Abstract
Comorbidities significantly affect the prognosis and outcomes of patients with hematological malignancies. We have previously reported the impact of comorbidities on the International Prognostic Scoring System (IPSS) score. The aim of this study was to determine whether comorbidities continued to have a significant impact when patients were reclassified according to the Revised-IPSS (IPSS-R). The medical records of 600 consecutive myelodysplastic syndrome patients who presented to MD Anderson Cancer Center between January 2002 and June 2004 were reviewed. The Adult Comorbidity Evaluation-27 (ACE-27) was used to assess the severity of comorbid conditions. Four hundred and two (67%) patients were male. Median age at presentation was 66.6 years (17–94). Mean duration of follow-up was 54 months (1–100). Five hundred and two (84%) patients died, and 54 (9%) patients underwent stem cell transplantation. Overall median survival was 16.8 months (1–100). Median survival by IPSS-R was 47, 34, 21, 16, and 6 months for patients in very low, low, intermediate, high, and very high-risk groups, respectively (P < 0.001). The ACE-27 comorbidity score significantly impacted the median survival of patients in the intermediate (P < 0.001), high (P = 0.045), and very high (P = 0.004) IPSS-R groups; but did not significantly impact the median survival in the low (P = 0.11) and very low (P = 0.49) IPSS-R groups. The ACE-27 comorbidity score significantly impacted the median survival of patients ≤65 years (P < 0.001) but did not significantly impact those >65 years (P = 0.18). Assessment of comorbidity may enhance the prognostic ability of the IPSS-R. Am. J. Hematol. 89:509-516, 2014.
Introduction
Myelodysplastic syndromes (MDS) comprise a group of heterogeneous clonal hematopoietic myeloid malignancies with varied clinical course and distinct natural histories [1,2]. Ineffective hematopoiesis and peripheral cytopenias are common in MDS and contribute significantly to the clinical morbidity and mortality associated with this disorder.
The heterogeneous prognosis of patients with MDS requires the use of prognostic systems for risk stratification. A number of such scoring systems are in use for classification and prognosis of MDS including the French–American–British, the revised WHO classification, and the International Prognostic Scoring System (IPSS) [3–5]. Other prognostic models have been proposed recently including the lower-risk MDS model and the global MD Anderson models [6,7]. The IPSS first published in 1997 has remained the primary system for prognostication of de novo, untreated MDS for nearly two decades. However, the IPSS has a number of limitations, including that the IPSS is not an accurate predictor of prognosis in patients with lower risk MDS and that it has limited cytogenetic classification [6–10]. These have resulted in the development of the Revised-IPSS (IPSS-R) that includes the new MDS cytogenetic classification and provides a more precise evaluation of cytopenias and percentage of bone marrow blasts [11]. Because the IPSS-R was developed in 7,000 patients from multiple countries, it is now accepted as the standard system for prognostication of untreated MDS and has been incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [12–16].
Most current cancer classification systems in general and MDS in particular do not routinely incorporate patient-based prognostic factors. These refer to aspects of the general health of the patient, defined by the frequency and pathophysiological severity of other diseases, illnesses, or medical conditions co-existent with the disease under study. These other conditions are referred to as comorbidities [17]. Comorbidities may be preexisting or may arise during the treatment of the underlying malignancy, but are not adverse effects of cancer therapy [18]. Previous studies have shown that comorbidities impact the outcomes of patients with cancer including patients with prostate, breast, head and neck, gastrointestinal, gynecological, and urinary malignancies [19–24]. Outcomes tend to be directly correlated to the presence of comorbidities with inferior outcomes more likely to be observed in patients with comorbid conditions. Furthermore, outcomes are associated with number and severity of comorbidities [25]. We have previously reported the impact of comorbidities using Adult Comorbidity Evaluation (ACE-27) in patients with MDS classified according to the IPSS score [26]. The aim of this study was to determine whether comorbidities continued to have a similar impact when patients were reclassified according to the IPSS-R.
Methods
We performed a retrospective review of 600 adult MDS patients who presented to MD Anderson Cancer Center (MDACC) between January 2002 and December 2004. Individual comorbid ailments and information defining the severity of comorbid health was extracted using the Adult Comorbidity Evaluation 27 (ACE-27), a 27-item validated comorbidity index for patients with cancer [25,27]. The ACE-27 allows accurate and efficient collection of comorbid health information from the medical records of patients with cancer [28]. Derived from the Kaplan–Feinstein comorbidity index, the ACE-27 takes into consideration the severity of individual organ decompensation and the negative prognostic impact attributable to each comorbid ailment and categorizes comorbid conditions into one of three levels of comorbidity: grade 1 (mild), grade 2 (moderate), and grade 3 (severe). The single highest ranked comorbid ailment was identified and used to calculate the final comorbidity score: none, 0; mild, 1; moderate, 2; or severe, 3. If instead of a single severe comorbid ailment, two or more moderate ailments affecting different organ systems were identified, the overall comorbidity score would be severe.
For each patient, we obtained demographic data including age, sex, race, and MDS-specific staging information based on the IPSS-R [11]. We also collected information on stem cell transplantation (SCT), leukemic transformation, mortality, and survival. Additionally, we collected each patient's date of presentation to MDACC and date of death or time of last follow-up. The study was approved by the institutional review board at MDACC.
