Abstract
The conventional method for creating targeted contrast agents is to conjugate separate targeting and fluorophore domains. In this study we report a new strategy based on incorporation of targeting moieties into the non-resonant structure of pentamethine and heptamethine indocyanines. Using the known affinity of phosphonates for bone minerals as a model system, we have synthesized two families of bifunctional molecules that target bone without the need for a traditional bisphosphonate. With peak fluorescence emission at ≈ 700 nm or ≈ 800 nm, these molecules can be used for FLARE dual-channel imaging. Longitudinal FLARE studies in mice demonstrate that phosphonated near-infrared fluorophores remain stable in bone for over 5 weeks, and histological analysis demonstrates incorporation into bone matrix. Taken together, we describe a new strategy for creating ultracompact, targeted, near-infrared fluorophores for various bioimaging applications.
Keywords: Near-Infrared Fluorophores, Functional Groups, Near-Infrared Fluorescence Imaging, Optical Imaging, Image-Guided Surgery
Near-infrared (NIR) fluorescence imaging of bone minerals, as first introduced in 2001,[1] required that a bisphosphonate or other bone targeting ligand [2–4] be conjugated covalently to an NIR fluorophore, creating a bifunctional molecule for biomedical imaging.[4] Although preparative scale synthesis,[5] hybrid optical/nuclear agents,[6] and various clinical applications[7,8] of these molecules were subsequently described, the basis chemical strategy remain unaltered.
However, the resonance structures within pentamethine and heptamethine indocyanines tolerate a host of substituent modifications that have little or no effect on optical performance of the fluorophore.[2,3] We therefore hypothesized that multiple copies of small substituents (ligands), which singly have low affinity for a target tissue, could be incorporated into the polymethine cyanine core (a fluorophore) in such a way as to generate a final molecule with both high affinity targeting and NIR fluorescence (i.e., a targeted contrast agent).[9–11] In this study, we explored this hypothesis by using the phosphonate group as the primary modification, and also studied the ability of other substituents, like sulfonates, to modify performance of the final molecules.
As shown in Figure 1a, four types of phosphonated NIR fluorophores were prepared from a pentamethine core for 700 nm fluorescence and a heptamethine core for 800 nm fluorescence, with the addition of sulfonates as indicated. The synthetic methods for P700 and P800 are slightly modified from the cGMP synthesis of ZW800-1 reported previously by our group.[12,13] Prior to measurement of optical properties and in vivo performance, each NIR fluorophore was purified to ≥ 95% as measured using 210 nm absorbance (Supporting Information Figure S1). Physicochemical and optical properties of all NIR fluorophores are summarized in Figure 1b. By varying the side chains of the polymethine core, it was possible to systematically modify hydrophobicity, polarity, and electron resonance without affecting optical performance. Energy-minimized 3D structures showed similar distributions of charge and hydrophobicity over the molecular surface.
Figure 1.
Synthetic scheme (a) and optical and physicochemical properties (b) of P700 and P800 NIR fluorophores.
The specificity of these new NIR fluorophore for the biologically important calcium salts hydroxyapatite (HA), calcium carbonate (CC), calcium phosphate (CP), calcium oxalate (CO), and calcium pyrophosphate (CPP) was measured. As shown in Figure 2, all of P700 and P800 fluorophores have a strong affinity for HA compared, but specificity was significantly different in molecules with additional sulfonates attached. In particular, high affinity for CP, roughly equal to that for HA, was seen with P700SO3 and P800SO3, but not P700H and P800H. Although the synergy of sulfonates with phosphonates for bone bonding is not well understood, sulfonate groups improve solubility in aqueous media and increase ionization of hydroxyl groups on the fluorophore due to strong electron withdrawing effects.[14] One hypothesis is that two phenyl sulfonate groups bind a calcium ion by salt formation while the phosphonate group competes with serum phosphate ions and serves as a monodentate ligand for calcium binding.[15]
Figure 2.
Calcium salt binding properties of (a) P700 and (b) P800 NIR fluorophores. SBR was calculated by the fluorescence intensity of each fluorophore sample versus the signal intensity of each control sample. All NIR fluorescence images have identical exposure and normalizations. Abbreviations used are: HA, hydroxyapatite; CC, calcium carbonate; CP, calcium phosphate; CO, calcium oxalate, and CPP, calcium pyrophosphate. Images are representative of n = 3 independent experiments.
