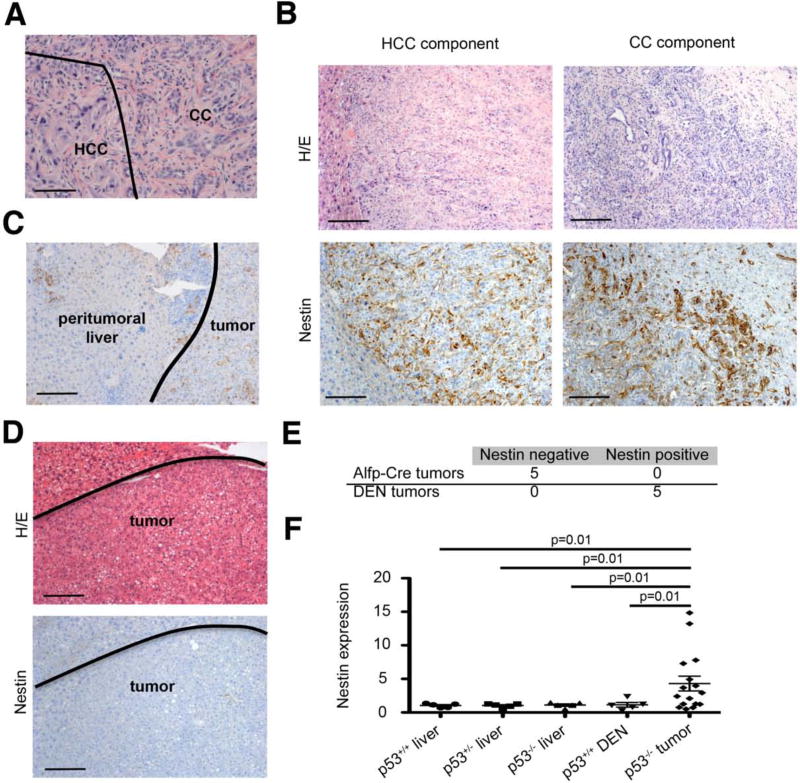
Conditional p53 deletion in the murine liver (using the albumin promoter combined with alpha-fetoprotein enhancer (Alfp-cre)) produces tumors with a mixed HCC/CC histology (Katz et al., 2012) (Fig. 1A). To determine if these tumors express the progenitor cell marker nestin, we performed immunostaining with an antibody specifically recognizing the murine nestin protein. Surprisingly, high nestin expression was identified in both the HCC and the CC components (Fig. 1B). Moreover, some nestin-positive cells were identified in adjacent peritumoral tissue (Fig. 1C), indicating expansion of non-cancerous nestin-positive cells. By contrast, chemically induced liver tumors produced by the carcinogen diethylnitrosamine (DEN), which rarely contain p53 mutations (Rumsby et al., 1994), did not express nestin (Fig. 1D and 1E). Similarly, Nestin mRNA levels were significantly higher in p53 null mixed HCC/CC tumors compared to DEN-induced HCCs (Fig. 1F). Thus, liver specific p53 deletion triggers the formation of bi-lineage liver tumors with high nestin expression.
Figure 1. Liver-specific p53 deletion leads to mixed HCC/CCs with high Nestin expression.
(A) H/E sections from liver tumors of Alfp-Cre p53 fl/fl mice. HCC tumor part and CC tumor part are indicated. Bar= 50 μm (B) Immunohistochemical staining for nestin in HCC- and CC tumor regions. Bars= 100 μm (C) Nestin immunohistochemistry of peritumoral regions. Bar indicates 100 μm (D) H/E and nestin-IHC of DEN-induced tumors. Bars= 100 μm (E) Quantification of nestin expression in Alfp-Cre p53 fl/fl tumors and DEN-induced tumors (F) Quantitative PCR analysis of Nestin expression in normal liver or tumor samples. See also Table S1.
Nestin levels are high in human tumors where they correlate with poor patient prognosis
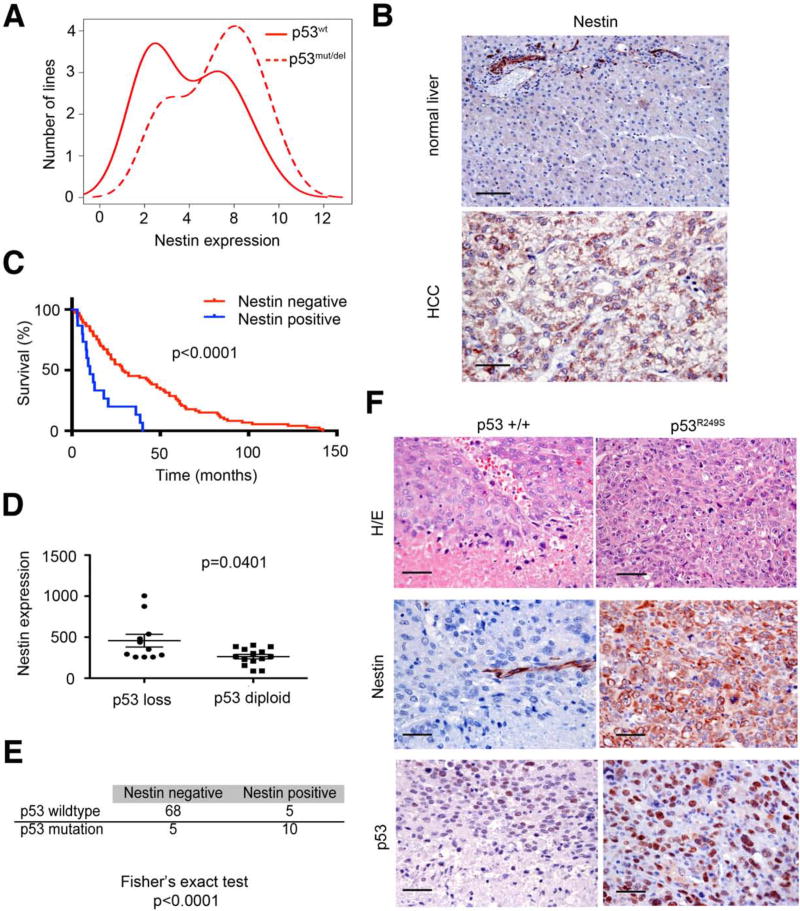
As a first step towards understanding factors that influence Nestin expression in liver cancers, we examined Nestin expression in human liver tumor cell lines using the cancer cell line encyclopedia database. As observed above, cell lines with p53 mutation or genomic loss at the p53 locus show higher Nestin mRNA levels than those with wild-type p53 (Fig. 2A). These results were confirmed by nestin IHC on 88 primary HCCs with different etiology (Suppl. Table S1) and 47 primary human CCs (Suppl. Table S2, S3). Hence, 17% of the HCCs and 40% of the CCs exhibited nestin-positive tumor cells (Figure 2B, Suppl. Fig S1A). In contrast, nestin protein was not expressed in hepatocytes of healthy livers and could only be detected in endothelial cells of blood vessels (Fig. 2B). Additionally, Nestin mRNA levels were significantly higher in HCCs and CCs compared to surrounding liver tissue (data not shown, Suppl. Fig. S1B). We also analyzed a small number of human tumors with mixed HCC/CC histopathology, and noted a trend for higher Nestin expression in those classified with “stem cell features” (2/3) compared to those with classical HCC/CC histopathology (1/5) (Suppl. Fig. S1D). Importantly, patients harboring tumors with high nestin levels displayed a much worse clinical outcome with a median survival of 10 months compared to 29 months for patients with nestin-negative tumors (Fig. 2C). Therefore, like p53 mutations, nestin is overexpressed in a subset of all liver cancer types where it is associated with poor patient prognosis.
