Abstract
Contraction of small artery (diameters typically less than 250 μm) vascular smooth muscle cells (VSMCs) plays a critical role in local control of blood flow and arterial pressure through its affect on vascular caliber. Specifically, contraction of small arteries in response to increased intraluminal pressure is referred to as the myogenic response and represents an important role for mechanotransduction. Critical questions remain as to how changes in pressure are sensed by VSMCs and transduced across the cell membrane to tune the contractile state of the cell. Recent studies suggest a pivotal role for interactions between VSMCs and extracellular matrix (ECM) proteins. Thus, pressure-induced deformation of ECM proteins and their cell surface receptors (for example, integrins) may initiate contraction and cytoskeletal remodeling through modulation of ion channels, membrane depolarization, increased intracellular Ca2+ and actomyosin crossbridge cycling. Importantly, it is argued that the contractile properties of small artery VSMCs reflect an intimate and integrated interaction with their extracellular environment and the three-dimensional structure of the vessel wall.
1. Introduction
Mechanotransduction refers to processes by which mechanical stimuli are converted into biochemical reactions eliciting a cellular response. The ability to respond to mechanical stimuli is a property of cells, in general, and is not simply limited to cells classically viewed as mechanoreceptors. This In Focus article will restrict its consideration of mechanotransduction to how arteriolar smooth muscle senses and responds to acute changes in intraluminal pressure (referred to as myogenic responsiveness) and in doing so contributes to the control of local hemodynamics through acute modulation of vessel diameter. It is acknowledged that even in the context of a VSMC's response to a change in intravascular pressure, that mechanical activation likely involves multiple signaling pathways. For example, in addition to modulation of contraction, pathways are activated that lead to alterations in gene expression and both short and longer-term synthetic and phenotypic changes underlying vascular remodeling (Martinez-Lemus et al., 2009, Mulvany, 2012). The scope of this article is limited to the events underlying acute pressure-induced vasoconstriction, and for more general reviews on mechanotransduction the reader is referred to (Orr et al., 2006, Ingber, 2010).
2. Cell Lineage and Plasticity
From a developmental perspective, VSMCs are derived from multiple progenitor cell types rather than originating from a single cell type. Using lineage-tracking approaches in the embryo, VSMCs have been shown to be derived from neural crest cells, mesothelial cells of the proepicardial organ and of mesodermal origin (Majesky, 2007). The neural crest-derived VSMCs were shown to populate the aortic arch while cells of the proepicardial organ specifically gave rise to the coronary vasculature. Additionally, the mesodermal precursor cells underlie formation of the splanchnic vessels (Majesky, 2007). Evidence for regionally distinct precursor cells has further been shown in genetically manipulated animals where specific gene deletion results in the lack of development of only certain regional vascular elements. In the mature vasculature, VSMCs may also be derived from multiple sources including pluripotent circulating cells, endothelial cell transition, pericytes and adventitial myofibroblasts, although the relative role(s) of these populations remains controversial (Majesky et al., 2012, Majesky et al., 2011). Conceivably the diversity of precursor cells may contribute to diversity at the level of cell function as well as structure of the vascular wall. These differences in derivation likely contribute to heterogeneity in responsiveness to environmental factors and in the susceptibility to pathophysiological abnormalities. Little information currently exists, however, as to how such diversity specifically impacts microvascular smooth muscle.
While mature arteries appear as relatively quiescent structures, VSMCs show a remarkable degree of plasticity, remodeling in response to local hemodynamic conditions, physical forces and hormonal and cytokine factors. Changes in intraluminal pressure, shear stress and prolonged vasoconstriction/vasodilation lead to changes in wall thickness and lumen diameter(Bakker et al., 2005, Martinez-Lemus et al., 2009). Thus, in hypertension small arteries show eutrophic remodeling around a constricted lumen while chronic increases in shear stress leads to widening of the vessel lumen. Remodeling may also occur under more physiological conditions as repositioning of cells within the vessel wall, to maintain a smaller lumen while lengthening from the contracted state, has been demonstrated after only 4 hrs of noradrenaline exposure ((Martinez-Lemus et al., 2004)). These latter events may serve to support contraction while also representing a transition from acute contraction to structural adaptation.
