Abstract
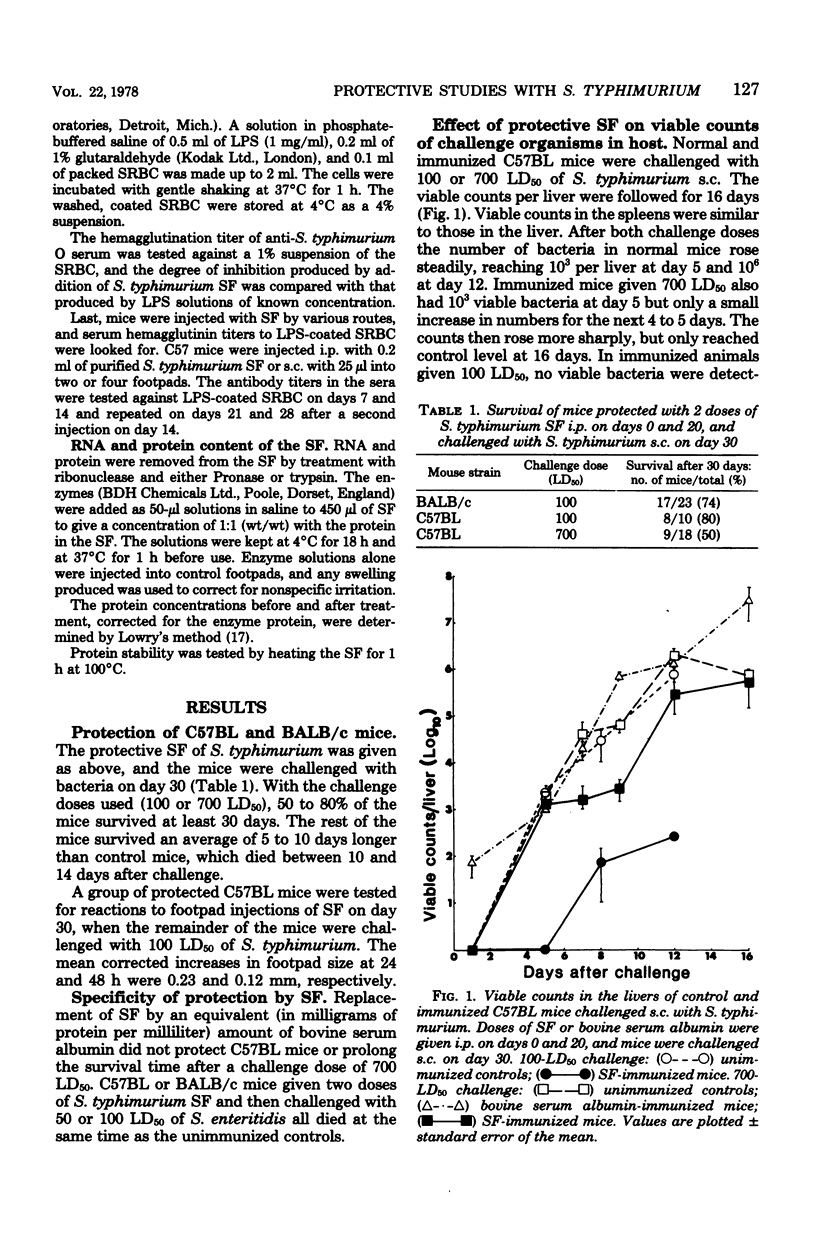
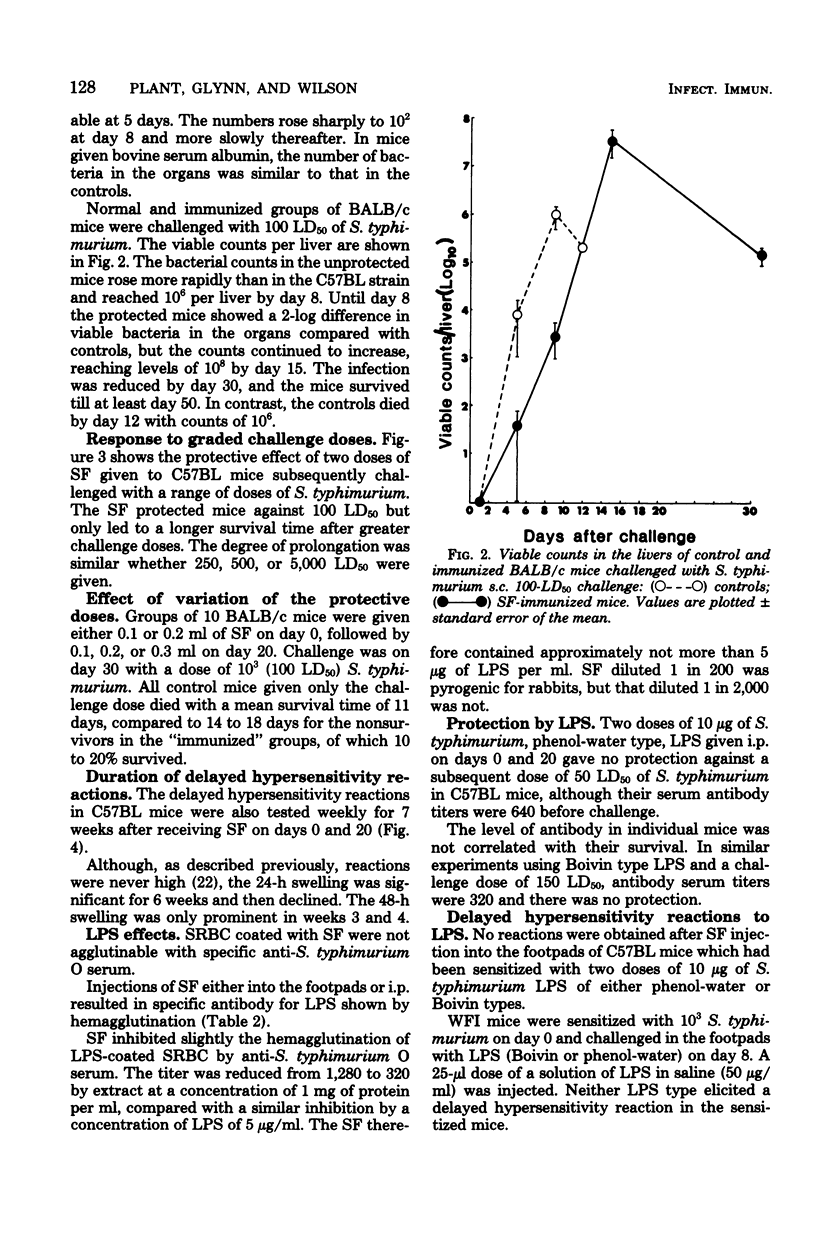
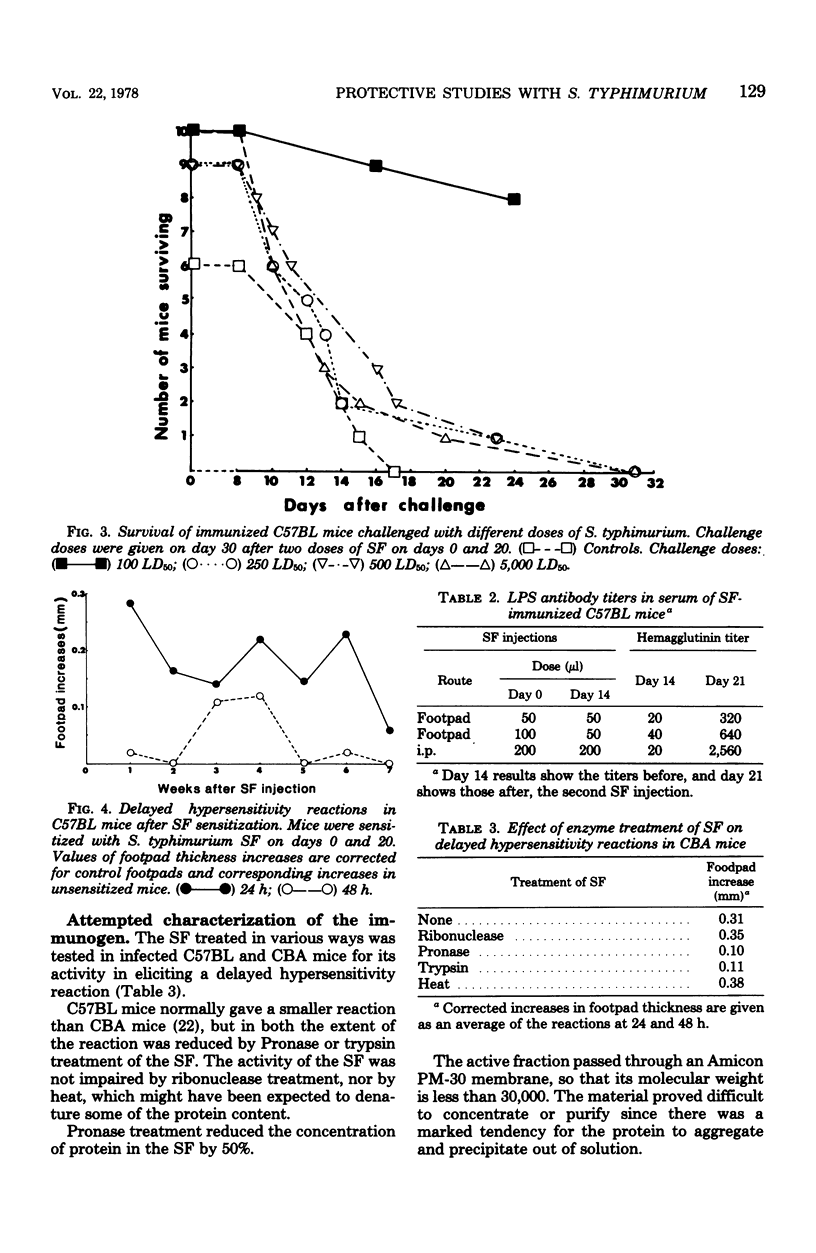
A supernatant factor prepared from 48-h cultures of Salmonella typhimurium has been used to immunize mice against subsequent challenge with normally lethal doses of S. typhimurium. The mouse strains used, C57BL and BALB/c, were sensitive to S. typhimurium with 50% lethal doses of less than 50 organisms. Two doses of supernatant factor, given intraperitoneally 20 days apart, protected mice against a subcutaneous challenge dose 10 days later of 100 50% lethal doses of S. typhimurium, resulting in 50 to 80% survival. The viable counts were reduced initially in organs of immunized mice compared with controls, and the multiplication of bacteria was delayed, although the final levels found in the organs would normally have been lethal. Protection obtained was specific for S. typhimurium in that no increased survival was shown after Salmonella enteritidis challenge of immunized mice. Although lipopolysaccharide was demonstrated in the supernatant factor, lipopolysaccharide alone did not protect challenged mice. Supernatant factor produced delayed-type hypersensitivity reactions in mice sensitized with nonlethal doses of Salmonella. The nature of the