Figure 2. Nestin expression is associated with poor prognosis and correlates with p53 loss of function in human HCCs.
(A) Nestin mRNA expression in human liver cancer cell lines with intact p53 (p53wt) or loss of p53 function (p53mut/del). (B) Nestin immunohistochemistry of normal liver tissue and HCC tissue. Bars= 50 μm (C) Survival analysis of liver cancer patients stratified into Nestin-positive and Nestin-negative groups. (D) Nestin mRNA expression in human HCCs with p53 loss or diploid p53 status. (E) Co-occurrence of nestin expression and p53 mutation in HCC samples. Fishers exact test was used for measuring association. (F) Nestin expression and p53 expression in a wild-type-p53 expressing (p53 +/+, left panel) and p53 mutant expressing (p53R249S, right panel) tumor nodule of a multinodular HCC. p53 mutation was determined by Sanger sequencing. Bars indicate 50 μm. See also Figure S1.
We next compared p53 status as assessed by array-based CGH and/or sequencing of p53 exons 5-8 to nestin expression as determined from publically available transcriptional profiling data (Neumann et al., 2012). Nestin levels were significantly higher in HCC samples that displayed p53 mutations or reduced copy number at the p53 locus (Fig. 2D, 2E, Suppl. Fig. S2C). One particularly informative HCC sample harbored a single nestin-positive nodule in an otherwise multi-focal tumor (Fig. 2F). Remarkably, DNA sequencing of microdissected tissue revealed a p53 S249A mutation in the nestin-positive nodule, whereas all the nestin-negative nodules harbored wild-type p53 (Fig. 2F). These studies support a direct relationship between loss of p53 and nestin expression in human liver cancer.
p53 represses Nestin transcription
We next investigated the molecular basis underlying the relationship between p53 status and nestin expression in the liver. To report Nestin transcription, we used a transgenic mouse in which activation of the Nestin promoter drives expression of green fluorescent protein (GFP) (Gleiberman et al., 2005). Analysis of adult liver tissues revealed GFP expression limited to individual cells within the interlobular bile duct and the canal of Hering (Suppl. Fig. S2A) – the suspected location of the liver progenitor cell niche in the adult (Fellous et al., 2009). Immunofluorescence staining indicated that expression of the liver progenitor cell markers A6 and EpCAM overlapped with the Nestin-driven GFP, whereas expression of the hepatocyte markers CK8 and asialoglycoprotein receptor (HR) did not (Suppl. Fig. S2B). These observations reinforce the notion that, in normal livers, nestin is expressed in undifferentiated progenitor cell populations.
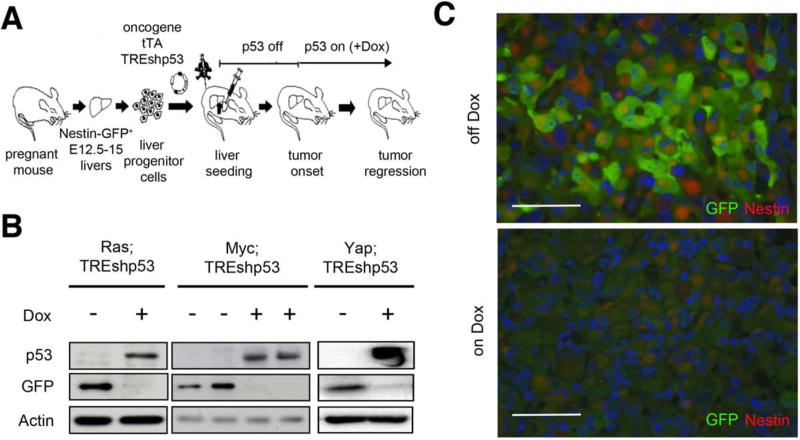
To determine whether there was a direct relationship between p53 and nestin expression during liver tumorigenesis, we took advantage of a liver carcinoma model that we previously used to establish a role for p53 in tumor maintenance (Xue et al., 2007). We isolated E12-15 liver progenitor cells (LPCs) from fetal Nestin promoter-GFP mice and infected them with retroviruses co-expressing different oncogenes (H-RasG12D, Myc or YapS127A), each coupled to a tetracycline transactivator (tTA) that drives reversible shRNA expression. These vectors were co-expressed with a second retrovirus containing a doxycycline-regulated shRNA targeting p53 (schematized in Fig. 3A). Modified fetal liver cells were injected into livers of recipient mice, and after tumor onset, the mice were fed a Doxycycline (Dox)-containing diet to silence the shRNA and reactivate p53. After 8 days, tumor tissue was isolated and analyzed for GFP expression using immunoblotting of liver extracts or by immunofluorescence of tissue sections.
Figure 3. Re-expression of p53 abolishes Nestin expression in vivo.
(A) Schematic workflow of the generation of p53-regulatable liver tumors using Nestin-promoter GFP hepatoblasts. (B) GFP and p53 immunoblots from tumors of mice fed Dox-containing diet for 8 days or nomal chow. (C) Immunofluorescence staining for GFP (green) and Nestin (red) of Ras;TREshp53 tumors off-Dox or 8 days on Dox. Bars=200 μm. See also Figure S2.
Whereas the parental hepatoblast populations were negative for GFP-expression (not shown), tumors with p53 depletion (off-Dox) showed a strong GFP signal irrespective of the driving oncogene, suggesting p53 suppression derepressed the Nestin promoter or, alternatively, selected for a subset of cells with intrinsically high nestin levels. Consistent with the former possibility, tumors re-expressing p53 following Dox addition quickly silenced GFP (Fig. 3B, C). Thus, p53 can repress Nestin-promoter activity in vivo.