Statistical Analysis
The primary end point of this study was overall survival from day of presentation to MDACC until death from any cause or last date of follow-up. Observations were censored for patients last known to be alive. Patients who received SCT were censored on the date of the procedure. Descriptive statistics of the study population, including frequencies, means (with corresponding standard deviations), medians (with corresponding ranges), and proportions were computed. Demographic and clinical factors associated with overall survival were analyzed using the Kaplan–Meier method, and groups were compared using the two-sided log-rank test (log-rank test for trend only where ≥ three groups were entered in logical order, such as groups in IPSS-R and ACE-27 score). In addition, we also performed Kaplan–Meier analysis to examine separately the association of the each system of comorbidities, that is, cardiovascular, respiratory, etc., with survival, as well as the grades of severity of comorbidities as determined by the ACE-27 within the IPSS-R subgroups of risk (very low, low, intermediate, high, and very high) with survival. Subgroup analysis was also performed for the age groups (≤65 years, >65 years). A multivariate Cox proportional hazards regression model was used to weigh the impact of age, IPSS-R, and ACE-27 score on survival. Score points were obtained based on regression coefficients from the final multivariate model. A prognostic model incorporating patient baseline comorbidity was thus derived according to IPSS-R risk categories and age groups. We performed survival landmark analyses to illustrate the effect of the prognostic model on the probability of survival at 12, 24, 36, and 60 months of patients evaluated at baseline and at 6 months landmark A P-value of <0.05 (two-tailed) was considered statistically significant. Statistical analyses were carried out using IBM SPSS Statistics 21 for Windows (SPSS Inc., Chicago, IL).
Results
Patient characteristics
A total of 600 adult patients with MDS were included in this study. The patient characteristics are shown in Table I. 67% were male, and 86% were white. The mean time from diagnosis to referral to MDACC was 4.9 months (range, 0–71). The median age at presentation to MDACC was 66 years (range, 17–94). Median follow-up for this cohort was 85 months (95% confidence interval [CI]; 64–106). A total of 34 (6%), 117 (20%), 131 (23%), 129 (22%), and 165 (29%) patients were IPSS-R very low, low, intermediate, high, and very high, respectively. One hundred and twenty (20%) patients had therapy-related MDS. During the follow-up a total of 123 (21%) underwent leukemic transformation.
TABLE I. Patient Characteristics at Presentation (N = 600).
| Characteristic | N (%) |
|---|---|
| Age group | |
| ≤65 yr | 268 (45) |
| >65 yr | 332 (55) |
| Sex | |
| Male | 402 (67) |
| Female | 198 (33) |
| Marital status | |
| Married | 465 (78) |
| Not married | 135 (22) |
| Race | |
| White | 517 (86) |
| Non-White | 82 (14) |
| ACE-27 score at MDACC presentation | |
| No comorbidity | 137 (23) |
| Mild decompensation | 254 (42) |
| Moderate decompensation | 127 (21) |
| Severe decompensation | 82 (14) |
| Comorbiditiesa | |
| Cardiovascular | 328 (55) |
| Respiratory | 53 (9) |
| Gastrointestinal | 40 (7) |
| Renal | 14 (2) |
| Diabetes mellitus | 97 (16) |
| Neurological | 35 (6) |
| Psychiatric | 48 (8) |
| Reumathologic | 17 (3) |
| Immunologic | 1 (0.2) |
| Other malignancy | 168 (28) |
| Substance abuse | 32 (5) |
| Obesity | 1 (0.2) |
| IPSS-R at MDACC presentation | |
| Very low | 34 (6) |
| Low | 117 (20) |
| Intermediate | 131 (23) |
| High | 119 (22) |
| Very high | 165 (29) |
| Received stem cell transplant | 54 (9) |
| Leukemia transformation | 123 (21) |
| Previous malignancy | 183 (31) |
| Received chemotherapy and/or radiation therapy | 120 (20) |
Abbreviations: ACE-27, Adult Comorbidity Evaluation-27; MDACC, MD Anderson Cancer Center; IPSS-R, Revised International Prognostic Scoring system.
The frequency of comorbidities do not add up to 600 because a patient may have more than one comorbidity.
Survival by patient and disease characteristics
A total of 502 (84%) patients died during study period, and 54 (9%) patients underwent SCT. The median overall survival was 16.8 months (95% CI; 14.05–19.55). In univariate analysis we observed advanced age, presence of leukemic transformation (defined by ≥20% bone marrow blasts), and absence of stem cell transplant were associated with inferior survival (Table II). Likewise advanced IPSS-R was associated with inferior survival (Fig. 1).