active factor, found to be partially protein, has yet to be elucidated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badakhsh F. F., Herzberg M. Deoxycholate-treated, nontoxic, whole-cell vaccine protective against experimental salmonellosis of mice. J Bacteriol. 1969 Nov;100(2):738–744. doi: 10.1128/jb.100.2.738-744.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C., Eylan E. Cross-protection induced in mice by immunizations with proteins of related bacteria species. Zentralbl Bakteriol Orig A. 1976 Jan;234(1):46–52. [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in nonvaccinated mice challenged by various routes. J Bacteriol. 1969 Feb;97(2):667–675. doi: 10.1128/jb.97.2.667-675.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Immunity to enteric infection in mice. Infect Immun. 1970 Mar;1(3):243–250. doi: 10.1128/iai.1.3.243-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. Effect of cyclophosphamide on delayed hypersensitivity to Staphylococcus aureus in mice. Immunology. 1977 Nov;33(5):767–776. [PMC free article] [PubMed] [Google Scholar]
- HOBSON D. Resistance to reinfection in experimental mouse typhoid. J Hyg (Lond) 1957 Sep;55(3):334–343. doi: 10.1017/s0022172400037244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Early antibody response in mice to either infection or immunization with Salmonella typhimurium. J Bacteriol. 1967 Mar;93(3):773–778. doi: 10.1128/jb.93.3.773-778.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACLEOD D. R. Immunity to Salmonella infection in mice. J Hyg (Lond) 1954 Mar;52(1):9–17. doi: 10.1017/s0022172400027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Variability of protection in inbred mice induced by a ribosomal vaccine prepared from Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):652–659. doi: 10.1128/iai.14.3.652-659.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- Rowley D., Auzins I., Jenkin C. R. Further studies regarding the question of cellular immunity in mouse typhoid. Aust J Exp Biol Med Sci. 1968 Aug;46(4):447–463. doi: 10.1038/icb.1968.38. [DOI] [PubMed] [Google Scholar]
- USHIBA D., SAITO K., AKIYAMA T., NAKANO M., SUGIYAMA T., SHIRONO S. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn J Microbiol. 1959 Apr;3:231–242. doi: 10.1111/j.1348-0421.1959.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Cell-mediated immune response to Salmonella typhimurium infection in mice: development of nonspecific bactericidal activity against Listeria monocytogenes. Infect Immun. 1976 Apr;13(4):1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