p53 regulates nestin expression in an Sp-1/3-dependent manner
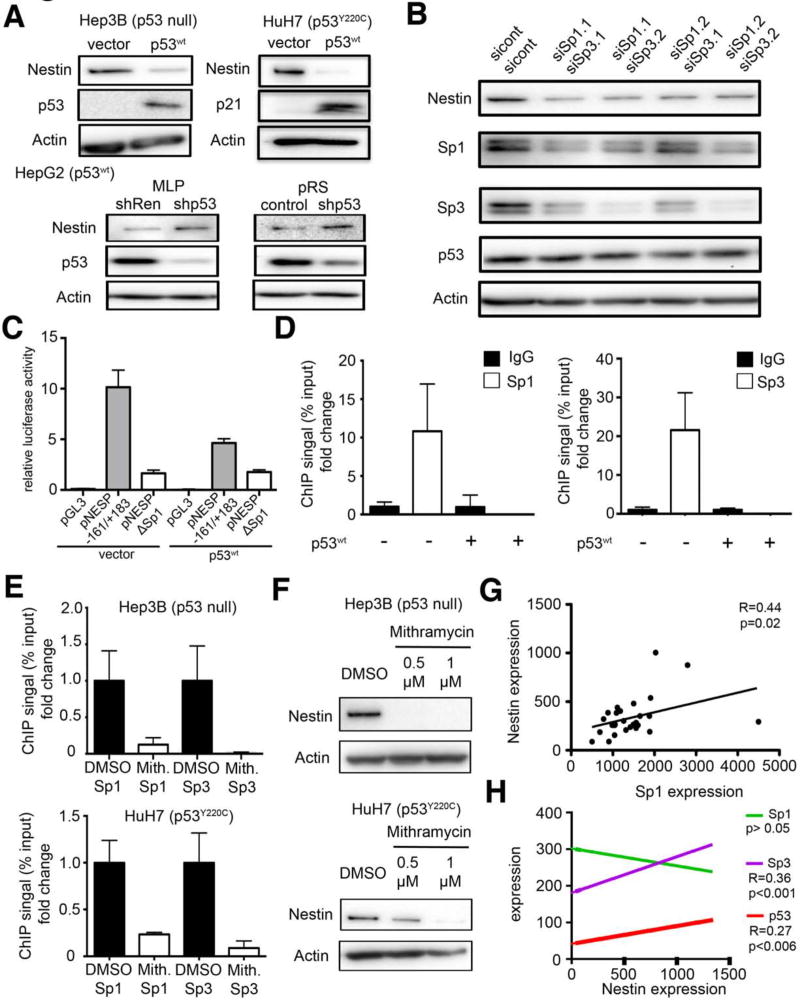
Studies suggest that p53 can act directly to repress mRNA transcription by binding p53 response elements in target promoters, or act indirectly by transcriptionally activating genes such as p21, E2F7, and miR-34 that ultimately reduce mRNA levels through transcriptional or post-transcriptional mechanisms (Ho and Benchimol, 2003). To assess the molecular basis for p53 mediated Nestin repression in human HCC cell lines, we explored factors that influence nestin expression in human HCC cell lines with deletion of p53 (Hep3B), harboring a Y220C mutation in p53 (HuH7) or expressing wild-type p53 (HepG2). Enforced expression of wild-type p53 (p53wt) caused a substantial decrease in Nestin mRNA (data not shown) and protein expression (Fig. 4A) in Hep3B cells and HuH7 cells; conversely, suppression of wild-type p53 in HepG2 cells using RNAi caused an increase in nestin protein levels (Fig. 4A). Confirming the specificity of these effects, neither enforced expression of two different p53 mutants (R175H and R248W) in p53-null Hep3B cells, nor knockdown of mutant p53 (R249S) in PLC cells, altered nestin expression (Suppl. Fig. S3A). Thus, p53 can repress nestin in human HCC cells.
Figure 4. p53 regulates nestin expression in a Sp1/Sp3 dependent manner.
(A) Nestin protein expression in Hep3B and HuH7 cells infected with a vector expressing p53wt or an empty vector (vector) and in HepG2 cells after RNAi mediated p53 knockdown. p53 and p21 expression confirmed functional wildtype p53 expression. (B) Immunoblot for Nestin after siRNA knockdown of Sp1 and Sp3 in HuH7 cells.. (C) Luciferase assay of murine Nestin promoter constructs in the presence or absence of wild-type p53 in NIH3T3 cells (D) ChIP analysis for Sp1 and Sp3 binding to the Nestin promoter in the presence or absence of wild-type p53 in HuH7 cells (E) ChIP analysis for Sp1 and Sp3 binding to the Nestin promoter 18h after treatment with 1 μM Mithramycin or control (DMSO) in Hep3B and HuH7 cells as indicated. (F) Western blot analysis of Nestin expression in Hep3B and HuH7 cells 48h after Mithramycin treatment. (G) Dot plot showing association of Nestin mRNA expression and Sp1 expression in human HCCs, as determined by Spearman correlation. (H) Association of Nestin mRNA expression with Sp1, Sp3, and p53 mRNA expression in cholangiocarcinomas.. Spearman correlation was used to determine statistical significance. See also Figure S3.
Using different informatics approaches, we identified a potential p53 binding site in the mouse Nestin gene (data not shown). However, this site was not conserved in the human Nestin promoter and, since p53 can suppress nestin in human cells, we hypothesized that p53 most likely represses nestin through an indirect mechanism. Still, its repressive effect was not mediated through p21, E2F7, or miR-34, because Huh7 cells co-expressing potent shRNAs targeting p21 or E2F7 still repressed nestin in response to wild-type p53 (Suppl. Fig. S3B), and no predicted mir34 binding sites were found in the 3'UTR of the murine or human transcripts (data not shown).
Another mechanism whereby p53 can repress transcription is by antagonizing other transcriptional activators. For example, p53 can bind Sp1 leading to the repression of several Sp1-inducible genes involved in embryonic development and angiogenesis (Kong et al., 2013; Zhang et al., 2000). Interestingly, the murine Nestin promoter harbors two Sp1 binding sites upstream of the transcription start site (TSS), which are conserved in the human Nestin gene and crucial for nestin expression (Cheng et al., 2004) (see also Suppl. Fig. S3E). To test whether Sp1 (and its related family member Sp3) controls nestin in HCC cells, we used siRNAs capable of knocking down either Sp1 or Sp3 and tested their ability to modulate nestin expression in p53 mutant HuH7 cells. Although none of the Sp1 and Sp3 siRNAs were capable of potent Sp1/3 knockdown (Suppl. Fig. S3C), co-suppression of both genes substantially reduced nestin protein (Fig. 4B) and mRNA (Suppl. Fig. S3D) expression.
To investigate whether p53 influences the activity of the Nestin promoter via Sp1, we transfected NIH3T3 cells with murine Nestin promoter constructs harboring intact or mutated Sp1-binding sites (Cheng et al., 2004) together with either a p53wt expression vector or an empty vector control. Confirming the Sp1 dependence of the nestin promoter (Cheng et al., 2004), cells transfected with reporter construct harboring intact Sp1 binding sites (NESP -161/+183) showed luciferase activity, whereas cells harboring the construct with deleted Sp1 sites (NESP ΔSp1) did not (Fig. 4C). Importantly, co-expression of p53 substantially decreased reporter output from the NESP -161/+183 but had no effect on NESP ΔSp1 activity (Fig. 4C).
Chromatin immunoprecipitation (ChIP) experiments using antibodies directed to either Sp1 or Sp3 revealed specific binding of each protein to the consensus sites in p53 mutant human HuH7 cells. Consistent with a role for p53 in modulating this activity, enforced expression of wild-type p53 triggered a release of Sp1 and Sp3 from the nestin promoter (Fig. 4D). Similarly, treatment of Hep3B (p53 null) and Huh7 (p53 mutant) cells with mithramycin, a small molecule capable of binding the GC rich regions found in Sp1 binding sites (Bond et al., 2004), abolished Sp1/3 binding to the Nestin promoter in a dose dependent manner (Fig. 4E, F). Together, these results indicate that Sp1 and Sp3 bind and activate the Nestin promoter in HCC cells in a manner that is antagonized by functional p53.
