Abstract
The choroid plexus (CP) is increasingly recognized as an important contributor to central nervous system (CNS) inflammation by recruitment of inflammatory cells and release of inflammatory cytokines. Here we investigate the role of the CP epithelium (CPE) as a source of three pro-inflammatory molecules of potential importance in inflammation after acute spinal cord injury (SCI): IL-1β, TNF-α, and hsp70. Immunohistochemical (IHC) staining for these three proteins was performed on 4th ventricular CPE from 4 dogs euthanized 12–48 h after spontaneous acute SCI, and from 4 neurologically normal dogs euthanized for other reasons. IHC staining was quantified using Aperio ImageScope software. IHC staining in the CPE of dogs with acute SCI was 2.2, 1.6 and 1.5 times higher than that of normal dogs, for IL-1β, TNF-α, and hsp70, respectively. Increases were statistically significant (p < 0.1) for IL-1β and TNF-α, and closely approached significance for hsp70. These findings indicate that the CPE could serve as an important source of these inflammatory mediators after SCI. There was also an inverse correlation between IL-1β and hsp70 staining and duration of clinical signs in acute SCI, suggesting that increased expression of these proteins by the CPE may be of particular importance in the immediate-early inflammatory response after acute SCI.
Keywords: Canine, Spinal cord injury, Inflammation, Choroid plexus, Heat shock protein 70
1. Introduction
Spinal cord injury (SCI) is accompanied by primary inflammatory changes in the central nervous system (CNS). This inflammation not only serves various purposes such as removal of cellular debris, but also causes secondary damage which has a negative impact on functional recovery (Wang et al., 2004; Ankeny et al., 2009; Peng et al., 2009; Olby, 2010). This secondary damage occurs over days, weeks and even months after the initial insult, but the exact mechanisms and mediators of this detrimental inflammatory response have yet to be fully defined.
The choroid plexus (CP) is a specialized CNS tissue located within the ventricular system of the brain. It is highly vascular and composed of a single sheets of cuboidal epithelial cells. Traditionally, roles ascribed to the CP include production of cerebrospinal fluid (CSF) and contribution to the blood–CSF barrier; however, recent studies have implicated the CP as a potentially important mediator of inflammatory responses within the CNS. For example, the CP has been shown to play an important role in the autoimmune inflammation associated with experimental allergic encephalitis (EAE) and its human counterpart, multiple sclerosis (MS) (Englehardt et al., 2001; Vercellino et al., 2008). Because the CP forms an important interface between the CSF and the peripheral vascular system, the potential for the CP to serve as both a key entry point for leukocytes into the CNS, as well as a source of inflammatory mediators during various disease states seems obvious, albeit previously underappreciated. Indeed, choroid plexus epithelial cells (CPE) have recently been shown to be a source of pro-inflammatory cytokines, such as IL-6, IL-8, TNF-α, and IL-1β, released into the CSF associated with peripheral systemic inflammation (Marques et al., 2009; Mitchell et al., 2009). Increases in IL-1β and TNF-α mRNA and protein expression in the CPE have also been recently documented in a rodent model of traumatic brain injury (Szmydynger-Chodobska et al., 2009), demonstrating the potential importance of the CPE in the inflammatory response to traumatic CNS injury.
The objective of the present study was to characterize the immunohistochemical (IHC) changes occurring in the CPE after spontaneously occurring acute SCI in dogs. In particular, we examined expression of IL-1β, TNF-α, and hsp70. These three proteins have been previously implicated as markers or mediators of a robust inflammatory response of the spinal cord following acute SCI, spinal cord ischemia-reperfusion (IR) injury and meningomyelitis (Sharma et al., 2006; Awad et al., 2008; Hecker et al., 2008; Peng et al., 2009; Moore et al., 2012). Particularly related to SCI, extracellular release of hsp70, IL-1β, and TNF-α may represent important contributors to inflammation, and likely inhibit recovery; IL-1β by exerting a direct detrimental effect (Allan et al., 2005), and TNF-α and hsp70 by serving as danger associated molecular patterns (DAMPs) and TLR agonists that stimulate further inflammatory cytokine release (Calderwood et al., 2007).