TABLE II. Association of Patient and Disease Characteristics with Overall Survival.
| Characteristic | Total no. of patients | Death N (%) | Median survival (mo) | 95% CI | P (univariate) |
|---|---|---|---|---|---|
| Age (yr) | <0.001 | ||||
| ≤65 | 268 (45) | 141 (53) | 34.9 | 26.5–43.2 | |
| >65 | 332 (55) | 251 (76) | 15.4 | 11.5–19.3 | |
| ACE-27 score | <0.001 | ||||
| None, 0 | 137 (23) | 62 (45) | 45.8 | 27.3–64.3 | |
| Mild, 1 | 254 (42) | 177 (70) | 21.8 | 16.6–27.0 | |
| Moderate, 2 | 127 (21) | 90 (71) | 17.4 | 10.5–24.3 | |
| Severe, 3 | 82 (14) | 63 (77) | 10.0 | 6.6–13.4 | |
| Sex | 0.50 | ||||
| Male | 402 (67) | 268 (67) | 21 | 17.1–24.9 | |
| Female | 198 (33) | 124 (63) | 23.8 | 18.1–29.5 | |
| Race | 0.36 | ||||
| White | 517 (86) | 342 (66) | 22.8 | 20.1–25.5 | |
| Non-White | 82 (14) | 49 (60) | 16.8 | 8.7–24.9 | |
| SCT | <0.001 | ||||
| No | 546 (91) | 378 (69) | 20.9 | 17.3–24.5 | |
| Yes | 54 (9) | 14 (26) | NR | ||
| IPSS-R | <0.001 | ||||
| Very low | 34 (6) | 15 (44) | 68.5 | 58.4–78.6 | |
| Low | 117 (20) | 75 (64) | 44.5 | 37.6–51.4 | |
| Intermediate | 131 (23) | 91 (69) | 27.1 | 19.7–34.5 | |
| High | 129 (22) | 85 (66) | 19.7 | 14.1–25.3 | |
| Very high | 165 (29) | 110 (67) | 7.9 | 5.1–10.7 | |
| Regimen | 0.02 | ||||
| Supportive care | 280 (47) | 209 (75) | 19.7 | 13.4–26 | |
| Hypomethylating | 69 (12) | 43 (62) | 31.7 | 22.8–40.6 | |
| Chemotherapy | 125 (20) | 66 (53) | 20 | 16.4–23.6 | |
| Experimental | 126 (21) | 74 (59) | 22.2 | 14.8–29.6 | |
| Leukemic transformation | <0.001 | ||||
| No | 484 (81) | 392 (81) | 17.6 | 14.1–21.1 | |
| Yes | 116 (19) | 0 | NA |
Abbreviations: ACE-27, Adult Comorbidity Evaluation-27; SCT, Stem Cell Transplantation; IPSS-R, Revised International Prognostic Scoring system; CI, Confidence interval.
Figure 1.
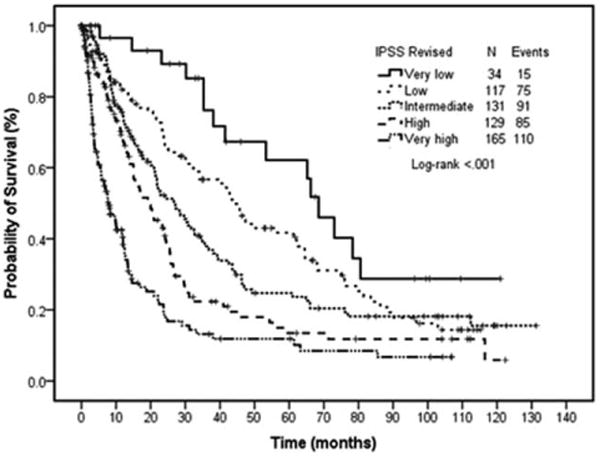
Overall survival by IPSS-R score. Survival curves by IPSS-R. Each line represents survival according to IPSS-R score. Patients with very low IPSS-R have the longest survival, whereas those with very high have the shortest survival.
ACE-27 comorbidity score in MDS and correlation with survival
ACE-27 scores at presentation were as follows: ACE-27 grade 0 in 137 patients (23%); grade 1 in 254 (42%); grade 2 in 127 (21%); and grade 3 in 82 (14%). The most frequently noted medical comorbidities included cardiovascular disorders, concurrent other malignancy/malignancies, and endocrine disorders identified in 55%, 28%, and 16% of MDS patients at presentation to MDACC (Table III). Other comorbidities including psychiatric illnesses and substance abuse were identified in 8% and 5%, respectively.
TABLE III. Median Survival by Kaplan–Meier Estimate for Comorbidity.