In order to explore bone targeting in vivo, phosphonated NIR fluorophores were administered intravenously into mice, and their biodistribution and clearance measured over 4 h and 24 h. As shown in Figure 3a–b, P700H and P800H exhibited hepatic clearance from liver to duodenum, while P700SO3 and P800SO3 were eliminated from the body by renal clearance into urine without nonspecific uptake in non-bone tissues and organs due to their low logD and high polarity.[12] By 24 h post-injection, all P700 and P800 NIR fluorophores were eliminated from the body and only bone signal was evident. Interestingly, P700SO3 and P800SO3 showed longer blood half-lives (t1/2β; ≈ 50 min) compared to P700H and P800H (26.6 min and 38.8 min, respectively; Supporting Information Table S1). These results highlight the effects of charge, hydrophobicity, and polarity of a targeted agent on its in vivo behavior.[12] Because the bone targeting and biodistribution of P700SO3 and P800SO3 were most favorable, we selected these sulfonated fluorophores for further evaluation and optimization. To determine the dose of P700SO3 and P800SO3 that achieved the highest signal-to-background ratio (SBR), agents were intravenously administered in the range of 1 to 25 nmol and imaged at 4 h post-injection. Although increasing dose amplified bone signal, there was diminishing return at higher dose due to increased background (Supporting Information Figure S2).
Figure 3.
In vivo biodistribution and bone tissue imaging using P700 (a) and P800 (b) NIR phosphonates in mice. Each NIR fluorophore was intravenously injected into 20 g CD-1 or NCRNU nude mice (10 nmol; 0.4 mg/kg) 4 h and 24 h prior to imaging. Abbreviations used are: BD, bile duct; Bl, bladder; Du, duodenum; He, Heart; In, intestine; JB, jaw bone; Ki, kidneys; Li, liver; Lu, lungs; Mu, muscle; Pa, pancreas; SJ, shoulder joint; Sp, spleen; St, stomach, and Ur, ureter. Scale bars = 1 cm. Images are representative of n = 3 independent experiments.
To confirm that these agents performed well across species, particularly large animals with tissues and organs the size of humans, we injected 1 μmol (0.03 mg/kg) of P700SO3 and P800SO3 into 35 kg Yorkshire pigs. Both phosphonated NIR fluorophores resulted in high signal in bone at 4 h post-injection with little nonspecific uptake in other tissues or organs (Figure 4), while commercially available BoneTag™ agents showed high background signal in skin, liver, and kidneys (Supporting Information Figure S3). Blood half-lives of P700SO3 and P800SO3 in pigs were 50.2 and 69.7 min, respectively, suggesting that a 4–6 h elimination period would be optimal in future human studies (Figure 4b).
Figure 4.
In vivo biodistribution and bone tissue imaging using P700SO3 and P800SO3 NIR phosphonates in pigs. (a) 30 pmol/g of P700SO3 and P800SO3 were intravenously injected into 35 kg Yorkshire pigs 4 h prior to imaging. (b) Blood clearance (%ID/g) and blood half-life (mean ± 95% confidence intervals) of P700SO3 and P800SO3 in pigs. All NIR fluorescence images have identical exposure and normalizations. Scale bars = 1 cm. Images are representative of n = 3 independent experiments.