2. Materials and methods
2.1. Tissue samples and histology
Immunohistochemical (IHC) staining for IL-1β, TNF-α and hsp70 were investigated in the CPE of 4 dogs with acute SCI secondary to intervertebral disc extrusion (IVDE). These dogs represented submissions to the Ohio State University College of Veterinary Medicine diagnostic pathology service, Columbus, OH. Clinical injury severity at the time of euthanasia was quantified using a previously published scale ranging from 1 to 5, with a score of 5 indicating a dog with paraplegia and absence of nociception (Sharp and Wheeler, 2005). All dogs with SCI were euthanized between 12 and 48 h of onset of clinical signs. Complete necropsies were performed on each dog and brain and spinal cord tissue were processed for routine histologic evaluation. Tissues were formalin-fixed and paraffin-embedded. Routine hematoxylin and eosin (H&E) was used to evaluate representative sections of brain and spinal cord, including sites of spinal cord compression and the choroid plexus of the 4th ventricle. Tissue from 4 dogs free of neurologic disease and euthanized and submitted for necropsy for other reasons were used as controls.
2.2. Immunohistochemistry (IHC)
Sections of fourth ventricular CP from both SCI dogs and normal controls underwent IHC staining for IL-1β and TNF-α, and hsp70. Specificity, source, and dilution of the antibodies are listed in Table 1. After deparaffinization, sections were treated with 3% hydrogen peroxide for 10 min. Species-specific biotin conjugated second step antibodies were added at a working dilution of 1:100–1:200. Peroxidase labeling was visualized with 3,3-diaminobenzidine. Sections were counterstained with hematoxylin. Appropriate isotype controls were used to eliminate nonspecific staining as a reason for IHC positivity. A section of spinal cord from the lesion epicenter of each dog with acute SCI was also stained with each antibody using an identical method, allowing comparison of proximate versus distal inflammatory responses.
Table 1.
List of antibodies used, including name, specificity, source, and dilution.
| Antibody | Specificity | Source | Dilution |
|---|---|---|---|
| Rabbit polyclonal | IL-1β | Abbiotec, San Diego, CA | 1:100 |
| Rabbit polyclonal | TNF-α | Abbiotec, San Diego, CA | 1:50 |
| Mouse polyclonal | hsp70/hsp72 | Enzo Life Sciences, Farmingdale, NY | 1:50 |
2.3. Evaluation of IHC images
Digital quantitative analysis of IHC staining was performed. Individual slides were digitized using the Aperio ScanScope (Aperio Technologies, Vista, CA, USA), and digital images were analyzed using Aperio ImageScope software. For each tissue examined, a region of interest (ROI) containing only CPE was manually defined by a single investigator (SAM) using 20× magnification on the ImageScope software. A pre-programmed algorithm for analysis of IHC staining consisted of the following: hue value of 0.1 (consistent with recognition of brown pixels), hue width of 0.5 and color saturation of 0.04. These parameters allowed for consistent identification of brown pixels (positive immunoperoxidase signal) and consistent exclusion of pixels containing other colors. Positive pixel counts were performed, and a mean positivity was reported for each ROI. Mean positivity was calculated as the number of positive pixels identified by the algorithm, divided by the number of total pixels in the ROI. Possible values ranged from 0 to 1.0. A single slide containing 4th ventricular CPE was evaluated from each dog, and three regions of CPE were randomly chosen for evaluation on each slide. The three values obtained for each available tissue section were averaged to produce a single value for mean positivity from each dog examined. The same process was repeated for spinal cord tissue from each dog with acute SCI. ROIs for spinal cord included the ependymal lining of the central canal, spinal cord gray matter, and spinal cord white matter.
2.4. Statistical analysis
For each of the IHC stains, mean positivity for sections of CPE from the group of SCI dogs was compared to that of the normal controls and to spinal cord tissue using an unpaired t-test. Correlations between various clinical parameters and degree of IHC staining in the SCI dogs were also examined using a Pearson correlation. p < 0.1 was considered statistically significant. All values are presented as mean ± standard error of the mean (SEM).
3. Results and discussion
The group of normal dogs consisted of 4 animals with no clinical or histopathologic evidence of neurologic disease. One dog died from a pulmonary thromboembolism identified at necropsy, one was euthanized due to severe pneumonia, another due to pancreatitis, and one dog had no apparent cause of death. Mean positivity for the CPE of normal dogs was 0.284 ± 0.09, 0.423 ± 0.10, and 0.302 ± 0.07 for IL-1β, TNF-α and hsp70, respectively. Staining patterns for all three proteins were most consistent with a primarily cytoplasmic distribution within the CPE, although a small amount of apparent nuclear staining was also present.