Wild-type p53 can interact directly with Sp1, suggesting a way in which p53 might repress gene expression in a manner that is disrupted in cancer cells. To confirm this interaction can occur in liver cancer cells, we co-transfected wild-type p53 into p53 mutant HuH7 cells, and assessed the ability of Sp1 to co-immunoprecipitate with p53. Interestingly, the transduced wild type p53 bound Sp1 whereas the endogenous mutant p53 did not (Suppl. Fig. S3F). Nonetheless, we could not detect p53 bound to the Nestin promoter, suggesting this interaction with Sp1 occurs off chromatin (data not shown). p53/Sp1/3 complexes have been reported to interfere with a self-amplification process, leading to diminished Sp1/3 levels (Tapias et al., 2008). Concordantly, we observed reduced Sp1, Sp3, and nestin protein levels in response to p53wt transduction into HuH7 cells (Suppl. Fig. S3G). Consistent with the role of Sp1/3 in activating nestin, Nestin mRNA expression significantly correlated with Sp1 levels in primary HCCs (Fig. 4G) and Sp3 levels in CCs (Fig. 4H). Therefore, p53 represses Nestin at least in part by antagonizing Sp1 and Sp3.
p53 restricts hepatocyte transformation and plasticity
To further investigate the relationship between p53 and nestin expression in liver tumorigenesis, we used a transposon-based system that enables the production and analysis of liver carcinomas with defined genetic alterations in the mouse (Yant et al., 2000). In this approach, plasmids harboring a recombinant Sleeping Beauty (SB) transposon vector and transposase are injected into the liver using hydrodynamic tail vein injection, which leads to selective uptake by hepatocytes (Bell et al., 2007). Transient expression of transposase in the transduced hepatocytes facilitates integration of the transposon vector into genomic DNA, allowing stable and heritable transgene expression. Of note, morphological and lineage tracing studies indicate that only differentiated hepatocytes and not cholangiocytes or progenitor cells are transduced using this approach (Fan et al., 2012).
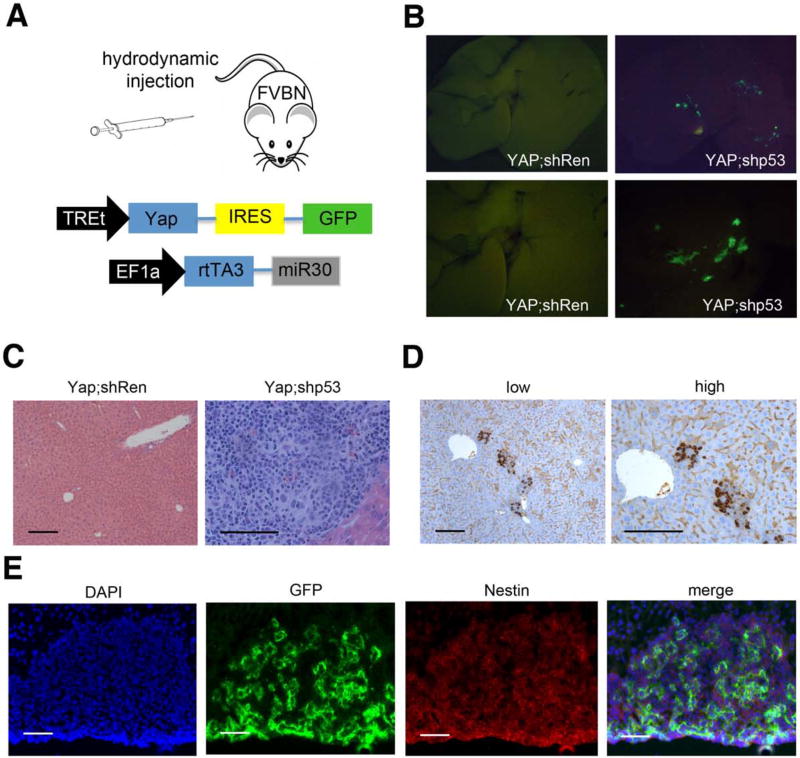
We chose to use Yap as a tumor promoting oncogene, as it is altered in both HCC and CC (Li et al., 2012) and can drive hepatocellular carcinoma in orthotopic or transgenic models (Dong et al., 2007; Zender et al., 2006). A two vector system was used to ensure the expression of Yap together with an shRNA in the same cell: in one vector, Yap and a GFP reporter were co-expressed under the control of the inducible TREtight (TREt) promoter; in the other, the mir30 shRNA cassette was placed downstream of a reverse tetracycline transactivator (rtTA3) expressed from the constitutive EF1a promoter (Fig. 5A). Hence, only cells receiving both vectors are capable of inducing Yap expression upon Dox addition, and then these cells can be visualized using the co-expressed GFP reporter. For these experiments, we incorporated either a control shRNA targeting Renilla firefly luciferase (shRen) or a well-characterized shRNA capable of potently suppressing p53 (shp53).
Figure 5. Yap;shp53 leads to undifferentiated nestin-positive tumors in adult hepatocytes.
(A) Schematic of transposon vectors used in this experiment. (B) Fluorescence images of whole livers 6 weeks after hydrodynamic transposon delivery into mice (n=5). (C) Representative H/E stainings of Yap;shp53 livers and Yap;shRen 6 weeks after injection. Bars indicate 100 μm (D) Nestin immunohistochemistry of liver sections from Yap;shp53 injected mice. Bars= 100 μm (E) Immunofluorescence of liver sections of Yap;shp53 injected mice. GFP marks expression of the YAP containing transposon vector, Nestin expression is detected by antibody staining. Bars indicate 50 μm. See also Figure S4.
Hydrodynamic tail vein injection was used to target the above vector combinations to the livers of FVBN mice. As anticipated from previous reports (Fan et al., 2012), analysis of livers for GFP-positive cells shortly after transduction identified the transduced cells as HNF4a-expressing hepatocytes, and no transduced cells were observed in CK19-positive cholangiocytes. Similarly, injection of dsRed expressing vectors into Nestin promoter-GFP mice revealed no overlap of dsRed and nestin expression (Suppl. Fig. 4A). Six weeks after injection and Dox addition, mice receiving YAP and the control shRen transposons showed no GFP fluorescence, suggesting these cells were eventually cleared. Accordingly, no overt pathology was observed (left panels Fig. 5B and C). Thus, Yap alone was unable to drive tumorigenesis over the observation period.
Livers from mice receiving the combination of Yap and a p53 shRNA displayed a dramatically different phenotype. Visual inspection and histological analyses (right panels Fig. 5B and C) revealed the presence of many GFP-positive foci consisting of small undifferentiated tumor cells that displayed markers of bipotential liver progenitors, having weak expression of the epithelial marker CK19 and high expression of the stem cell marker CD133 (data not shown). These tumors appeared aggressive, showing numerous mitotic figures and invasive growth (Fig. 5C). Consistent with the ability of p53 to repress nestin, most Yap/shp53-expressing tumors were also nestin-positive (Fig. 5D, 5E). Importantly, similar tumors were produced using an orthogonal approach whereby a cre-ER transgene was substituted for the p53 shRNA and the same experiment performed using p53-floxed mice (p53fl/fl) (Suppl. Fig. S4B): only animals treated with tamoxifen (to activate cre and delete p53) developed tumors, all of which were GFP-positive, undifferentiated, and highly expressed Nestin (Suppl. Fig. S4C-E). Together, these data demonstrate that p53 loss facilitates YAP induced tumorigenesis by allowing dedifferentiation of adult hepatocytes into progenitor-like cells capable of malignant expansion. Thus, p53 appears to restrict cellular plasticity in response to an oncogenic stimulus.
