Abstract
The mucopolysaccharidoses (MPS) are genetic lysosomal storage diseases. Peripheral bone dysplasia and spinal involvement are the predominant orthopedic damage. The risk of spinal cord compression due to stenosis of the craniocervical junction is well known in these patients, whereas the thoracolumbar kyphosis is often well tolerated over a long period of time. Thus, signs of spinal cord compression at this level occur later and more insidiously. The authors describe three cases of patients with thoracolumbar kyphosis who suffered from postoperative spinal cord compression in the absence of direct surgical trauma. Analysis of these cases and review of the literature helped identify causal factors resulting in spinal cord ischemia. The risk of perioperative spinal cord complications related to a thoracolumbar kyphosis must be discussed with patients with MPS and their families prior to any surgery, including extra-spinal procedures.
Introduction
Mucopolysaccharidoses (MPS) are genetic lysosomal storage diseases related to the accumulation of glycosaminoglycans resulting from congenital enzymatic deficiency. Dysostosis multiplex, along with spinal cord damage, is predominant and forms part of a picture of progressive multi-organ impairment appearing as of age one (Kleigman and Muenzer 2004; Feillet et al. 2006). Stenosis of the craniocervical junction and thoracolumbar kyphosis are the characteristic spinal deformities and are responsible for spinal cord compression at both levels (White and Harmatzc 2010). More recently, myelopathy due to cervicothoracic stenosis has been described in patients with Morquio syndrome (Baratela et al. 2014). We report on three children afflicted with MPS and thoracolumbar kyphosis showing no neurological impairment (two Hurler syndrome, or MPS type I, and one Morquio syndrome, or MPS type IV) who suffered perioperative spinal cord damage in the absence of direct surgical trauma.
Case Reports
Patient 1 was a 12-year-old child suffering from Morquio syndrome with recurrent bilateral genu valgum despite repeated treatment by temporary epiphysiodesis. At age 5, the child had been treated for craniocervical stenosis. At age 12, preoperative orthopedic assessment also revealed a T12 apical thoracolumbar kyphosis of 40° in prone position, reduced to 22° when in supine position. A 45° T2 apical craniothoracic kyphosis was present in prone position which remained unchanged when in supine position (Fig. 1). Preoperative neurological assessment was normal. Distal femoral and proximal tibial varisation osteotomies were carried out under general anesthesia, with no observable perioperative adverse event. Since it was an extra-spinal surgery, evoked potentials have not been monitored before and during surgery. On day 2 postoperatively, L1-L2 motor paraplegia appeared which slowly progressed over three days. No sensory disturbance was observed; damage appeared to be an anterior spinal artery syndrome. Emergency MRI showed no worsening of the two kyphotic zones. The diameter of the medullary canal remained unchanged with persistence of perimedullar fluid spaces next to both kyphotic zones. An extended intramedullary hyperintensity, however, was observed from T1 to T3 which did not correspond to the clinical topography. It was compatible, on the other hand, with ischemia or temporary spinal cord compression. There was no further neurosurgical indication, and the patient had partially recovered six months postoperatively, albeit persistence of sphincter disorders.
Fig. 1.

Preopertive radiograph (a) and MRI (b and c) of patient 1. T12 apical thoracolumbar kyphosis of 40° in prone position, (a) reduced to 22° when in supine position. 45° T2 craniocervical kyphosis unchanged when in supine position (a and b)
Patient 2 was a 4-year-old child afflicted with Hurler syndrome with advanced apical T12, greater than 90° (Fig. 2). No neurological complications existed, and preoperative evoked potentials were normal. Two-stage surgical correction was planned, with initial posterior instrumentation of T12-L2, followed by anterior graft. The first surgery was carried out monitoring somatosensory and motor evoked potentials. A slight modification of motor evoked potentials was observed during the procedure, but which returned to normal with repositioning of the spinal electrode. On recovery, the child developed an asymmetric paraplegia with partial sensory loss, more pronounced on the left side, requiring emergency surgery to remove the hardware. A spine cast was ordered to maintain reduction of this unstable thoracolumbar kyphosis after hardware removal, despite any MRI indication of spinal cord compression (Fig. 3). There was no improvement of neurological deficit with definitive installation of a complete L2 paraplegia with a complete sensory loss.
Fig. 2.