3. Arterial Vascular Smooth Muscle Function
3.1 Contraction
The general process of VSM contraction occurs by either, or a combination of, electromechanical and pharmacomechanical coupling. The former refers to stimuli directly initiating a change in membrane potential (Em) (for example, application of KCl or cell stretch) while the latter largely refers to receptor-mediated stimuli that involve activation of trimeric G-protein signaling and generation of second messengers (including, IP3, DAG). Of relevance to the current topic, pressure-induced vasoconstriction of arterioles appears to utilize both electrical and second messenger-based mechanisms of SMC activation. Ultimately both pathways lead to Ca2+-dependent activation of myosin light chain kinase and actomyosin crossbridge cycling. Evidence also exists for these mechanisms of contraction being supported by modulation of Ca2+ sensitivity, thin filament regulation, and cytoskeletal remodeling (Cole and Welsh, 2011).
3.2 Mechanotransduction
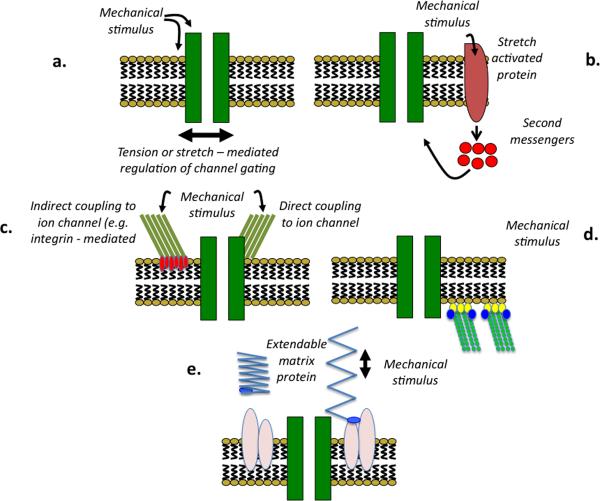
A change in intraluminal pressure presumably exerts a mechanical force on a cellular component, or components, that acts as a mechanosensor to initiate signal transduction events. Although it is uncertain as to what variable is sensed by VSM cells, support has been provided for pressure – induced changes in wall tension, deformation of cell membrane proteins (for example receptors and ion channels); conformational changes in intra-and extracellular proteins including matrix and cytoskeletal proteins; ECM – mediated integrin activation and activation of other cellular junctions such as cadherins (Figure 1). Importantly, these models while presented as independent likely exhibit significant interaction (Hill et al., 2007).
Figure 1.
Putative mechanisms underlying mechanosensation in small artery myogenic signaling. Panel A: direct effect of the mechanical stimulus on an ion channel or its local environment resulting in a change in channel gating. Panel B: ECM proteins transmit mechanical forces to ion channels or other membrane elements. Panel C: mechanically-induced activation of second messenger-based pathways with indirect activation of cation channels. Panel D: mechanical activation in response to an increase in pressure is transmitted from the cytoskeleton to cation channels that underlie subsequent depolarization. Panel E: mechanical deformation leading to altered matrix protein conformation and exposure of previously hidden (matricryptic) binding motifs.
Thus, the location of a putative myogenic mechanosensor is likely to be at the level of the cell membrane, cellular adhesion sites (matrix and/or intercellular junctions) or the cytoskeleton. All of which are sites that conceivably are mechanically linked and impacted by the complex three-dimensional structure of the vascular wall (Figures 1 and 2).
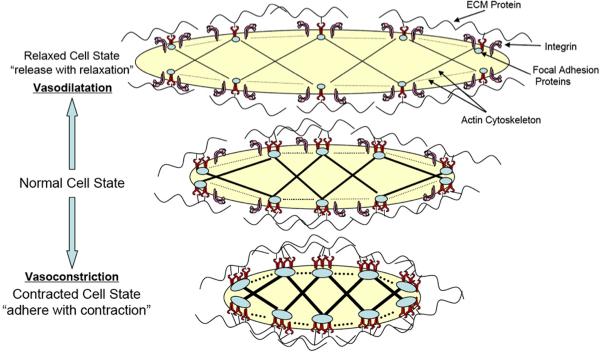
Figure 2.
Schematic diagram showing the interaction between ECM, integrins and cytoskeleton as a VSMC undergoes contraction or relaxation. Integrins are envisaged to undergo both changes in clustering and affinity. Analogous models have been presented by Gunst and colleagues in airway smooth muscle (Gunst and Zhang, 2008).