Nestin is required for tumorigenesis
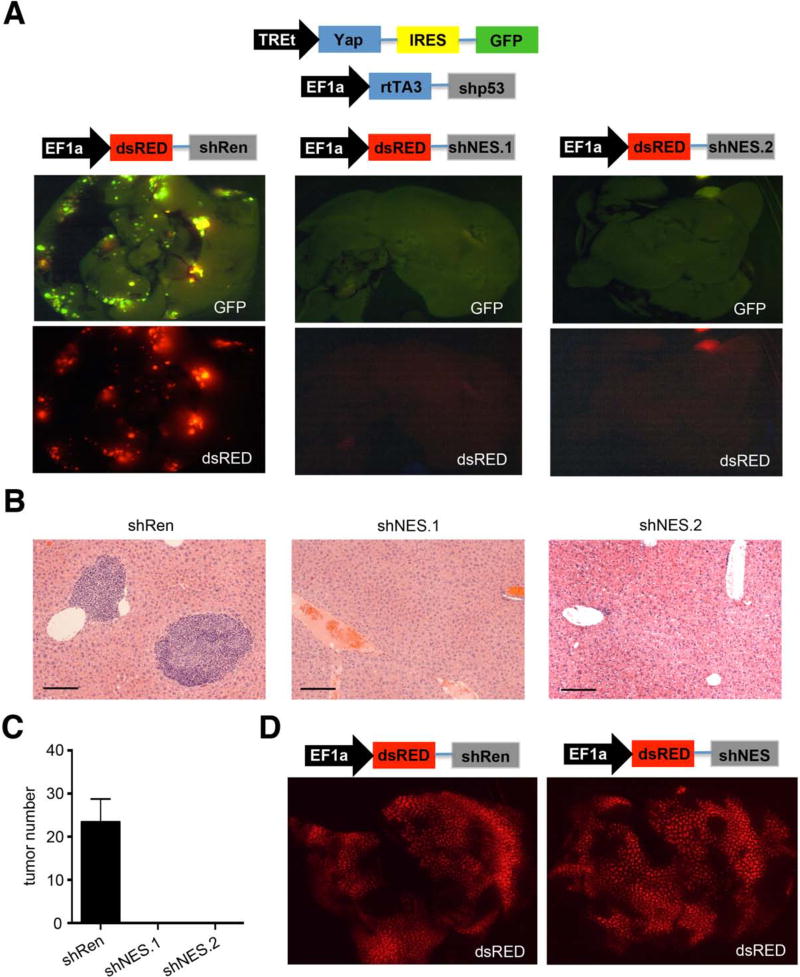
Although nestin has been considered simply a marker of stem and progenitor cells, its tight link to p53 expression in the liver raised the possibility it plays a more active role in tumorigenesis. To test this, we injected transposons encoding YAP and p53 shRNA into murine livers together with an excess of a third EF1a-driven transposon vector that co-expresses either a nestin or control (Renilla luciferase) shRNA with dsRED (Fig. 6A). Expression of Yap/p53shRNA was again traced using GFP, whereas dsRED provided a surrogate marker for nestin or Renilla shRNA expression. These combinations were introduced into mice by hydrodynamic transfection, and livers were analyzed six weeks later.
Figure 6. Nestin is important for tumor initiation in vivo.
(A) Schematic of injected transposons vectors used in this experiment. Respective GFP and dsRED fluorescence images of livers from transposon injected mice 6 weeks after injection (n=5-7 mice per experiment), as indicated. (B) H/E stainings of the livers. Bars indicate 100 μm (C) Quantification of tumor numbers of animals. Error bars represent SEM (n=5-7 per group). (D) dsRED expression in normal livers 6 weeks after injection of indicated transposon vectors. See also Figure S5.
The addition of the control dsRED-linked shRNA had no impact on the disease course induced by the Yap/shp53 combination, and multiple GFP/dsRED double positive tumors with an undifferentiated histology were detected in the livers of recipient mice (Fig. 6A and B). By marked contrast, the inclusion of two independent dsRED-linked nestin shRNAs completely blocked tumor formation, such that the recipient livers showed no dsRED/GFP foci, and no tumors were identified by histology (Fig. 6C). The inhibitory effect of nestin shRNAs on tumor formation was not merely a toxic side-effect of RNAi in hepatocytes (Grimm et al., 2006), as mice receiving dsRED-linked nestin shRNAs without Yap and the p53 shRNA showed many dsRED positive cells that were retained in the liver for at least six weeks without producing any overt liver pathology (Fig. 6D; data not shown).
p53 mutant Huh7 cells transduced with potent two independent Nestin shRNAs (Suppl. Fig. 5A) showed an accumulation of cells in G2/M-phase of the cell cycle (Suppl. Fig. S5C) and an increase in phospho-H3 positive cells, indicative of impaired progression through mitosis (Suppl. Fig. S5D). Accordingly, nestin depleted cells were less able to form colonies when placed at low density (Suppl. Fig. S5B) and displayed decreased tumorigenic potential upon injection into immunocompromised mice (Suppl. Fig. S5E). While these results indicated nestin is necessary for tumorigenesis of p53 mutant murine and human HCC cells it is not sufficient: hence, retroviral transduction of Nestin cDNA into myc;p53+/+ hepatoblasts was unable to mirror p53 loss in promoting transformation and had no additional impact on the proliferative capacity of myc;p53-/- hepatoblasts in vitro (Suppl. Fig. S5F). Therefore, nestin is required for the dedifferentiation and malignant expansion of p53-deficient cells and apparently contributes to tumor maintenance.
Additional oncogenic “hits” program p53-deficient progenitor tumors into HCCs or CCs
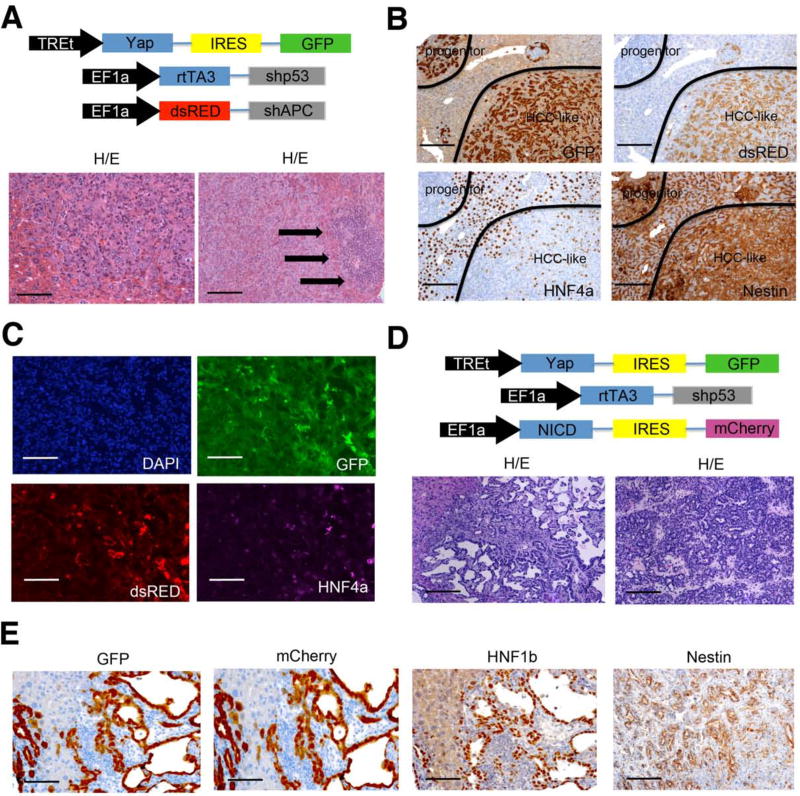
Although nestin expression is normally restricted to a putative stem/progenitor cell compartment in the liver, our results demonstrate that deletion of p53 in hepatocytes facilitates cellular plasticity in response to Yap leading to the generation of undifferentiated tumors with high nestin expression. Nonetheless, tumors with a similar histology are not observed in humans, perhaps because lesions that enforce lineage specification cooperate with p53 loss during tumorigenesis. We therefore hypothesized that these undifferentiated cells might be driven to HCC or CC by enforcing Wnt or Notch pathway activation, which drive hepatocyte and cholangiocyte differentiation (Boulter et al., 2012) and are frequently activated in HCC and CC, respectively. To do this, we produced dsRED tagged transposon vectors expressing an APC shRNA, which deregulates Wnt signaling, or the notch intracellular domain (NICD), which constitutively activates Notch signaling, and performed hydrodynamic transduction with the YAP/GFP and p53 shRNA transposons as described above (Fig 7A, 7D).