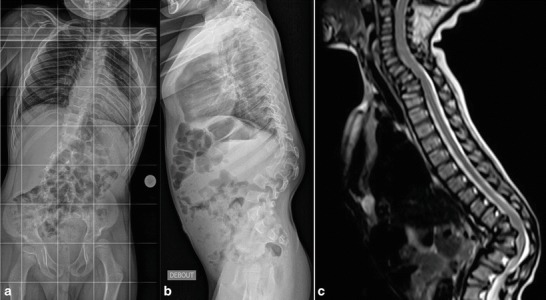
Preoperative radiographies (a and b) and MRI (c) of patient 2 (Hurler syndrome). Kyphosis greater than 90° with retrolisthesis of the apical T12 vertebra and infiltrated, bulging appearance of intervertebral discs in the spinal cord
Fig. 3.

Immediate postoperative condition of patient 2. (a) Anteroposterior radiograph of instrumentation T12-L2. (b) MRI before removal of hardware, absence of indication of spinal cord compression (artefacts related to the hardware). (c) MRI after removal of hardware showing no sign of spinal cord compression
Patient 3 was a 13-year-old child also suffering from Hurler syndrome who had previously undergone surgery for thoracolumbar kyphosic instability treated by T10-L2 arthrodesis with instrumentation. He consulted for right hip dysplasia. The treatment approach associated a Dega pelvic osteotomy with a proximal femoral varisation and derotation osteotomy under spinal anesthesia (the epidural catheter was removed just after anesthesia). Respiratory distress occurred in the early hours postoperatively, attributed to pulmonary atelectasis, followed by progressive tetraparesis. Emergency CT-scan revealed a compressive posterior epidural hematoma extending from T1 to T6 but no pathological process at the cervical level. Decompression was carried out, evacuating the hematoma with a T1 to T3 laminotomy. Surgical verification was also carried out in the zone of spinal anesthesia (lower lumbar), revealing no indication of hemorrhagic stigmata. A laminectomy was nevertheless performed. No recovery was observed and the patient died from tetraparesis complications.
For these three patients, a complete retrospective study of anesthesia records failed to find an etiology, including hemodynamic.
Discussion
These three cases illustrate the spinal cord fragility of patients suffering from MPS. This fragility is most likely multifactorial, as few common elements were seen in the cases observed. Medullary complications can occur from spinal surgery as well as in extra-spinal surgical correction.
Thoracolumbar kyphosis is a characteristic deformity of MPS, occurring most frequently in type I, type VI (Maroteaux–Lamy syndrome), and type IV. It develops from poor bone growth in the anterior–superior aspect of the cranial lumbar vertebrae. This process leads to anterior wedging with retrolisthesis of the apical vertebra, as well as to posteriorly herniated infiltrated intervertebral discs bulging at the thoracolumbar junction (White and Harmatzc 2010). This kyphosis, moreover, is characterized by its hypermobility (Dalvie et al. 2001a) resulting from the weakening of the intrinsic ligamentary properties due to glycosaminoglycan infiltration. A lesser degree of kyphosis, moreover, is confirmed by diverse radiographic assessment in supine position (Dalvie et al. 2001a). This narrowing of the canal in a particularly mobile spine has already been identified in the literature as a major risk factor in spinal cord compression (White and Harmatzc 2010; Dalvie et al. 2001a, b; Ebara et al. 2003).
Analysis of these three observations leads us to believe that narrowing associated with spinal hypermobility plays a key role in the origin of this medullary damage. But this interrelation is not the sole factor, as evidenced by the two cases of extra-spinal surgery, notably where kyphosis had previously been stabilized. Other factors, particularly vascular and hemostatic, may also impact: these patients could present a risk of compressive epidural hematoma, as in our third case, potentially due to vascular walls infiltration that could make them more fragile and alter endothelial function (Kleigman and Muenzer 2004; Aydin et al. 2006; Kelly et al. 2013), with risk of spontaneous compressive hematoma. This is, however, still an hypothesis which has not been clearly stated. The role of spinal anesthesia in the development of epidural hematoma in our third case has not been established, but taking into consideration the global picture of vascular factors and anomalies of blood crasis, it seems preferable to avoid this type of anesthesia in these patients.
Moreover, a thickening of perispinal tissue, through infiltration of epidural fat and the dura mater, is regularly observed in spinal surgery (White and Harmatzc 2010). The infiltrative hypertrophy of the dura mater results in compressive spinal sheathing, without systematic bone canal narrowing. Hypertrophy of the ligamentum flavum has already been reported twice in the literature as being responsible for spinal compression in Type VI MPS (Sheridan et al. 1992; Mut et al. 2005). Furthermore, spinal sheathing is a factor in the onset of spinal ischemia from compression of the spinal arteries.