The group of dogs with SCI consisted of 4 dogs with acute IVDE affecting the T3-L3 spinal cord segment. The duration of clinical signs ranged from 12 to 48 h prior to euthanasia, with a mean duration of 28.5 h. Severity of injury ranged from grade 3 to grade 5 (Sharp and Wheeler, 2005) with a mean injury severity of 4 (equating to paraplegia with nociception intact). All sections of CPE appeared histologically normal when evaluated via H&E staining. Spinal cord sections from these dogs contained variable degrees of hemorrhage, necrosis, and inflammatory cell infiltrates, consistent with acute SCI. Acute disc herniation was confirmed at necropsy in each case.
Mean positivity for the CPE of dogs with SCI was 0.630 ± 0.01, 0.660 ± 0.05, and 0.407 ± 0.08 for IL-1β, TNF-α and hsp70, respectively. When comparing SCI dogs to normal control dogs, significant increases is IHC staining of the CPE were identified for IL-1β (p = 0.014), and TNF-α (p = 0.038). CPE staining for hsp70 was also higher in dogs with SCI. This relationship did not achieve statistical significance, but approached it (p = 0.176) (Figs. 1 and 2). There was not a significant correlation between injury severity and degree of IL-1β, TNF-α or hsp70 staining in the SCI group; however, there was an inverse correlation between duration of clinical signs and degree of IL-1β, TNF-α and hsp70 staining. This relationship was statistically significant for hsp70 (r = −0.90, p = 0.054), and IL-1β (r = −0.88, p = 0.059), but not for TNF-α.
Fig. 1.
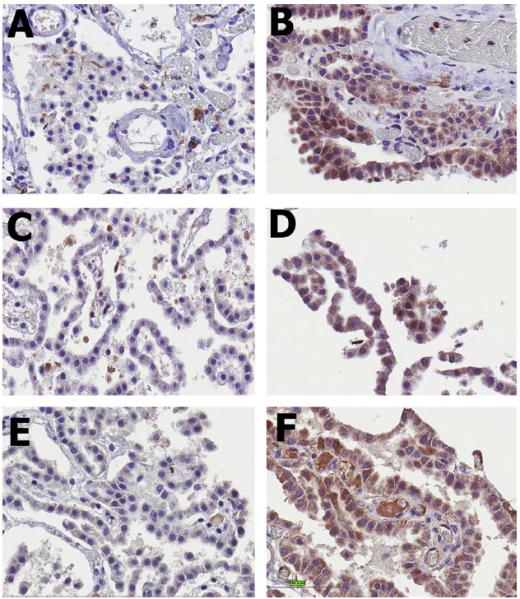
IHC staining results at 40× magnification for the CP of dogs with acute spinal cord injury (SCI – B, D and F) compared to normal control dogs (A, C and E). IL-1β (A and B), hsp70 (C and D) and TNF-α (E and F) staining were all increased in dogs with acute SCI. This relationship achieved statistical significance only for IL-1β and TNF-α (p < 0.1).
Fig. 2.
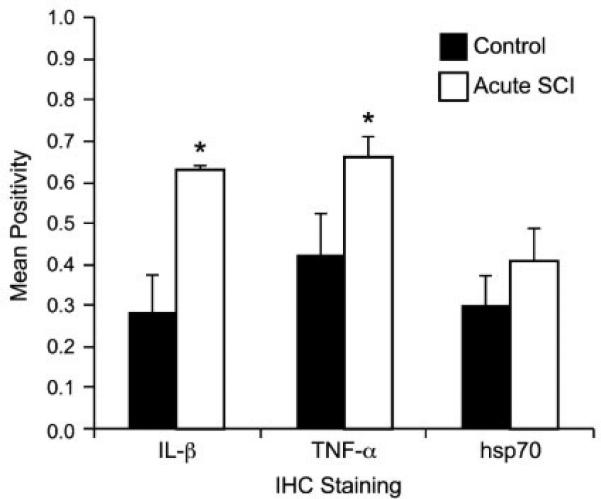
Mean positivity for IL-1β, TNF-α and hsp70 (±SEM) in the CP of dogs with acute spinal cord injury (SCI) compared to normal control dogs. Statistically significant differences (p < 0.1) are indicated with an asterisk.
To assess the relative importance of the CPE as a source of IL-1β, TNF-α and hsp70 in the injured CNS, sections of spinal cord located at the lesion epicenter were also evaluated for the dogs with acute SCI. Differences in mean positivity between the CPE and regions of spinal cord evaluated are detailed in Table 2. IHC staining of the CPE was significantly higher than for the ependymal lining of the central canal, spinal cord gray matter and white matter at the lesion epicenter for IL-1β (p = 0.023, 0.003, <0.001, respectively), and for TNF-α (p = 0.008, 0.004, <0.001, respectively). This was also the case for hsp70 staining in the spinal cord gray matter (p = 0.02) and white matter (p = 0.013); however, there was a significant increase in hsp70 staining in the ependymal cells lining the central canal of the spinal cord when compared to the CPE (p = 0.065).