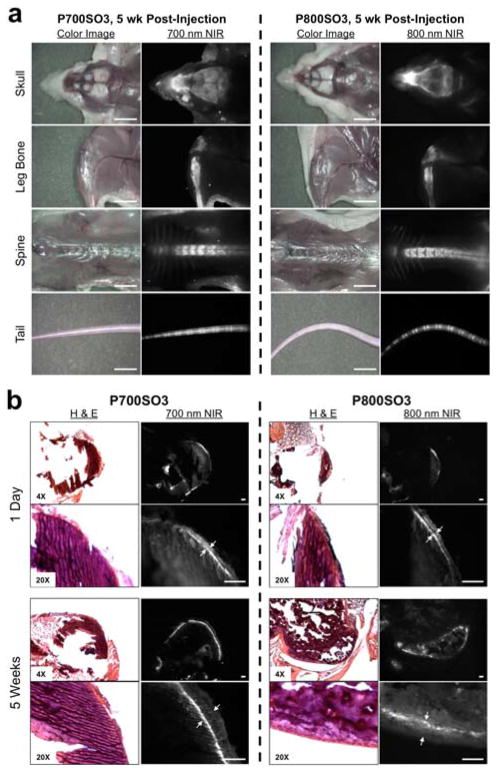
We performed serial imaging of all NIR fluorophores in NCRNU nude mice over the course of 5 weeks. As shown in Figure 5a, P700SO3 and P800SO3 had two-fold higher fluorescent signal compared to P700H and P800H. Of note, SBR values for P700SO3 are relatively lower than P800SO3 due to skin autofluorescence in the 700 nm channel. Importantly, SBR values in spine were stable over 5 weeks after a single intravenous injection (Supporting Information Figure S4). Magnified bone imaging of sacrificed and de-skinned mice 5 weeks post-injection revealed exquisite imaging of bone tissue (Figure 5a). Microscopic analysis of cross-sectioned bones obtained at 1 day and 5 weeks post-injection revealed that P700SO3 and P800SO3 were stably incorporated into the bone matrix, with additional normal bone matrix deposited on top of the NIR fluorophores over time (Figure 5b).
Figure 5.
Stable incorporation of phosphonated NIR fluorophores into bone matrix. (a) P700SO3 and P800SO3 were injected intravenously into 20 g NCRNU nude mice (10 nmol; 0.4 mg/kg) 5 wk prior to imaging. Scale bars = 1 cm. (b) H&E and NIR imaging of resected bone tissues at day 1 and 5 wk post-injection of P700SO3 and P800SO3. Scale bars = 100 μm. All NIR fluorescence images for each condition have identical exposure times and normalizations.
In this study, we hypothesized that chemical substituents with low affinity for a target tissue could be incorporated into the non-resonant backbone of an NIR fluorophore to create a new contrast agent with high affinity.[16] Based on the results, this strategy appears to work well, at least with the phosphonate model system employed. We did, however, see significant modulation of contrast agent performance from additional side groups. The addition of sulfonate groups improved biodistribution and clearance, resulting in a higher signal, lower background, and more complete elimination of unbound dose from the body.
The improvement in SBR through sulfonation, though, came at the price of specificity. P700H and P800H had a pattern of binding to calcium salts most similar to a conventional bisphosphonate conjugated to a NIR fluorophore,[1,5,17,18] i.e., high HA-specificity. However, P700SO3 and P800SO3 exhibited high binding to CP as well as HA. For in vivo imaging of bone and urinary crystals,[8,19] CP binding might actually be an advantage, because otherwise binding is restricted to sites of osteoblastic activity. However, if HA specificity is the goal, then a conventional bisphosphonate-NIR fluorophore conjugate would be best.
Importantly, the new molecules we describe in this study appear to be stably incorporated into the bone matrix without interfering with normal bone deposition (Figure 5b). In fact, NIR fluorescence of bone remained intact and stable for at least 5 weeks post-injection. This long-term stability could be ideal for tissue engineering experiments where neo-ossification needs to be followed for days and months. For studies of drugs that modulate either osteoblast or osteoclast activity, long-acting contrast agents would permit quantitative, ratiometric imaging of bone growth and resorption of over time.
Finally, we purposely engineered a single carboxylic acid into each molecule, which permits conjugation to other functional molecules and therapeutics. For example, one could use this chemical group to conjugate drugs that modulate osteoblasts or osteoclasts, or to conjugate diagnostic or therapeutic radioisotopes, creating trifunctional theragnostic agents. In addition, non-hormonal phosphonates increase bone strength, and repeated injections of this theragnostic agent will improve bone density by preventing the loss of minerals.
Experimental Section
Synthesis of P700 and P800 NIR fluorophores
All chemicals and solvents were of American Chemical Society grade or HPLC purity. Starting materials were purchased from Sigma-Aldrich (Saint Louis, MO) and Fisher Scientific Inc. (Pittsburgh, PA) and used without purification. Final products were separated by preparative HPLC system (Waters, Milford, MA, USA) equipped with a PrepLC 150 mL fluid handling unit, a manual injector (Rheodyne 7725i), a 2487 dual wavelength absorbance detector (Waters) and an evaporative light scatter detection (ELSD, Richards Scientific, Novato, CA, USA). See Supporting Information for detailed chemical syntheses and analyses.