| Comorbidity | Total no. patients | Death N (%) | Median survival (mo) | 95% CI | P (univariate) |
|---|---|---|---|---|---|
| Cardiovascular disease | <0.001 | ||||
| Yes | 328 (55) | 239 (73) | 16.4 | 12.6–20.2 | |
| No | 272 (45) | 153 (56) | 30.8 | 22.6–39 | |
| Other malignancy | <0.001 | ||||
| Yes | 168 (28) | 120 (71) | 13.1 | 8.8–17.4 | |
| No | 432 (72) | 272 (63) | 25.7 | 21.9–29.5 | |
| Endocrine | 0.009 | ||||
| Yes | 97 (16) | 73 (75) | 19.7 | 12.4–27 | |
| No | 503 (84) | 319 (63) | 23.4 | 19.9–26.9 | |
| Respiratory | 0.37 | ||||
| Yes | 53 (9) | 42 (79) | 21.9 | 10.4–33.4 | |
| No | 547 (91) | 350 (64) | 22.4 | 19.5–25.3 | |
| Psychiatric | 0.36 | ||||
| Yes | 53 (9) | 32 (60) | 29.8 | 16.9–42.7 | |
| No | 552 (92) | 360 (65) | 22.1 | 18.7–25.5 | |
| Gastrointestinal | 0.05 | ||||
| Yes | 40 (7) | 31 (78) | 19.7 | 10.4–29 | |
| No | 560 (93) | 361 (64) | 22.4 | 19.6–25.2 | |
| Neurologic | 0.43 | ||||
| Yes | 35 (6) | 25 (71) | 26.6 | 3–50.2 | |
| No | 564 (94) | 367 (65) | 22.2 | 19.2–25.2 | |
| Renal | 0.07 | ||||
| Yes | 14 (2) | 11 (79) | 11.8 | 0–25.7 | |
| No | 586 (98) | 381 (65) | 22.4 | 19.6–25.2 | |
| Reumathologic | 0.51 | ||||
| Yes | 17 (3) | 9 (53) | 14 | 6.3–21.7 | |
| No | 583 (97) | 383 (66) | 22.2 | 19.6–24.8 | |
| Substance abuse | 0.61 | ||||
| Yes | 32 (5) | 22 (63) | 23.1 | 4.4–41.8 | |
| No | 568 (95) | 370 (65) | 22.2 | 19.5–24.9 | |
| Obesity | 0.61 | ||||
| Yes | 1 (0.7) | 1 (100) | 75 | NR | |
| No | 599 (99) | 391 (65) | 22.2 | 19.2–25.2 | |
| Immunologic | 0.67 | ||||
| Yes | 1 (0.7) | 0 | NA | NA | |
| No | 599 (99) | 392 (65) | 22.2 | 19.2–25.2 |
Abbreviations: CI, confidence interval.
Median survival according to ACE-27 scores was: 45.9 months for grade 0, 21.8 months for grade 1, 17.4 months for grade 2, and 10.0 months for grade 3 (P < 0.001) (Fig. 2). Among comorbid ailments, only cardiovascular disease, other malignancies, renal, and endocrine disorders were associated with inferior survival. Patients with cardiovascular disease had an inferior survival (16.4 vs. 30.8 months; P < 0.001). Similarly, presence of concurrent non-MDS malignancy was associated with worse survival (13.1 vs. 25.7 months; P < 0.001) (Table III).
Figure 2.
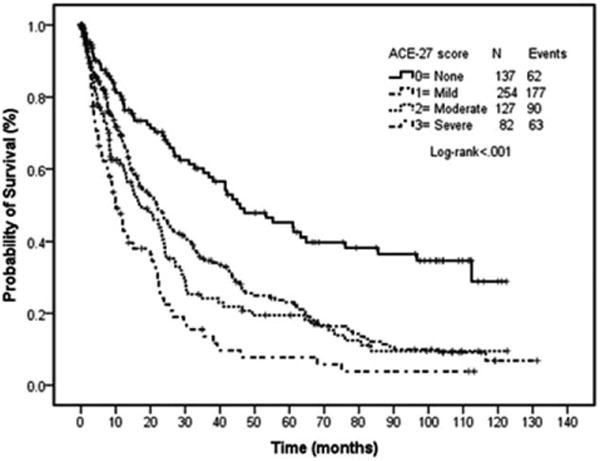
Overall survival by ACE-27 comorbidity score. Survival curves by ACE-27. Each line represents survival according to ACE-27 score. Patients with no comorbidity (ACE-27 score, 0) have the longest survival, whereas those with severe comorbidity (ACE-27 score, 3) have the shortest survival.
Survival in IPSS-R groups by ACE-27 score
The median overall survival in the five IPSS-R groups was significantly different (P < 0.001), ranging from 68.5 months in the very low risk IPSS-R patients to 7.9 months in the very high-risk IPSS-R patients (Fig. 1). We then evaluated the impact of the ACE-27 comorbidity score on median overall survival for each individual IPSS-R group. The ACE-27 comorbidity score was significantly associated with OS in the subgroups of patients with intermediate (P < 0.001), high (P < 0.001), and very high (P < 0.001) IPSS-R groups; but was not associated with OS in the low (P = 0.14) and very low (P = 0.21) subgroups of IPSS-R (Fig. 3A–E).
Figure 3.