Table 2.
Mean positivity of IHC staining for IL-1β, TNF-α and hsp70 in the CPE of the 4th ventricle and the “fold” increase of this value compared to ependymal cells, gray matter and white matter located in the lesion epicenter in the spinal cord of dogs with acute SCI.
| IL-1β | TNF-α | hsp70 | |
|---|---|---|---|
| Mean positivity of CPE | 0.630 | 0.660 | 0.406 |
| Fold increase compared to: | |||
| Ependymal cells | 2.2 | 2.7 | 0.6 |
| Gray matter | 3.2 | 2.9 | 5.3 |
| White matter | 6.5 | 6.2 | 12.4 |
Our results demonstrate significant increases in IL-1β, TNF-α, and possibly hsp70 staining in the CPE of dogs with acute SCI compared to that of normal control dogs. Additionally, these responses appear to be significantly more robust that those appreciated within the gray and white matter of the spinal cord lesion epicenter. To the authors’ knowledge, this is the first study demonstrating a potential role of the CP in the inflammatory response following acute SCI in the dog. IL-1β, TNF-α and hsp70 have all been previously implicated as potential mediators of CNS inflammation in acute SCI and immune-mediated inflammation (Wang et al., 2004; Sharma et al., 2006; Peng et al., 2009; Moore et al., 2012), and the pathologic significance of each of these molecules after SCI has been studied to various degrees. IL-1β and TNF-α responses have been documented in various pathologic conditions of the canine spinal cord, including chemically induced inflammation and ageing (Horais et al., 2003; Chung et al., 2010). Additionally, laboratory models of SCI have repeatedly demonstrated IL-1β and TNF-α responses within the CNS, and improved outcome with attenuation of these responses (Wang et al., 2004; Peng et al., 2009; Sawai et al., 2010). Hsp70 responses have been less thoroughly evaluated; however, the inducible form of hsp70 is normally near the lower limit of detection by ELISA in the CSF of health dogs, and rises in conditions of cellular ischemia or inflammation (Awad et al., 2008; Moore et al., 2012).
An inducible hsp70 response has been documented in rodent models of SCI, and attenuation of this response appears to reduce tissue pathology and improve outcome; however, the sources of hsp70 in the CNS, as well as its role in the inflammatory sequel to acute SCI require further investigation (Sharma et al., 1995, 2006). Evaluation of extracellular hsp70 release in various other disease models implicates this protein as an important inflammatory mediator and contributor to tissue damage. Apparent mechanisms of action include interacting with Toll-like receptors (TLR), inducing pro-inflammatory cytokine expression via the NF-KB pathway, up-regulating macrophage-mediated phagocytosis, and enhancing apoptosis (Clemons and Anderson, 2006; Kovalchin et al., 2006; Yokota et al., 2010).
Given the results of the current study, it appears that the CPE could serve as a potential source for extracellular release of IL-1β, TNF-α, and hsp70 after acute SCI in dogs. For hsp70, ependymal cells lining the central canal may also serve as an important source, a phenomenon previously suggested in a canine model of IR injury of the spinal cord (Awad et al., 2008). In the present study, CSF concentrations of each protein could not be evaluated as definitive proof of extracellular release because CSF samples were not available from any of the dogs included in this study.
Given the inverse correlation between hsp70 and IL-1β staining and the duration of clinical signs in SCI, these two molecules, in particular, may play an important role in the immediate-early inflammatory response after SCI in dogs. Extracellular release of pro-inflammatory cytokines such as IL-1β and hsp70 from either the CPE or ependymal cells into the CSF would allow for dissemination of inflammatory signals throughout the neuroaxis. Ultimately this could contribute to the chronic pro-inflammatory state known to exist in the CNS long after the acute traumatic insult of SCI has passed. Given these findings, the role of the choroid plexus in the inflammatory response after canine SCI warrants further investigation.
Acknowledgments
The project described was supported by the Gray Lady Foundation. The content is solely the responsibility of the authors.
Footnotes
Conflict of interest statement
The authors have no financial or personal relationships that could inappropriately influence or bias the content of the paper.