The Cell Membrane
The mechanical forces exerted by intraluminal pressure are proposed to directly impact either the protein or lipid components of the cell membrane. Deformation of ion channel proteins or other integral membranes proteins could result in changes of protein activity that initiate a response to the initial mechanical stimulus. For example, recent evidence suggests mechanosensitivity of G protein coupled receptors (GPCRs) including the angiotensin II receptor (Mederos y Schnitzler et al., 2008). In these studies a direct mechanical effect on the GPCRs was demonstrated to be agonist independent although in the example of the AT1 receptor, pressure-induced activation of Gαq/11 could be inhibited by losartan a specific AT1 receptor blocker. It was proposed that mechanical force directly alters the conformation of the receptor proteins inducing an activate state. Interestingly, this mode of mechanical activation is not limited to the AT1 receptor as it could also be demonstrated for several other GPCRs including those for histamine and vasopressin. The effect was, however, not totally non-selective as mechanical activation of the Gs – coupled β2 adrenoceptor could not be demonstrated.
Subsequent activation of phospholipases and generation of second messengers may then lead to modulation of ion channels and levels of membrane potential (Spassova et al., 2006, Park et al., 2003). Importantly, phospholipase –based signaling has been implicated in the activation of cation channels including those comprised of Trp proteins. For example, activation of TrpC6 currents could conceivably lead to membrane depolarization, opening of VGCC and myogenic contraction. This would, therefore link a number of earlier observations, including that acute TrpC6 knockdown inhibits myogenic responsiveness (Welsh et al., 2002), without the Trp channel having to be mechanosensitive per se. Similarly, TrpM4 has been implicated in myogenic signaling and are activated by events downstream of phosholipase activation (Earley et al., 2004, Ebner and Chen, 1995). As these events can also occur subsequent to classical agonist-induced signaling conceivably they may interact with myogenic signaling via stretch-induced release of agonists (Kauffenstein et al., 2012) or synergistic intracellular signaling mechanisms (Liu et al., 1994). Additional information on trimeric G-protein involvement in myogenic signaling can be found in recent reviews by Storch et al. and Kauffenstein et al. (Kauffenstein et al., 2012, Storch et al., 2012).
VSMC – matrix adhesion through integrins
ECM protein binding to cell membrane integrins provide a direct link from the extracellular matrix environment and a mechanism for bi-directional transmission of physical forces. Additionally, integrins can act as recipients of specific matrix protein-coded information and assist in signal amplification (outside-in and inside-out signaling). As an example, physical forces may impact the outside-pathway via strain applied to existing linkages or may cause conformational changes in (and unfolding of) ECM proteins to uncover cell membrane binding motifs. The latter concept is supported by studies demonstrating `matricryptic' sites buried in the three dimensional structure of native matrix proteins (Davis, 2010) and those showing that specific matrix proteins such as fibronectin possess domains that can be extended on application of force (Erickson, 2002).
Specifically implicating a role for integrins in arterioles, integrin-recognizing synthetic RGD peptides decrease VSMC intracellular Ca2+ and cause vasodilation (Mogford et al., 1996, D'Angelo et al., 1997). Demonstrating specificity, these actions are prevented by function blocking antibodies directed at the β3 integrin subunit (Mogford et al., 1996). Further support for integrins as sensors for the myogenic response was similarly provided by studies of isolated arterioles showing that blockade of αvβ3 or α5β1 integrins, with antibodies, abolishes myogenic constriction to step increases in intraluminal pressure (Martinez-Lemus et al., 2005). Other important evidence for integrins being possible initiating events in arteriolar mechanotransduction, is that integrin activation leads to phosphorylation and activation of ion channels (including VGCCs and BKCa) previously demonstrated to be important to myogenic responsiveness (Wu et al., 2008).
Atomic force microscopy (AFM) has been used to demonstrate of a novel role for fibronectin in integrin-mediated myogenic-like behavior of single VSMCs (Sun et al., 2008). On binding fibronectin-coated AFM probes to the cell, focal adhesions were formed, characterized by the co-localization of submembranous actin, integrins and focal-adhesion-related proteins (FAK and paxillin) at the adhesion site. Controlled retraction of the AFM probe caused local membrane stretch and deformation of underlying cytoskeletal structures to which the cells subsequently responded with a localized `contraction' that effectively counteracted the applied stretch. Importantly, this myogenic-like cellular response was inhibited by a myosin light chain MLC kinase inhibitor (ML-7), cytochalasin D or function-blocking antibodies to α5β1- and αvβ3-integrins (Sun et al., 2008).