NIR fluorescence imaging system
The dual-NIR channel FLARE imaging system has been described in detail previously.[20–22] In this study, 4 mW/cm2 of 670 nm excitation light and 11 mW/cm2 of 760 nm excitation light were used with white light (400–650 nm) at 40,000 lx. Color and NIR fluorescence images were acquired simultaneously with custom software at rates up to 15 Hz over a 15 cm diameter field of view. The imaging system was positioned at a distance of 18 inches from the surgical field. For each experiment, camera exposure time and image normalization was held constant.
Quantitative analysis
At each time point, the fluorescence and background intensity of a region of interest (ROI) over each tissue was quantified using custom FLARE software. The signal-to-background ratio (SBR) was calculated as SBR = fluorescence/background, where background is the signal intensity of neighboring tissues, such as muscle or skin, obtained over the imaging period. All NIR fluorescence images for a particular fluorophore were normalized identically for all conditions of an experiment. At least three animals were analyzed at each time point. Statistical analysis was carried out using the unpaired Student’s t-test or one-way analysis of variance (ANOVA). Results were presented as mean ± s.d. and curve fitting was performed using Prism version 4.0a software (GraphPad, San Diego, CA).
Histology and NIR fluorescence microscopy
Bone tissues were placed in 2% paraformaldehyde in PBS for 30 min before mounting in Tissue-Tek OCT compound (Fisher Scientific, Pittsburgh, PA) and flash-freezing in liquid nitrogen. Frozen samples were cryosectioned (10 μm per slice), observed by NIR fluorescence microscopy, and also stained with hematoxylin and eosin (H&E). NIR fluorescence microscopy was performed on a 4-filter Nikon Eclipse TE300 microscope system as previously described.[9,23,24] The microscope was equipped with a 100 W mercury light source (Chiu Technical Corporation, Kings Park, NY), NIR-compatible optics, and a NIR-compatible 10X Plan Fluor objective lens and a 100X Plan Apo oil immersion objective lens (Nikon, Melville, NY). Images were acquired on an Orca-AG (Hamamatsu, Bridgewater, NJ). Image acquisition and analysis was performed using iVision software (BioVision Technologies, Exton, PA). Two custom filter sets (Chroma Technology Corporation, Brattleboro, VT) composed of 650 ± 22 nm and 750 ± 25 nm excitation filters, 675 nm and 785 nm dichroic mirrors, and 710 ± 25 nm and 810 ± 20 nm emission filters were respectively used to detect P700SO3 and P800SO3 signals in the frozen tissue samples.
Supplementary Material
Footnotes
This study was supported by the following grants from the National Institutes of Health: NCI BRP grant #R01-CA-115296 and NIBIB grants #R01-EB-010022 and #R01-EB-011523. We thank David Burrington, Jr. for editing, and Eugenia Trabucchi for administrative assistance. John V. Frangioni, M.D., Ph.D.: Dr. Frangioni is currently CEO of Curadel, LLC, which has licensed FLARE imaging systems and contrast agents from the BIDMC.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
Contributor Information
Dr. Hoon Hyun, Division of Hematology/Oncology, Department of Medicine and Department of Radiology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, SL436A, Boston, MA 02215 (USA), Fax: (+1) 617-667-0981
Dr. Hideyuki Wada, Division of Hematology/Oncology, Department of Medicine and Department of Radiology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, SL436A, Boston, MA 02215 (USA), Fax: (+1) 617-667-0981
Dr. Kai Bao, Division of Hematology/Oncology, Department of Medicine and Department of Radiology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, SL436A, Boston, MA 02215 (USA), Fax: (+1) 617-667-0981
Dr. Julien Gravier, Division of Hematology/Oncology, Department of Medicine and Department of Radiology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, SL436A, Boston, MA 02215 (USA), Fax: (+1) 617-667-0981
Dr. Yogesh Yadav, Department of Chemistry, Georgia State University, Atlanta, GA 30303 (USA)
Dr. Matt Laramie, Department of Chemistry, Georgia State University, Atlanta, GA 30303 (USA)
Dr. Maged Henary, Department of Chemistry, Georgia State University, Atlanta, GA 30303 (USA)