Figure 7. Progenitor cells can differentiate to HCC or CC in vivo.
(A) Schematic of transposon vector combination used to generate HCCs and representative H/E stainings of tumors after 6 weeks. Arrows indicate small progenitor tumor cells. Bar= 100 μm (B) GFP (Yap;shp53), dsRED (shAPC), HNF4a, and nestin staining of progenitor like tumor cells and HCC-like tumor cells. Bars indicate 200 μm (C) Immunofluorescence images of a tumor nodule of Yap/shp53/shAPC injected mice. GFP indicates Yap/shp53, dsRED indicates shAPC, HNF4a was detected by Alexa-648 secondary antibody. DAPI was used for counterstaining. Bars reflect 100 μm (D) Schematic of transposon vectors used to generate CC and representative HE sections of liver tumors 6 weeks after injection. (E) GFP (Yap;shp53), mCherry (NICD), HNF1b, and nestin immunohistochemistry of cholangiocarcinomas. Bars indicate 100 μm.
Histological analyses of the Yap/p53shRNA/APCshRNA transduced livers performed six weeks after injection contained many epithelial tumors composed of polygonal cells growing in a solid-trabecular pattern without a desmoplastic stroma – features characteristic of HCC (Fig. 7A). More detailed microscopic analysis of the same tissue also revealed small undifferentiated tumors similar to those observed in mice transduced with just YAP and shp53 (Fig. 7A, arrows). Interestingly, the HCC-like lesions showed co-expression of GFP and dsRED, whereas undifferentiated tumor regions only expressed GFP indicating they did not express shAPC (Fig. 7B). In agreement, the hepatocyte-specific marker HNF4a was expressed in the GFP/dsRED-double positive HCC-like tumor zones and double negative normal hepatocytes, but not in the GFP single positive undifferentiated tumors (Fig. 7C). Regardless of their differentiation state, all tumors expressed high nestin levels (Fig. 7C). Thus, APC loss can drive p53-deficient progenitor-like lesions towards a those with features of hepatocyte differentiation while retaining high nestin expression.
Co-expression of NICD with Yap and shp53 produced a markedly distinct result. Histopathological examination revealed tumors that displayed the typical ductal and papillary growth pattern and stromal involvement of CC (Fig. 7D) and expressed GFP, mCherry, and the cholangiocyte marker HNF1b (Fig. 7E). Despite their differentiated phenotype but consistent with CCS harboring mutant p53, these murine CCs retained high nestin expression (Fig. 7E). Collectively, these results demonstrate that p53, in part through repressing nestin, restricts both cell plasticity and tumorigenesis in the liver. As a consequence, p53-deficient hepatocytes can produce tumors that adopt characteristics of distinct cell fates depending on initiating and cooperating oncogenic events.
Discussion
Many studies have focused on the activity of p53 as a transcriptional activator and its target genes linked to cell cycle arrest, apoptosis, senescence, and other anti-proliferative processes (Vousden and Lane, 2007). Here we demonstrate that p53 acts via Sp1/3 to repress nestin expression and that nestin is required for p53-inactivation to promote liver tumorigenesis. Further, p53 loss enables dedifferentiation of mature hepatocytes in response to an oncogenic stimulus leading to the expansion of malignant reprogrammed progenitor cells capable of acquiring features of HCC or CC upon acquiring lineage-specific oncogenic lesions. Nonetheless, these tumors retain high nestin expression and an aspect of “stemness” that, in patients, is associated with a poor prognosis. Consequently, our results identify nestin as a key player in liver carcinogenesis and have implications for p53 action in tumor suppression.
Nestin is a stem cell marker that functionally contributes to liver tumorigenesis
A direct role for nestin in modulating liver cancer was unexpected given its established role as a marker of stemness in neural progenitor cells and malignant gliomas (Chen et al., 2012; Mignone et al., 2004). Nonetheless, hints that nestin plays a broader role in normal stem cell biology and cancer come from studies showing nestin marks progenitor cell populations outside the central nervous system and that its inhibition can impair the proliferation and survival of certain cancer cell lines in culture (Krupkova et al., 2010). While our data confirm these observations for HCC, the molecular mechanism by which nestin contributes to liver tumorigenesis remains to be determined. Consistent with our observations, nestin is required for cell proliferation, migration, and invasion in other contexts, for example, during neuronal cell development and in glioblastoma and lung cancer cell lines (Lu et al., 2011; Sahlgren et al., 2006; Takakuwa et al., 2013).
Since nestin is not normally expressed in adult but is induced in response to oncogenic stress, the requirement for nestin in proliferation may reflect its ability to promote cell reorganization after mitosis in rapidly proliferating cells (Takakuwa et al., 2013). Accordingly, our limited analysis confirms that nestin is required for efficient progression through the G2/M phase of the cell cycle in human HCC cells. Regardless of the precise mechanism, our data indicate that nestin is not merely a stem/progenitor cell marker but instead actively participates in cancer progression.
p53-mediated gene repression and tumor suppression
Our results have implications for the biochemical properties of p53 that contribute to its action in cancer suppression. Despite intensive efforts to prove its relevance, it remains unclear whether transcriptional activation by p53 is sufficient to mediate tumor suppression (Brady et al., 2011). In light of this conundrum, it is intriguing that p53 can also repress transcription and, consistent with its importance, p53-mediated repression of Nestin in the liver restricts cellular plasticity and limits tumorigenesis. Hence, p53 loss leads to high nestin expression in p53 mutant tumors, and nestin ablation impairs tumor formation.
p53 represses nestin through an indirect mechanism that involves its ability to bind Sp1 and impair its function. Whether all p53 mutants are defective in binding Sp1 remains a topic of debate, though there is general agreement that p53 mutations disable its ability to repress Sp1 target genes (Zhang et al., 2000). Interestingly, two other pluripotency genes – Nanog and CD44 – are also repressed by p53 (Godar et al., 2008; Lin et al., 2005), hinting towards a broader role for p53-mediated gene repression in maintaining cell identity. While this repression is thought to result from direct p53 effects, both Nanog and CD44 have functional SP1 sites in their promoters and could be subject to SP1 antagonism by p53 as well (Wu and Yao, 2006; Zhao et al., 2013). Beyond Nestin, p53/Sp1 represses the expression of other genes such including VEGF, IGF-1R, and hTERT whose increased expression has been widely linked to tumorigenesis. It thus seems likely that p53 limits tumor growth by coordinating both gene activation and repression programs.
p53 restricts cellular plasticity during tumorigenesis
Our results also have ramifications for the biological properties of p53 that contribute to tumor suppression. p53 loss in the liver enables the aberrant expansion of oncogene-expressing cells. Unexpectedly, those malignant cells that do emerge have high nestin levels and thus retain some progenitor-like characteristics, suggesting that these cells might be particularly sensitive to the action of p53. Consistent with this view, p53 re-expression in p53-deficient embryonic carcinoma cells triggers differentiation (Lutzker and Levine, 1996), and p53 loss enables myeloid progenitor cells to acquire an indefinite state of self-renewal in response to certain oncogenic events, thereby contributing to leukemogenesis. Together, these observations imply that a key function of p53 in tumor suppression involves its ability to restrict the self-renewal of multi-potent progenitor cells. Indeed, the first p53 function that was described was its ability to restrict cellular immortalization (Harvey and Levine, 1991), a process characterized by the indefinite ability of cultured cells to self-renew.