Involvement of matrix proteins in myogenic signaling is likely more complex than the outside-in directed signaling through integrins described above. Matrix proteins are known, for example, to bind cell surface proteins other than integrins. In addition, integrin binding affinity can be modulated by intracellular mechanisms (Kim et al., 2011), raising the possibility that integrin – ECM protein interactions may be modulated by inside-out signaling mechanisms during myogenic or agonist induced changes in activation. In this case integrin involvement may be viewed as an adaptive response to the contractile state, perhaps reinforcing or stabilizing the contractile state and adhesion sites.
Intercellular Junctions - cadherins
Although mechanosensitivity can be demonstrated at the level of a single VSMC, evidence from a number of systems suggests that mechanotransduction can be enhanced in multicellular preparations suggesting the importance of cell-cell junctions (Schwartz, 2010). In relation to this, interest has developed in the cadherins, a super family of calcium-dependent, transmembrane cell – cell adhesion proteins that form adherens junctions and play a major role in many multicellular activities requiring a degree of coordination between neighboring cells (Leckband and Prakasam, 2006, Aberle et al., 1996). Cadherin molecules homotypically or heterotypically bind cadherins on neighboring cells and are coupled to catenins on the cytoplasmic side of the membrane. The cadherin-catenin complex directly interacts with the actin cytoskeleton and, like integrins, provides a nucleation site for scaffolding with a variety of signaling molecules (Aberle et al., 1996). Thus this pathway has many of the same features that the integrin-extracellular matrix pathway possesses to make it an attractive pathway for mechanosensation.
As discussed with regard to integrins, it is conceivable that involvement of cadherins in smooth muscle mechanotransduction could be a primary event (that is, being involved in the initial mechanosensory steps) or may be secondarily (inside-out signaling) activated to reinforce the mechanical response, for example, to a change in intraluminal pressure. In recent studies, the role of N-cadherin in arteriolar myogenic responses has been considered using function blocking antibodies and inhibitory peptides (Jackson et al., 2010). Using both approaches myogenic responsiveness of cannulated cremaster muscle arterioles was attenuated. In contrast, pressure-induced changes in Ca2+i were not altered. This latter observation raises questions as to where N-cadherins are involved in the Ca2+i signaling cascade. As cadherins link to the actin cytoskeleton via catenins it is conceivable that their involvement lies in events controlling Ca2+ sensitivity or inside-out signaling directed at acutely strengthening/remodeling of cell–cell adhesions. Interestingly, functional blocking antibodies directed at either β1 or β3 integrins similarly blocked myogenic constriction without inhibiting pressure-induced changes in Ca2+i (Jackson et al., 2010). While in a myogenic signaling context it is uncertain why blockade of either N-cadherins or integrins (containing β1 or β3 sub-units) blocks constriction it has been proposed that these junctional molecules maintain tissue homeostasis through a cooperative cytoskeleton–based mechanism (Brunton et al., 2004, Leckband and Prakasam, 2006). Further studies will be required to establish the specific involvement of cadherins in myogenic signaling and determine the nature of their interaction with integrins.
VSMC Cytoskeleton
While a contribution of cytoskeletal proteins to mechanotransduction has long been suggested, its precise role in arteriolar myogenic constriction has remained uncertain. While this reflects current limitations for dynamic study of the cytoskeleton in functional arteriole preparations, it is an attractive hypothesis given the known links between putative mechanosensory elements (including, integrins, cadherins, ion channels) and the cytoskeleton as well as the potential for cytoskeletal rearrangement secondary to pressure-induced signaling events such as the activation of RhoA.
Initial studies in this area relied on the use of pharmacological agents that either disrupt or stabilize the actin cytoskeleton. Cipolla and colleagues showed that rat cerebral small arteries were less able to withstand the distending forces exerted by intraluminal pressure following treatment with the actin depolymerizing agent, cytochalasin B (Cipolla and Osol, 1998) while the actin stabilizer, jasplakinolide, enhanced myogenic tone (Cipolla et al., 2002). Using confocal fluorescence microscopy Flavahan et al. (Flavahan et al., 2005) showed myogenic constriction of isolated rat tail arteries to be associated with G- to F-actin transition. Further, as intraluminal pressure was increased from 10 mmHg to 60 and 90 mmHg, actin redistributed from the cell periphery (adjacent to the plasma membrane) to the cell interior. Both the redistribution of the actin fibers and myogenic constriction were prevented by actin depolymerization with cytochalasin D. These data appear to demonstrate that the actin cytoskeleton provides an important intracellular structural mechanism for resisting the distending forces imparted by intraluminal pressure and facilitating myogenic responsiveness and adaptation to mechanical forces.