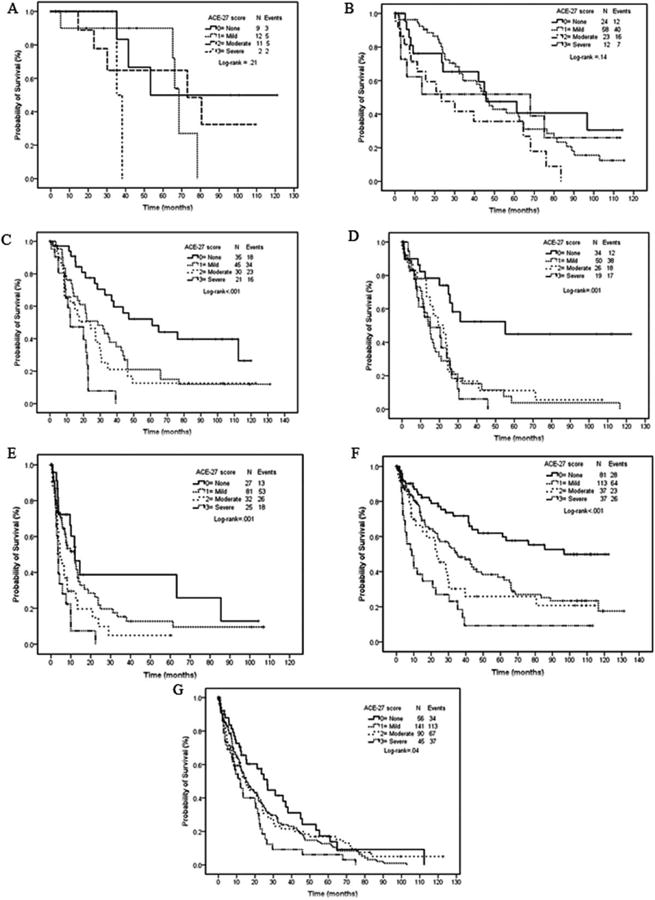
Survival by ACE-27 in subgroups of IPSS-R score (A, B, C, D, E) and age (F and G). (A) very low IPSS-R; (B) low IPSS-R; (C) intermediate IPSS-R; (D) high IPSS-R; (E) very high IPSS-R; (F) age ≤ 65 years, and (G) >65 years. (A) Survival curves by comorbidity score and IPSS-R: very low IPSS-R. (B) Surviva curves by comorbidity score and IPSS-R: low IPSS-R. (C) Survival curves by comorbidity score and IPSS-R: intermediate IPSS-R. (D) Survival curves by comorbidity score and IPSS-R: high IPSS-R. (E) Survival curves by comorbidity score and IPSS-R: very high IPSS-R. (F) Survival curves by comorbidity score and age: age 65 years or younger. (G) Survival curves by comorbidity score and age: older than 65 years.
Survival in different age groups by ACE-27 score
We conducted a subgroup analysis examining survival by ACE-27 comorbidity score for groups of patients ≤65 years versus >65 years. The ACE-27 comorbidity score was significantly associated with OS in both groups of patients (P < 0.001 and P = 0.04, respectively) (Fig. 3F,G).
Multivariate analysis incorporating ACE-27 in MDS
The covariates of interest identified by univariate analysis and included in a multivariate Cox proportional hazards analysis were age, IPSS-R, and comorbidity grade (Table IV). Age (P < 0.001), ACE-27 scores (P < 0.001), and IPSS-R risk group at presentation (P = 0.001) maintained significant association with OS.
TABLE IV. Cox Proportional Hazard Regression Model with Factors Predicting Survival.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P |
| Age (yr) | ||||||
| ≤65 | 1 | 1 | ||||
| >65 | 1.98 | 1.648–2.382 | ≤0.001 | 1.96 | 1.606–2.388 | ≤0.001 |
| ACE-27 | ||||||
| No comorbidity | 1 | 1 | ||||
| Mild decompensation | 1.59 | 1.250–2.029 | ≤0.001 | 1.42 | 1.098–1.825 | 0.007 |
| Moderate decompensation | 1.87 | 1.421–2.469 | ≤0.001 | 1.57 | 1.167–2.099 | 0.003 |
| Severe decompensation | 2.75 | 2.024–3.726 | ≤0.001 | 2.58 | 1.874–3.537 | ≤0.001 |
| IPSS-R | ||||||
| Very low | 1 | 1 | ||||
| Low | 1.40 | 0.87–2.248 | 0.17 | 1.17 | .719–1.892 | 0.53 |
| Intermediate | 1.78 | 1.112–2.839 | 0.02 | 1.64 | 1.023–2.628 | 0.04 |
| High | 2.51 | 1.575–4.011 | ≤0.001 | 2.23 | 1.389–3.578 | ≤0.001 |
| Very high | 4.46 | 2.814–7.055 | ≤0.001 | 4.41 | 2.774–7.023 | ≤0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval ACE-27, Adult Comorbidity Evaluation-27; IPSS-R, Revised International Prognostic Scoring system.
Based on the coefficients of this multivariate survival model a score was assigned to each factor by dividing the respective coefficients from the multivariate survival model by 0.3 and then rounding to the nearest integer (Table V). A prognostic model was constructed with values obtained from the previous weights and the values were used to stratify the risk of death. The new prognostic score had values from 0 to 11. The final prognostic model incorporated four categories labeled as: low risk (values 0–4), intermediate risk 1 (value 5–6), intermediate risk 2 (values 7–8), and high risk (values >8) (Table VI). The new prognostic model accurately predicted survival for the entire group with no overlapping confidence intervals for the four groups (Figs. 4 and 5).
TABLE V. Final Multivariate Survival Model and Risk Score.
| Prognostic factor | Coefficient | Score |
|---|---|---|
| Age >65 yr | 0.753 | 2 |
| ACE-27 | ||
| Mild/moderate | 0.513 | 2 |
| Severe | 1.075 | 4 |
| IPSS-R | ||
| Low/intermediate | 0.489 | 2 |
| High | 0.968 | 3 |
| Very high | 1.658 | 5 |
Abbreviations: Adult Comorbidity Evaluation-27; IPSS-R, Revised International Prognostic Scoring system.
TABLE VI. Final Prognostic Score Incorporating Comorbidities, Age Group, and IPSS-R.
| Risk group | N = 576 (%) | Death N (%) | Median survival (mo) | 95% CI | 5 yr (%) | P |
|---|---|---|---|---|---|---|
| Low (values 0–4) | 168 (29) | 83 (49) | 53.3 | 35.9–70.7 | 49.4 | ≤0.001 |
| Intermediate-1 (values 5–6) | 168 (29) | 117 (70) | 26.9 | 21.4–32.4 | 23 | |
| Intermediate-2 (values 7–8) | 137 (24) | 98 (72) | 13.1 | 10.7–15.5 | 9 | |
| High >8 | 103 (18) | 78 (76) | 6.6 | 4.9–8.3 | 3.3 |
Abbreviations: CI, confidence interval.