Beyond limiting the ability of premalignant cells to self-renew, our results imply that p53 can restrict the plasticity of premalignant cells in vivo and that this property is important for its action as a tumor suppressor. Cellular plasticity is typically defined as the ability of cells to change identity through a process of dedifferentiation, differentiation or both, and contributes to regenerative processes in plants, invertebrates, and amphibians (Sugimoto et al., 2011). Emerging data suggest that a similar potential exists in mammals, for example, as evidenced by the dedifferentiation of luminal secretory cells into basal stem cells in the mouse lung during injury repair (Tata et al., 2013). Indeed, recent studies in mice suggest that dedifferentation can accompany tumorigenesis and that differentiated cells can serve as the cell of origin of cancer (Friedmann-Morvinski et al., 2012; Schwitalla et al., 2013).
Using a transgenesis method that stably expresses genes in adult hepatocytes, we found that p53 loss confers extraordinary cellular plasticity during liver tumorigenesis. Specifically, p53 suppression in hepatocytes allows dedifferentiation and the emergence of nestin-positive progenitor cell tumors in response to Yap, an oncogene that is overexpressed in human HCC and CC and can drive hepatocellular carcinoma in mice (Dong et al., 2007). Remarkably, such p53-deficient tumors can reacquire features of differentiated hepatocytes or even cholangiocytes with the addition of lineage specific lesions that deregulate the Wnt and Notch pathways, respectively. Importantly, p53 loss alone confers no lineage bias per se, but instead enables the expansion of progenitor like cells that take on distinct cell fates through different cooperating genetic events.
Of the oncogene combinations we analyzed, p53 loss was unique in its ability to produce tumor with features of undifferentiated progenitor cells. It seems likely that the appearance of these lesions also involves specific functions of YAP, as p53-deficient tumors driven by Myc or oncogenic K-ras acquire features of HCC and CC, respectively (Saborowski et al., 2013). Nonetheless, irrespective of the cooperating oncogene, the resulting p53-deficient tumors acquire some progenitor-like characteristics as indicated by high nestin expression. Perhaps these observations explain the association between p53 mutations and the presence of stem cell signatures in certain human cancers (Markert et al., 2011; Mizuno et al., 2010) and why HCC and CC, which otherwise display distinct mutational landscapes, each display high rates of p53 mutations (Nault and Zucman-Rossi, 2011).
A role for p53 maintaining cell identify by restricting plasticity during cancer is consistent with disparate observations related p53 biology to cellular differentiation and stem cell biology. A primordial role for p53 in controlling cell plasticity in vivo has been noted during limb regeneration in the Salamander (Yun et al., 2013). Despite little evidence that p53 contributes to normal mammalian development, enforced p53 expression triggers differentiation in certain p53-deficient tumor cells (Soddu et al., 1994) and, conversely, p53 loss has been described as a factor that promotes de-differentiation during glioblastoma development in mice (Friedmann-Morvinski et al., 2012). Collectively, these observations imply that p53 helps maintain cell identity in cells encountering aberrant proliferative signals and support the emerging view that de-differentiation can be important for the etiology of certain tumor types.
Our results are particularly intriguing in light of p53 action in limiting the epigenetic reprogramming of differentiated cells into induced pluripotent stem (iPS) cells (Hong et al., 2009; Kawamura et al., 2009; Marion et al., 2009). While demonstrating a role for p53 in limiting plasticity, the cancer relevance of these observations was unknown, and indeed might merely reflect a benefit due to increased proliferation in the reprogramming process (Hanna et al., 2009). Our studies suggest that p53 loss enables the reprogramming of differentiated hepatocytes to malignant progenitors with multi-lineage potential that can acquire distinct cell fates through sustaining other mutations. While it seems likely that human liver carcinomas can arise from normal progenitor cells as well, our studies establish the importance of p53 in restricting cellular plasticity during liver carcinogenesis and define a tumorigenic path for the emergence of liver carcinomas with stem cell features and a poor prognosis.
Experimental procedures
Animals and treatments
The Nestin promoter-GFP mice were described before (Cheng et al., 2004). 8-10 week old female FVB/N mice were purchased from Charles River laboratories or Jackson laboratory. p53 fl/fl mice were obtained from our breeding colony and maintained on a Bl6 background. The Alfp-Cre p53 fl/fl animals were described recently (Katz et al., 2012). All animal experiments were approved by the MSKCC Institutional Animal Care and Use Committee (protocol 11-06-011).
Hydrodynamic tail vein injection
For hydrodynamic tail vein injection a sterile 0.9% NaCl solution/plasmid mix was prepared containing 10μg DNA of each Transposon vector together with CMV-SB13 Transpoase (1:5 ratio). Mice were injected with the 0.9% NaCl solution/plasmid mix into the lateral tail vein with a total volume corresponding 10 % of body weight in 5-7 seconds.
Human patient samples
Generation of mRNA and array-CGH data from human hepatocellular carcinomas (Neumann et al., 2012) and human cholangiocellular carcinomas (Andersen et al., 2012) was previously described. Pseudo-anonymized human FFPE tissue samples from HCCs, HCC-CCCs, and CCCs were provided by the Tissue Bank of the National Center for Tumor Diseases Heidelberg (Heidelberg, Germany) and the Institute of Pathology of the Greifswald University (Greifswald, Germany). Institutional Review Board approval was obtained at participating hospitals. All specimens were classified according to established criteria (World Health Organization, Union for International Cancer Control) by experienced pathologists (D.F.T., M.E., T. L.).
Statistical analysis
Data is presented as mean ± standard deviation if not otherwise stated. The Spearman rank coefficient or Fishers exact test were used for statistical measure of association as indicted. The statistical comparison between 2 groups was accomplished with the two tailed students t-test. All statistical tests were performed using the PRISM6 software.
Supplementary Material
Supplemental Information includes Extended Experimental Procedures, five figures, and two tables
Highlights.
Nestin overexpression in HCC and CC is linked with p53 mutations and poor prognosis
Nestin is repressed by p53 and is required for the emergence of p53 mutant tumors
p53 restricts expansion of malignant progenitors in response to oncogenic stress
p53 restrict cellular plasticity while specific lesions dictate malignant cell fate
Acknowledgments
We thank all members of the Lowe lab for stimulating discussion and in particular Dr. Charles J. Sherr for important suggestions and editing the manuscript. We thank D. Grace, J. Simon, the MSKCC animal facility, and MSKCC molecular cytology core for outstanding technical support. This work was supported by program project grants from the National Cancer Institute (S.W.L.). This study was supported by the National Center for Tumor Disease, Heidelberg, and grants of the German Research Foundation (DFG, SFB/TRR77) and the German Cancer Aid (T.L.). D.F.T. is funded by a postdoctoral fellowship of the German Research Foundation (DFG). S.W.L. is the Geoffrey Beene Chair for Cancer Biology and a Howard Hughes Medical Institute investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: D.F.T. designed and performed the majority of experiments, with W.X., T.V.M., and L.Z. contributing key data and ideas important for the early stages of this work. D.F.C., M.E., J.B.A., D.C., and T.L. analyzed human patient samples; L.E.D., A.B., and E.R.K provided critical reagents and ideas; S.F.K. and A.L. provided annotated tumor samples from Alfp-Cre p53fl/fl mice.; and S.W. and C.H.H. performed immunoprecipitation and chromatin immunoprecipitation, respectively. G.E. provided the nestin-GFP mouse and G.E. and S.W.L. helped conceive the study and supervised research. D.F.T. and S.W.L. wrote the manuscript with the input of all coauthors.