In recent studies, El-Yazbi et al. (El-Yazbi et al., 2011) have made the intriguing observation that under certain conditions acute arteriolar myogenic constriction can be dissociated from a change in the levels of phosphorylation of the myosin 20 kD regulatory light chain (pMLC20). Specifically, in the presence of serotonin to achieve maximal levels of pMLC20 (approximately 50% of total MLC20) an acute increase in pressure could still elicit myogenic contraction. This effect could be prevented by latrunculin B (a marine toxin which binds actin monomers and prevents polymerization) implicating a remodeling role for the actin cytoskeleton in this form of myogenic contraction.
An alternate hypothesis for cytoskeletal involvement in myogenic constriction is via linkages to critical mechanosensory or mechanotransducing elements. Thus, the actin cytoskeleton may couple to relevant ion channels by linker proteins such as filamin A (Sharif-Naeini et al., 2009). Consistent with this, disruption of the actin cytoskeleton enhances pressure-induced depolarization and Ca2+ entry via nifedipine – sensitive VGCCs (Gokina and Osol, 2002).
It is also conceivable that other elements of the cytoskeleton play a role in myogenic signaling or, similarly, facilitate its occurrence. Microtubules, for example, provide a resistive force in many cell type (Wang et al., 1993). Depolymerization of microtubules causes vasoconstriction that in some studies seems to involve Rho-A-dependent Ca2+ sensitization without an overt increase in Ca2+i (Platts et al., 2002). Vimentin- and desmin-deficient mice while showing normal myogenic responses exhibit alterations in other vasomotor properties including agonist sensitivity and impaired flow-dependent dilation (Loufrani et al., 2002, Schiffers et al., 2000).
3.3 Impact of the Local VSMC Environment
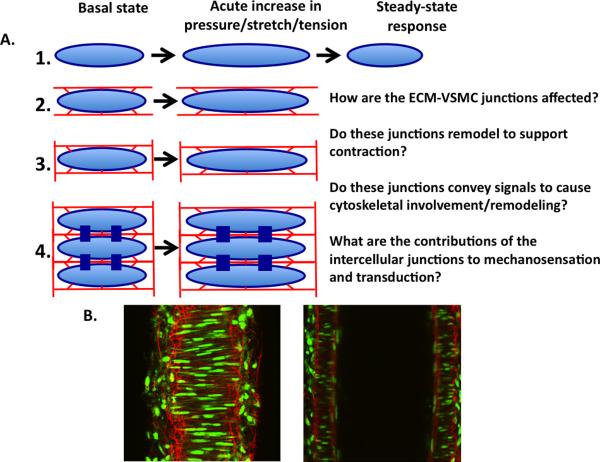
Although single arteriolar VSMCs display responsiveness to stretch, the role of the complex 3D mechanical environment in which they are situated is often not considered (Figure 3). An appreciation of this likely goes beyond the mechanosensation role of ECM-integrin interactions and intercellular adhesion molecules referred to above. Recent studies using confocal microscopy and 3D image analysis and reconstruction highlight both the complexity of the ECM and that elements of the ECM traverse the media and are not necessarily restricted to layers. In the case of elastin fibers, it has been shown that there is connectivity between the longitudinally-arranged adventitial fibers and the sheet-like IEL(Clifford et al., 2011) (Figure 3). These observations raise questions as to how this structure impacts both mechanosensation and the ultimate contractile behavior. From a functional perspective, these matrix elements potentially provide cell attachment sites that could participate in mechanosensation or cellular adhesion when altered by physical forces or pathology.
Figure 3.
Arteriolar VSMCs are embedded in a complex ECM environment. Panel A: schematic diagram illustrating the increasing complexity of ECM-VSMC interactions in single cells vs a multi-cellular section of the vessel wall. Panel B: confocal images of a cannulated mesenteric small artery at the mid-wall level (left) and through the center of the lumen (right). Red shows Alexa 633 hydrazide staining of elastin fibers and green shows cell nuclei (for details see (Clifford et al., 2011)).