Figure 4.
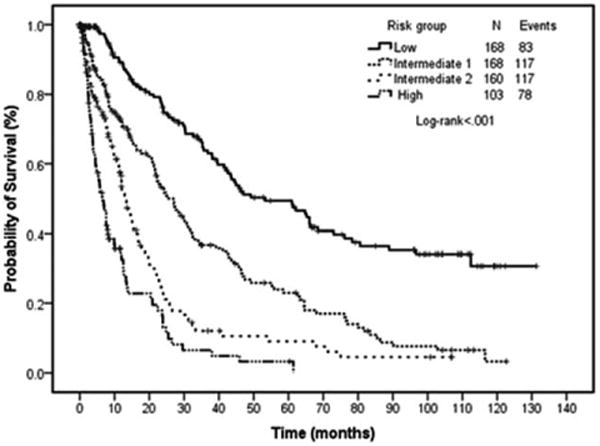
Survival by prognostic model risk group. Survival curves by proposed risk model for all patients. Patients in the low risk category have the longest survival (53 months) compared with those in the high risk category (7 months).
Figure 5.
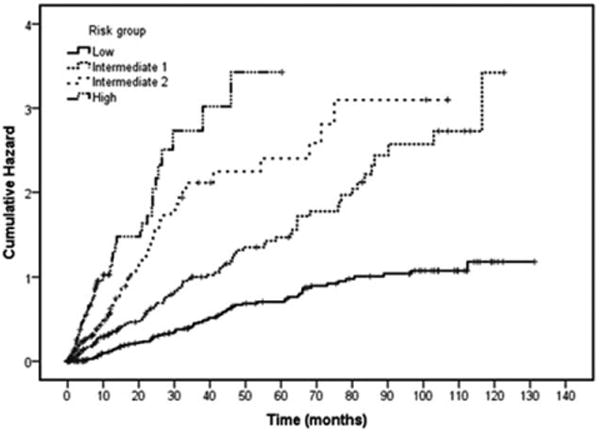
Cumulative hazard by prognostic model risk group. Cumulative hazard by proposed risk model for all patients. The cumulative hazard was highest for those in the high risk category compared with those in the low risk category.
The landmark analysis at baseline and 6 months is reported in Table VII and presents the survival probability of patients evaluated at baseline and also of those who had survived 6 months after the initial evaluation according to the score we developed which accounts for age, ACE-27, and RIPSS risk.
TABLE VII. Landmark Analysis at 6 months from Baseline Showing the Survival Probabilities of Patients at 12, 24, 36, and 60 months.
| Landmark time (from MDACC presentation) | Risk group | Probability of surviving at fixed time points (mo) | |||
|---|---|---|---|---|---|
| Months | 12 | 24 | 36 | 60 | |
| 0 | Low (0–4) | 89.2 | 75.5 | 63.8 | 49.4 |
| Int-1 (5–6) | 72.2 | 53.3 | 36.7 | 23 | |
| Int-2 (7–8) | 56.5 | 23.3 | 12 | 9 | |
| High (>8) | 32.5 | 11.4 | 6.5 | 3.3 | |
| 6 | Low (0–4) | 91.6 | 76.8 | 65.5 | 50.7 |
| Int-1 (5–6) | 85.1 | 62.9 | 43.3 | 27.1 | |
| Int-2 (7–8) | 71.4 | 29.7 | 16.1 | 12.1 | |
| High (>8) | 63 | 25.2 | 12.6 | 6.3 | |
Abbreviations: MDACC, MD Anderson Cancer Center.
Discussion
Our study clearly highlights the impact of comorbidities on the overall survival of patients with MDS independent of age and IPSS-R. We used the ACE-27 to stratify the individual comorbid ailments and define the severity of comorbid health. The ACE-27 was developed by Piccirillo et al. by modifying the Kaplan–Feinstein index based on the frequency of occurrence of comorbidities in newly diagnosed cancer patients [28]. The ACE-27 allows an accurate assessment of comorbidity by providing a more comprehensive list of comorbid conditions including cardiac arrhythmia, obesity, psychiatric disorders, alcohol, and substance abuse [25,28]. We have previously reported the relative impact of comorbidities and IPSS score in determining the survival of patients with MDS at MDACC. A total of 600 patients were evaluated in that report. Comorbidities were assessed using the ACE-27 score and MDS was staged using the IPSS. We noted that comorbidities significantly impacted the survival of patients independent of the IPSS score and age [26]. Herein, we have updated our report by including the recently developed IPSS-R score, which appears to be superior to the IPSS for staging and prognostication of MDS. By incorporating the effects of age, IPSS-R, and comorbidity we developed a new prognostic model that was able to clearly identify four groups of risk within MDS patients with distinct and non-overlapping survival.