Dr. John V. Frangioni, Division of Hematology/Oncology, Department of Medicine and Department of Radiology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, SL436A, Boston, MA 02215 (USA), Fax: (+1) 617-667-0981. Curadel, LLC, 377 Plantation Street, Worcester, MA 01605 (USA)
Dr. Hak Soo Choi, Email: hchoi@bidmc.harvard.edu, Division of Hematology/Oncology, Department of Medicine and Department of Radiology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, SL436A, Boston, MA 02215 (USA), Fax: (+1) 617-667-0981. Department of Cogno-Mechatronics Engineering, Pusan National University, Busan 609-735, South Korea
References
- 1.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. Nat Biotechnol. 2001;19:1148–1154. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 2.Kovar JL, Xu X, Draney D, Cupp A, Simpson MA, Olive DM. Anal Biochem. 2011;416:167–173. doi: 10.1016/j.ab.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Pautke C, Vogt S, Kreutzer K, Haczek C, Wexel G, Kolk A, Imhoff AB, Zitzelsberger H, Milz S, Tischer T. J Anat. 2010;217:76–82. doi: 10.1111/j.1469-7580.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowles EA, Kovar JL, Curtis ET, Xu H, Othman SF. BioResearch open access. 2013;2:186–191. doi: 10.1089/biores.2013.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhushan KR, Tanaka E, Frangioni JV. Angew Chem Int Ed Engl. 2007;46:7969–7971. doi: 10.1002/anie.200701216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhushan KR, Misra P, Liu F, Mathur S, Lenkinski RE, Frangioni JV. J Am Chem Soc. 2008;130:17648–17649. doi: 10.1021/ja807099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaheer A, Murshed M, De Grand AM, Morgan TG, Karsenty G, Frangioni JV. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1132–1136. doi: 10.1161/01.ATV.0000210016.89991.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 9.Choi HS, Gibbs-Strauss SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV. Nat Biotechnol. 2013;31:148–153. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashitate Y, Hyun H, Kim SH, Lee JH, Henary M, Frangioni JV, Choi HS. Theranostics. 2014;4:693–700. doi: 10.7150/thno.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park MH, Hyun H, Ashitate Y, Wada H, Park G, Lee JH, Njiojob C, Henary M, Frangioni JV, Choi HS. Theranostics. 2014;4:823–833. doi: 10.7150/thno.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH, Ashitate Y, Hyun H, Patonay G, Strekowski L, Henary M, Frangioni JV. Angew Chem Int Ed. 2011;50:6258–6263. doi: 10.1002/anie.201102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyun H, Bordo MW, Nasr K, Feith D, Lee JH, Kim SH, Ashitate Y, Moffitt LA, Rosenberg M, Henary M, Choi HS, Frangioni JV. Contrast Media & Mol Imaging. 2012;7:516–524. doi: 10.1002/cmmi.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozar A, Haren N, Chasseraud M, Louvet L, Maziere C, Wattel A, Mentaverri R, Morliere P, Kamel S, Brazier M, Maziere JC, Massy ZA. Journal of cellular physiology. 2008;215:47–54. doi: 10.1002/jcp.21283. [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi T, Yano K, Nakagawa S, Kaji F. Journal of colloid and interface science. 2003;260:19–25. doi: 10.1016/s0021-9797(02)00157-1. [DOI] [PubMed] [Google Scholar]
- 16.Hyun H, Park MH, Owens EA, Wada H, Henary M, Handgraaf HJM, Vahrmeijer AL, Frangioni JV, Choi HS. Nature Medicine. 2014 doi: 10.1038/nm.3728. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmatys KM, Cole EL, Smith BD. Molecular pharmaceutics. 2013;10:4263–4271. doi: 10.1021/mp400357v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama H, Kawase T, Okuda K, Wolff LF, Yoshie H. Acta radiologica. 2011;52:978–988. doi: 10.1258/ar.2011.110131. [DOI] [PubMed] [Google Scholar]
- 19.Kozloff KM, Weissleder R, Mahmood U. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2007;22:1208–1216. doi: 10.1359/jbmr.070504. [DOI] [PubMed] [Google Scholar]
- 20.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, Ngo L, Khamene A, Azar F, Frangioni JV. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashitate Y, Kim SH, Tanaka E, Henary M, Choi HS, Frangioni JV, Flaumenhaft R. J Vasc Surg. 2012;56:171–180. doi: 10.1016/j.jvs.2011.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gioux S, Choi HS, Frangioni JV. Mol imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 23.Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Nat biotechnol. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Nano Lett. 2009;9:2354–2359. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.