4. Associated Pathologies
A number of cardiovascular disorders exhibit alterations in myogenic responsiveness suggesting the potential for drug targets aimed at modulating vascular resistance with an intent of restoring local blood flow control and capillary and hydrostatic pressures (Hill et al., 2009). For example, cerebral and coronary vasospasms are important pathophysiological events suggested to be linked to abnormalities of mechanotransduction (Cipolla et al., 1997). Altered myogenic tone has also been implicated in the microvascular complications of diabetes (Hill and Ege, 1994), hypertension (Ahn et al., 2007), heart failure (Xu et al., 2009) and hearing disturbances (Reimann et al., 2011). Further, alterations in myogenic responsiveness may contribute to hypoperfused states such as occurs in sepsis (Meziani et al., 2007). Presumably some of these disturbances may relate to the changes in the ECM component of the vessel wall (e.g. deposition, glycation, stiffening) and its interaction with VSMCs. Discovery of the specific nature of such links could thus have a direct translational impact on these debilitating conditions.
Although myogenic responsiveness is altered in many diseases, it can be questioned as to whether there are specific alterations in the pathways underlying mechanotransduction. Difficulties in assessing this arise from many of the signaling molecules utilized for mechanotransduction (for example TRP channels, voltage-gated channels, PKC, Rho kinase etc) are also crucial for other vasoregulatory functions. Nevertheless, an ability to an ability to `re-set' myogenic tone would allow manipulation of systemic vascular resistance and pressure while also preserving existing interactions with neurohumoral regulatory mechanisms. Clearly, to achieve this aim knowledge of specific components of the mechanosensory pathway is required.
Cell Facts – arteriolar VSMC
Spindle-shaped, approximately 100μm in length and 5μm in width, contractile cells
Contraction regulates lumen diameter of blood vessels and hence flow and pressure
Activated by electrical, pharmacological and mechanical (e.g. stretch) stimuli
Contraction occurs via Ca2+-dependent activation of myosin light chain kinase
Contraction is modulated by changes in Ca2+ sensitivity, thin filament regulation, and cytoskeletal rearrangment
Acknowledgments
MAH and GAM are supported by the National Institutes of Health, USA (HL HL092241 and P01-HL095486).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ahn DS, Choi SK, Kim YH, Cho YE, Shin HM, Morgan KG, Lee YH. Enhanced stretch-induced myogenic tone in the basilar artery of spontaneously hypertensive rats. J Vasc Res. 2007;44:182–191. doi: 10.1159/000100374. [DOI] [PubMed] [Google Scholar]
- Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Osol G. Vascular smooth muscle actin cytoskeleton in cerebral artery forced dilatation. Stroke. 1998;29:1223–1228. doi: 10.1161/01.str.29.6.1223. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, Martinez-Lemus LA, Dora KA, Yang Y, Davis MJ, Pohl U, Meininger GA, Hill MA. Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress. Arterioscler Thromb Vasc Biol. 2011;31:2889–2896. doi: 10.1161/ATVBAHA.111.236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys. 2011;510:160–173. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Mogford JE, Davis GE, Davis MJ, Meininger GA. Integrin-mediated reduction in vascular smooth muscle [Ca2+]i induced by RGD-containing peptide. Am J Physiol. 1997;272:H2065–2070. doi: 10.1152/ajpheart.1997.272.4.H2065. [DOI] [PubMed] [Google Scholar]
- Davis GE. Matricryptic sites control tissue injury responses in the cardiovascular system: relationships to pattern recognition receptor regulated events. J Mol Cell Cardiol. 2010;48:454–460. doi: 10.1016/j.yjmcc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol. 1995;46:463–506. doi: 10.1016/0301-0082(95)00010-s. [DOI] [PubMed] [Google Scholar]
- El-Yazbi A, Walsh E, Walsh M, Cole W. Potential involvement of actin cytoskeleton reorganization in generation of the arterial myogenic response. FASEB Journal. 2011;25:1. [Google Scholar]
- Erickson HP. Stretching fibronectin. J Muscle Res Cell Motil. 2002;23:575–580. doi: 10.1023/a:1023427026818. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Bailey SR, Flavahan WA, Mitra S, Flavahan S. Imaging remodeling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol. 2005;288:H660–669. doi: 10.1152/ajpheart.00608.2004. [DOI] [PubMed] [Google Scholar]