The IPSS score based on clinical data obtained from 816 patients has been in place since 1997 and has been the standard for assessing prognosis in untreated MDS patients [5]. The IPSS is reproducible and easy to use. Unfortunately, it has limitations including poor prediction of survival in patients with lower risk disease and insufficient emphasis on cytogenetics. Cytogenetics have shown to greatly impact clinical outcomes and prognosis in patients with MDS [29]. To overcome these limitations the IWG-PM evaluated patients from multiple databases. They systematically and comprehensively evaluated the relative prognostic impact of a number of independently defined prognostic variables in newly diagnosed MDS patients. The newly developed IPSS-R incorporates clinical data from 7,012 patients and is more robust than the IPSS. The same major features identified in the IPSS, namely bone marrow blast percentage, cytopenias, and cyto-genetic subgroups retained major prognostic impact in the IPSS-R. However, by refining these features including further stratifying bone marrow blasts <5%, more precise cytogenetic subgrouping and considering not only the presence or absence but also the depth of cytopenias, the IPSS-R affords more precise prognostication of survival and leukemic transformation [11].
The IPSS-R score has been validated in patients receiving therapy for MDS, including hypomethylator-based therapy, intensive chemotherapy, and/or SCT. It is expected that the IPSS-R will be incorporated by most centers as the primary tool for assessing the prognosis of newly diagnosed MDS patients and for design and evaluation of major clinical trials in MDS until the role of recently identified molecular and flow cytometry information is better defined [30–36]. Given the more robust and precise nature of the IPSS-R, we wished to evaluate whether comorbidities would continue to show a significant impact on survival of MDS patients as they had for patients staged per the older IPSS or WHO scoring systems [26,37–39]. In spite of the increased prognostic accuracy of the IPSS-R model, comorbidities continued to have a significant impact on survival. The ACE-27 comorbidity score accurately stratified the survival of patients in intermediate, high, and very high IPSS-R groups; but did not significantly impact the OS in the low and very low groups. This may be attributable to the already improved survival and limited number of disease related events occurring in the lower risk patients who often have a relatively benign underlying MDS process. The relatively small number of patients with severe comorbidities in the low and very low risk groups may also have contributed to the lack of observable survival difference between these subgroups. Perhaps with longer follow-up a difference in survival may emerge among the favorable IPSS-R subsets. In high risk patients comorbidities may have multiple negative impacts, firstly by directly increasing the risk of death, secondly by restricting therapeutic options and enrollment on clinical trials, and thirdly by impairing tolerance to treatment. Conversely, Della Porta et al. noted that the MDS-comorbidity index significantly impacted overall survival in patients with very low, low, and intermediate WHO-classification based prognostic scoring system (WPSS) risk but not in patients with WPSS high risk. In their opinion the severity of the underlying disease in high-risk MDS patients overcame the clinical impact of mild or moderate comorbidities. Similarly, the ACE-27 comorbidity score significantly impacted the OS of patients <65 years as well as those older than 65 years. Thus, a more accurate clinical prognostication of patients with MDS may be achieved by integrating the IPSS-R and comorbidities score.
These findings led us to develop a prognostic model that included the three factors that significantly impacted survival in MDS, namely IPSS-R, age, and baseline ACE-27 comorbidity score. The new prognostic model accurately predicted survival for the entire group with no overlap among the four groups. Patients were well divided among the groups. Survival ranged from 53 months in the low-risk group to 7 months in the high-risk group. In contrast, our prior prognostic model based on comorbidities and the IPSS scoring system included only three risk groups with median survival ranging from 43 months in the low-risk group to 9 months in the high-risk groups [26]. The new prognostic model seemed to be especially accurate for patients who fell into the intermediate-risk group. According to our prior prognostic model these patients had a median predicted survival of 23 months. The new prognostic model introduced two intermediate-risk categories: namely intermediate-1 and intermediate-2. Predicted survival differed significantly between these two groups with a predicted median survival of 27 months for intermediate-1 patients versus 13 months for intermediate-2 patients. Thus, the newer prognostic model seems to provide clearer prognostic delineation between risk groups.
The retrospective nature of our study implies that the comorbidities could not be assessed in real time but had to be extracted from written records. The study was conducted in a single institution and needs to be validated in a multi-institutional analysis. Similarly, although we have included information on treatment, our study was not designed to predict outcomes based on treatment modality but to provide accurate prognostic information to patients and physicians at the time of diagnosis of MDS.
The IPSS-R is a refined and more accurate system for prognostication of newly diagnosed MDS. In spite of this, comorbidities continue to significantly impact the survival of patients with MDS. Hence, assessment and incorporation of comorbidity status at diagnosis of MDS is warranted.
Acknowledgments
This study was conducted following the guidelines of The University of Texas MD Anderson Cancer Center after local IRB approval.
Contract grant sponsor: It was supported in part by the Ruth & Ken Arnold Fund, the Edward P. Evans Foundation (GGM), the MD Anderson Cancer Center Leukemia Support Grant (CCSG) CA016672, and generous philanthropic contributions to the MD Anderson Moon Shots Program.
Footnotes
Author Contributions: GGM and ND developed the concept, recruited patients, wrote the manuscript and take primary responsibility for the article; GGM, HK, ND, EJ, and TK recruited the patients; MCT, SP, and ND participated in the statistical analysis; GGM, KN, KTH, HK, CBR, EJ, TK, CD, KTN, and ND coordinated the research; ND, GGM, HK, EJ, TK, CD, MCT, SP, CBR, and KN wrote the article.