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Suntres Z, Heijmans J, Smeak D, Bergdall-Costell V, Christofi FL, Magro C, Oglesbee MJ. Intracellular and extracellular expression of the major inducible 70 kDa heat shock protein in experimental ischemia-reperfusion injury of the spinal cord. Exp. Neurol. 2008;212:275–284. doi: 10.1016/j.expneurol.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Theriault JR. Extracellular heat shock proteins in cell signaling. Fed. Euro. Biochem. Soc. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Chung JY, Choi JH, Lee CH, Yoo KY, Won MH, Yoo DY, Kim DW, Choi SY, Youn HY, Moon SM, Hwang IK. Comparison of ionized calcium-binding adapter molecule 1-immunoreactive microglia in the spinal cord between you adult and aged dogs. Neurochem. Res. 2010;35:620–627. doi: 10.1007/s11064-009-0108-4. [DOI] [PubMed] [Google Scholar]
- Clemons NJ, Anderson RL. TRAIL-induced apoptosis is enhanced by heat shock protein expression. Cell Stress Chaperon. 2006;11:343–355. doi: 10.1379/CSC-206.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englehardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc. Res. Technol. 2001;52:112–129. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hecker JG, Sundram H, Zou Shaomin, Praestgaard A, Bavaria JE, Ramchandren Am McGarvey M. Heat shock proteins HSP70 and HSP27 in the cerebral spinal fluid of patients undergoing thoracic aneurysm repair correlate with the probability of post-operative paralysis. Cell Stress Chaperon. 2008;13:435–446. doi: 10.1007/s12192-008-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horais K, Hruby V, Rossi S, Cizkova D, Meschter C, Dorr R, Yaksh T.l. Effects of chronic intrathecal infusion of a partial differential opioid agonist in dogs. Toxicol. Sci. 2003;71:263–275. doi: 10.1093/toxsci/71.2.263. [DOI] [PubMed] [Google Scholar]
- Kovalchin JT, Wang R, Wagh MS, Azoulay J, Sanders M, Chandawarkar RY. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006;14:129–137. doi: 10.1111/j.1743-6109.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Coppola G, Falcao AM, Rodrigues AJ, Geschwind DH, Sousa N, Crreia-Neves M, Palha JA. Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. J. Cereb. Blood Flow Metab. 2009;29:921–932. doi: 10.1038/jcbfm.2009.15. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Yang HYT, Berk JD, Tran JH, Ladarola MJ. Monocyte chemotractant protein-1 in the choroid plexus: a potential link between vascular pro-inflammatory mediators and the CNS during peripheral tissue inflammation. J. Neurosci. 2009;158:885–895. doi: 10.1016/j.neuroscience.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Kim MY, Maiolini A, Tipold A, Oglesbee MJ. Extracellular hsp70 release in canine steroid responsive meningitisarteritis. Vet. Immunol. Immunopathol. 2012;145:129–133. doi: 10.1016/j.vetimm.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olby NJ. The pathogenesis and treatment of acute spinal cord injuries in dogs. Vet. Clin. North Am. Small Anim. 2010;40:791–807. doi: 10.1016/j.cvsm.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai H, Ohkawa Y, Yamada H, Kumamaru H, Harada A, Okano H, Yokomizo T, Iwamoto Y, Okada S. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS, Olsson Y, Westman A serotonin inhibitor, pchlorophenylalanine reduces the heat shock protein response following trauma to the spinal cord: an immunohistochemical and ultrastructural study in the rat. Neurosci. Res. 1995;21:241–249. doi: 10.1016/0168-0102(94)00855-a. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Gordh T, Wiklund L, Mohanty S, Sjoquist PO. Spinal cord injury induced heat shock protein expression is reduced by antioxidant compound H-290/51: an experimental study using light and electron microscopy in the rat. J. Neural. Transm. 2006;113:521–536. doi: 10.1007/s00702-005-0405-2. [DOI] [PubMed] [Google Scholar]
- Sharp NJ, Wheeler SJ. Small Animal Spinal Disorders: Diagnosis and Surgery. 2nd ed. Elsevier; 2005. p. 125. [Google Scholar]
- Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:1503–1516. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellino M, Votta B, Condello C, Piacentino C, Romagnolo A, Merola A, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathologic study. J. Neuroimmunol. 2008;199:133–141. doi: 10.1016/j.jneuroim.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Yokota S, Chiba S, Furuyama H, Fujii N. Cerebrospinal fluids containing anti-hsp70 augment hsp70-induced proinflammatory cytokine production in monocytic cells. J. Neuroimmunol. 2010;218:129–133. doi: 10.1016/j.jneuroim.2009.10.009. [DOI] [PubMed] [Google Scholar]