- Gokina NI, Osol G. Actin cytoskeletal modulation of pressure-induced depolarization and Ca(2+) influx in cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;282:H1410–1420. doi: 10.1152/ajpheart.00441.2001. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MA, Ege EA. Active and passive mechanical properties of isolated arterioles from STZ-induced diabetic rats. Effect of aminoguanidine treatment. Diabetes. 1994;43:1450–1456. doi: 10.2337/diab.43.12.1450. [DOI] [PubMed] [Google Scholar]
- Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol Sci. 2009;30:363–374. doi: 10.1016/j.tips.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Hill MA, Sun Z, Martinez-Lemus L, Meininger GA. New technologies for dissecting the arteriolar myogenic response. Trends Pharmacol Sci. 2007 doi: 10.1016/j.tips.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ingber DE. From cellular mechanotransduction to biologically inspired engineering: 2009 Pritzker Award Lecture, BMES Annual Meeting October 10, 2009. Ann Biomed Eng. 2010;38:1148–1161. doi: 10.1007/s10439-010-9946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TY, Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. N-cadherin and integrin blockade inhibit arteriolar myogenic reactivity but not pressure-induced increases in intracellular Ca. Front Physiol. 2010;1:165. doi: 10.3389/fphys.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Laher I, Matroughi K, Guerineau NC, Henrion D. Emerging role of G protein-coupled receptors in microvascular myogenic tone. Cardiovascular Research. 2012 doi: 10.1093/cvr/cvs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ye F, Ginsberg MH. Regulation of Integrin Activation. Annu Rev Cell Dev Biol. 2011 doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- Leckband D, Prakasam A. Mechanism and dynamics of cadherin adhesion. Annu Rev Biomed Eng. 2006;8:259–287. doi: 10.1146/annurev.bioeng.8.061505.095753. [DOI] [PubMed] [Google Scholar]
- Liu J, Hill MA, Meininger GA. Mechanisms of myogenic enhancement by norepinephrine. Am J Physiol. 1994;266:H440–446. doi: 10.1152/ajpheart.1994.266.2.H440. [DOI] [PubMed] [Google Scholar]
- Loufrani L, Matrougui K, Li Z, Levy BI, Lacolley P, Paulin D, Henrion D. Selective microvascular dysfunction in mice lacking the gene encoding for desmin. Faseb J. 2002;16:117–119. doi: 10.1096/fj.01-0505fje. [DOI] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr. The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr., Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. Faseb J. 2004;18:708–710. doi: 10.1096/fj.03-0634fje. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. Embo J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziani F, Kremer H, Tesse A, Baron-Menguy C, Mathien C, Mostefai HA, Carusio N, Schneider F, Asfar P, Andriantsitohaina R. Human serum albumin improves arterial dysfunction during early resuscitation in mouse endotoxic model via reduced oxidative and nitrosative stresses. Am J Pathol. 2007;171:1753–1761. doi: 10.2353/ajpath.2007.070316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogford JE, Davis GE, Platts SH, Meininger GA. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res. 1996;79:821–826. doi: 10.1161/01.res.79.4.821. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim Y, Lee YH, Earm YE, Ho WK. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res. 2003;93:557–564. doi: 10.1161/01.RES.0000093204.25499.83. [DOI] [PubMed] [Google Scholar]
- Platts SH, Martinez-Lemus LA, Meininger GA. Microtubule-dependent regulation of vasomotor tone requires Rho-kinase. J Vasc Res. 2002;39:173–182. doi: 10.1159/000057765. [DOI] [PubMed] [Google Scholar]
- Reimann K, Krishnamoorthy G, Wier WG, Wangemann P. Gender differences in myogenic regulation along the vascular tree of the gerbil cochlea. PLoS One. 2011;6:e25659. doi: 10.1371/journal.pone.0025659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffers PM, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi GE, Levy BI, De Mey JG. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch U, Mederos y Schnitzler M, Gudermann T. G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol. 2012;302:H1241–1249. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am J Physiol Cell Physiol. 2008;295:C268–278. doi: 10.1152/ajpcell.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by alpha5beta1 integrin activation in arteriolar smooth muscle. J Physiol. 2008;586:1699–1713. doi: 10.1113/jphysiol.2007.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Henning RH, Sandovici M, van der Want JJ, van Gilst WH, Buikema H. Enhanced myogenic constriction of mesenteric artery in heart failure relates to decreased smooth muscle cell caveolae numbers and altered AT1- and epidermal growth factor-receptor function. Eur J Heart Fail. 2009;11:246–255. doi: 10.1093/eurjhf/hfn027. [DOI] [PMC free article] [PubMed] [Google Scholar]