Conflict of interest: Nothing to report.
References
- 1.Garcia-Manero G. Myelodysplastic syndromes: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:692–701. doi: 10.1002/ajh.23264. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 4.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 6.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–543. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos FP, Kantarjian H, Garcia-Manero G, et al. The search for better prognostic models in myelodysplastic syndromes. Curr Hematol Malig Rep. 2011;6:13–21. doi: 10.1007/s11899-010-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugo SE, Bundrick SC, Hanson CA, et al. Independent validation of the MD Anderson Cancer Center risk model for myelodysplastic syndromes (MDS), and comparison to the International Prognostic Scoring System (IPSS) and the World Health Organization-based prognostic scoring system (WPSS) Blood. 2009;114:1467–1468. [Google Scholar]
- 10.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyo-type in MDS and correlation with subtypes: Evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–4395. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg PL, Attar E, Bennett JM, et al. Myelodysplastic syndromes: Clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2013;11:838–874. doi: 10.6004/jnccn.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcarcel D, Montesinos P, Sanchez-Ortega I, et al. A scoring system to predict the risk of death during induction with anthracycline plus cytarabine-based chemotherapy in patients with de novo acute myeloid leukemia. Cancer. 2012;118:410–417. doi: 10.1002/cncr.26273. [DOI] [PubMed] [Google Scholar]
- 14.Messa E, Gioia D, Evangelista A, et al. High predictive value of the revised international prognostic scoring system (IPSS-R): An external analysis of 646 patients from a multiregional Italian MDS Registry. Blood. 2012;120 ASH meeting abstract 1702. [Google Scholar]
- 15.Mishra A, Al Ali NH, Corrales-Yepez M, et al. Validation of the revised international prognostic scoring system (R-IPSS) for patients with myelodysplastic syndromes: Therapeutic implications. Blood. 2012;120 ASH meeting abstract 2816. [Google Scholar]
- 16.Cermak J, Mikulenkova D, Brezinova J, et al. A reclassification of Myelodysplastic Syndrome (MDS) patients of RAEB-1 subgroup according to IPSS-R improves discrimination of high risk patients and better predicts overall survival. A retrospective analysis of 49 patients. Blood. 2012;120 ASH meeting abstract 4957. [Google Scholar]
- 17.Piccirillo JF, Costas I. The impact of comorbidity on outcomes. ORL J Otorhinolaryngol Relat Spec. 2004;66:180–185. doi: 10.1159/000079875. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein A. Pre-therapeutic classification of comorbidity in chronic disease. J Chron Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 19.Albertsen PC, Fryback DG, Storer BE, et al. The impact of co-morbidity on life expectancy among men with localized prostate cancer. J Urol. 1996;156:127–132. [PubMed] [Google Scholar]
- 20.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 23.Wells CK, Stoller JK, Feinstein AR, et al. Comorbid and clinical determinants of prognosis in endometrial cancer. Arch Intern Med. 1984;144:2004–2009. [PubMed] [Google Scholar]
- 24.Miller DC, Taub DA, Dunn RL, et al. The impact of co-morbid disease on cancer control and survival following radical cystectomy. J Urol. 2003;169:105–109. doi: 10.1016/S0022-5347(05)64046-3. [DOI] [PubMed] [Google Scholar]
- 25.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of co-morbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 26.Naqvi K, Garcia-Manero G, Sardesai S, et al. Association of comorbidities with overall survival in myelodysplastic syndrome: Development of a prognostic model. J Clin Oncol. 2011;29:2240–2246. doi: 10.1200/JCO.2010.31.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. J Chron Dis. 1974;27:387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 28.Piccirillo JF, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Registry Manage. 2003;30:8–14. [Google Scholar]
- 29.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaemmanuil E, Cazzola M, Boultwood J, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365:1384–1395. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida K, Sanada M, Shiraishi Y, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 33.Thol F, Kade S, Schlarmann C, et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood. 2012;119:3578–3584. doi: 10.1182/blood-2011-12-399337. [DOI] [PubMed] [Google Scholar]
- 34.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson CA, Cote G, Quintas-Cardama A. The changing mutational landscape of acute myeloid leukemia and myelodysplastic syndrome. Mol Cancer Res. 2013;11:815–827. doi: 10.1158/1541-7786.MCR-12-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westers TM, Ireland R, Kern W, et al. Standardization of flow cytometry in myelodysplastic syndromes: A report from an international consortium and the European Leukemia Net Working Group. Leukemia. 2012;26:1730–1741. doi: 10.1038/leu.2012.30. [DOI] [PubMed] [Google Scholar]
- 37.Wang R, Gross CP, Halene S, et al. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res. 2009;33:1594–1598. doi: 10.1016/j.leukres.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Della Porta MG, Malcovati L. Clinical relevance of extra-hematologic comorbidity in the management of patients with myelodysplastic syndrome. Haematologica. 2009;94:602–606. doi: 10.3324/haematol.2009.005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeilstocker M, Karlic H, Nosslinger T, et al. Myelodysplastic syndromes, aging, and age: Correlations, common mechanisms, and clinical implications. Leuk Lymphoma. 2007;48:1900–1909. doi: 10.1080/10428190701534382. [DOI] [PubMed] [Google Scholar]