References
- Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. doi: 10.1053/j.gastro.2011.12.005. e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JB, Podetz-Pedersen KM, Aronovich EL, Belur LR, McIvor RS, Hackett PB. Preferential delivery of the Sleeping Beauty transposon system to livers of mice by hydrodynamic injection. Nature protocols. 2007;2:3153–3165. doi: 10.1038/nprot.2007.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nature medicine. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Kenzelmann Broz D, Basak S, Park EJ, McLaughlin ME, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–583. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Jin Z, Liu L, Yan Y, Li T, Zhu X, Jing N. Characterization and promoter analysis of the mouse nestin gene. FEBS letters. 2004;565:195–202. doi: 10.1016/j.febslet.2004.03.097. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, et al. Cholangiocarcinomas can originate from hepatocytes in mice. The Journal of clinical investigation. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous TG, Islam S, Tadrous PJ, Elia G, Kocher HM, Bhattacharya S, Mears L, Turnbull DM, Taylor RW, Greaves LC, et al. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology. 2009;49:1655–1663. doi: 10.1002/hep.22791. [DOI] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman AS, Encinas JM, Mignone JL, Michurina T, Rosenfeld MG, Enikolopov G. Expression of nestin-green fluorescent protein transgene marks oval cells in the adult liver. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;234:413–421. doi: 10.1002/dvdy.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Levine AJ. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes & development. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Ho J, Benchimol S. Transcriptional repression mediated by the p53 tumour suppressor. Cell death and differentiation. 2003;10:404–408. doi: 10.1038/sj.cdd.4401191. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis ben liver cancer. Oncogene. 2007;26:2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- Katz SF, Lechel A, Obenauf AC, Begus-Nahrmann Y, Kraus JM, Hoffmann EM, Duda J, Eshraghi P, Hartmann D, Liss B, et al. Disruption of Trp53 in livers of mice induces formation of carcinomas with bilineal differentiation. Gastroenterology. 2012;142:1229–1239. doi: 10.1053/j.gastro.2012.02.009. e1223. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisua Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzelmann Broz D, Attardi LD. In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis. 2010;31:1311–1318. doi: 10.1093/carcin/bgp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupkova O, Jr, Loja T, Zambo I, Veselska R. Nestin expression in human tumors and tumor cell lines. Neoplasma. 2010;57:291–298. doi: 10.4149/neo_2010_04_291. [DOI] [PubMed] [Google Scholar]
- Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver international : official journal of the International Association for the Study of the Liver. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nature cell biology. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Lan F, He Q, Lee A, Tang CZ, Dong L, Lan B, Ma X, Wu JC, Shen L. Inducible expression of stem cell associated intermediate filament nestin reveals an important role in glioblastoma carcinogenesis. International journal of cancer Journal international du cancer. 2011;128:343–351. doi: 10.1002/ijc.25586. [DOI] [PubMed] [Google Scholar]
- Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzker SG, Levine AJ. A functionally inactive p53 protein in teratocarcinoma cells is activated by either DNA damage or cellular differentiation. Nature medicine. 1996;2:804–810. doi: 10.1038/nm0796-804. [DOI] [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert EK, Mizuno H, Vazquez A, Levine AJ. Molecular classification of prostate cancer using curated expression signatures. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:21276–21281. doi: 10.1073/pnas.1117029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. The Journal of comparative neurology. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Spike BT, Wahl GM, Levine AJ. Inactivation of p53 in breast cancers correlates with stem cell transcriptional signatures. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22745–22750. doi: 10.1073/pnas.1017001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault JC, Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Seminars in liver disease. 2011;31:173–187. doi: 10.1055/s-0031-1276646. [DOI] [PubMed] [Google Scholar]
- Neumann O, Kesselmeier M, Geffers R, Pellegrino R, Radlwimmer B, Hoffmann K, Ehemann V, Schemmer P, Schirmacher P, Lorenzo Bermejo J, et al. Methylome analysis and integrative profiling of human HCCs identify novel protumorigenic factors. Hepatology. 2012;56:1817–1827. doi: 10.1002/hep.25870. [DOI] [PubMed] [Google Scholar]
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- Rumsby PC, Davies MJ, Evans JG. Screening for p53 mutations in C3H/He mouse liver tumors derived spontaneously or induced with diethylnitrosamine or phenobarbitone. Molecular carcinogenesis. 1994;9:71–75. doi: 10.1002/mc.2940090204. [DOI] [PubMed] [Google Scholar]
- Saborowski A, Saborowski M, Davare MA, Druker BJ, Klimstra DS, Lowe SW. Mouse model of intrahepatic cholangiocarcinoma validates FIG-ROS as a potent fusion oncogene and therapeutic target. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1311707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. The EMBO journal. 2006;25:4808–4819. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. The Journal of clinical investigation. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Soddu S, Blandino G, Citro G, Scardigli R, Piaggio G, Ferber A, Calabretta B, Sacchi A. Wild-type p53 gene expression induces granulocytic differentiation of HL-60 cells. Blood. 1994;83:2230–2237. [PubMed] [Google Scholar]
- Spike BT, Wahl GM. p53, Stem Cells, and Reprogramming: Tumor Suppression beyond Guarding the Genome. Genes & cancer. 2011;2:404–419. doi: 10.1177/1947601911410224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends in cell biology. 2011;21:212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Takakuwa O, Maeno K, Kunii E, Ozasa H, Hijikata H, Uemura T, Kasai D, Ohkubo H, Miyazaki M, Oguri T, et al. Involvement of intermediate filament nestin in cell growth of small-cell lung cancer. Lung Cancer. 2013;81:174–179. doi: 10.1016/j.lungcan.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Tapias A, Ciudad CJ, Roninson IB, Noe V. Regulation of Sp1 by cell cycle related proteins. Cell Cycle. 2008;7:2856–2867. doi: 10.4161/cc.7.18.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nature reviews Molecular cell biology. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Wu DY, Yao Z. Functional analysis of two Sp1/Sp3 binding sites in murine Nanog gene promoter. Cell research. 2006;16:319–322. doi: 10.1038/sj.cr.7310040. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant SR, Meuse L, Chiu W, Ivics Z, Izsvak Z, Kay MA. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nature genetics. 2000;25:35–41. doi: 10.1038/75568. [DOI] [PubMed] [Google Scholar]
- Yun MH, Gates PB, Brockes JP. Regulation of p53 is critical for vertebrate limb regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17392–17397. doi: 10.1073/pnas.1310519110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu D, Hu M, Xiong S, Lang A, Ellis LM, Pollock RE. Wildtype p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer research. 2000;60:3655–3661. [PubMed] [Google Scholar]
- Zhao Y, Zhang W, Guo Z, Ma F, Wu Y, Bai Y, Gong W, Chen Y, Cheng T, Zhi F, et al. Inhibition of the transcription factor Sp1 suppresses colon cancer stem cell growth and induces apoptosis in vitro and in nude mouse xenografts. Oncology reports. 2013;30:1782–1792. doi: 10.3892/or.2013.2627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information includes Extended Experimental Procedures, five figures, and two tables