This theory of medullary ischemia is most likely involved in the genesis of medullary complications. These patients, for the most part, suffer from cardiovascular damage and thoracic deformity which lead to reduced cardiac ejection fraction. Prone position decreases cardiac index and further contributes to reducing the flow (Brown et al. 2013). Spinal sheathing and narrowing of the medullary canal thus promote medullary ischemia, already generated by the abovementioned factors. The key motor symptomatology is most likely related to the predominance of anteromedullar compression (corticospinal tract). As Tong et al. underscore in their description of perioperative paraplegia in atlanto-axoidian stabilization in a patient with Morquio syndrome, spinal cord infarction remote from maximal compression argues for the role of ischemia in the onset of neurological symptoms (Tong et al. 2012).
Currently accepted practice is to surgically stabilize kyphosis, resecting bulging discs combining an anterior and a posterior approach, in one or two surgeries, before the appearance of neurological complications. The decision to operate must take into account the patient’s age, potential for growth and the evolution of the kyphosis.
These three cases raise the question of preventive spinal cord stabilization, prior to lower limb surgery, in order to decrease the risk of compression related to hypermobility, keeping in mind the nonnegligeble risk of spinal cord damage. Close collaboration between anesthesiologists and surgeons is important in maintaining sufficient medullary perfusion pressure and proscribing low cardiac output.
Perioperative monitoring of motor and sensory evoked potentials is an essential procedure in these risky surgeries (Mut et al. 2005; Tong et al. 2012). Nevertheless, our second case shows that it is not infallible, which makes us to hypothesize that the thickening of perimedullary tissue could alter data from the potentials.
Conclusion
The presence of craniocervical stenosis and thoracolumbar kyphosis in patients suffering from MPS must be seen as raising the risk of perioperative medullary complications, even in extra-spinal surgery. These complications are tied, on the one hand, to factors of compression (hypermobile spine, narowwing of the medullary canal accentuated by bulging discs; medullary sheathing from infiltrative hypertrophy of surrounding tissue) and, on the other hand, to ischemia (lowered perfusion pressure due to compression of spinal arteries and altered cardiovascular function). Informing patients and their families is essential prior to any surgical treatment, and neurological monitoring precautions should systematically be incorporated in case management. These precautions include prolonged extra-spinal surgery where the risk of hemorrhage might impact medullary perfusion pressure.
One Sentence Message
Thoracolumbar kyphosis in patients suffering from mucopolysaccharidoses is raising the risk of perioperative medullary complications, even in extra-spinal surgery.
Compliance with Ethics Guidelines
Conflict of Interest
All the authors of this chapter declare that there are no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients and their legal representative when necessary for being included in the study.
Details of the Contributions of Individual Authors
Nicolas Pauchard has reviewed all the patients.
Jean-Luc Jouve, Christophe Garin, and Pierre Journeau performed the surgical procedure for the patients and followed them.
Nicolas Pauchard, Pierre Journeau and Pierre Lascombes have written and reviewed the article.
Footnotes
Competing interests: None declared
Contributor Information
P. Journeau, Email: p.journeau@chu-nancy.fr
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Aydin M, Akarsu S, Kabakus N, Akpolat N. Mucopolysaccharidosis IIIB, cerebral vasculopathy and recurrent subdural hematoma. Indian Pediatr. 2006;43:437–440. [PubMed] [Google Scholar]
- Baratela WA, Bober MB, Thacker MM, Belthur MV, Oto M, Rogers KJ. Cervicothoracic myelopathy in children with Morquio syndrome A: a report of 4 cases. J Pediatr Orthop. 2014;34:223–228. doi: 10.1097/BPO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Brown ZE, Görges M, Cooke E, Malherbe S, Dumont GA, Ansermino JM. Changes in cardiac index and blood pressure on positioning children prone for scoliosis surgery. Anaesthesia. 2013;68:742–746. doi: 10.1111/anae.12310. [DOI] [PubMed] [Google Scholar]
- Dalvie S, Skinner J, Vellodi A, Noorden HH. Mobile thoracolumbar gibbus in Morquio type A: the cause of paraparesis and its management. J Pediatr Orthop B. 2001;10:328–330. doi: 10.1097/01202412-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Dalvie S, Skinner J, Noorden HH, Vellodi A. Anterior instrumented fusion for thoracolumbar kyphosis in mucopolysaccharidosis. Spine. 2001;26:E539–E541. doi: 10.1097/00007632-200112010-00020. [DOI] [PubMed] [Google Scholar]
- Ebara S, Kinoshita T, Yuzawa Y, Takahashi J, Nakamura I, Hirabayashi H. A case of mucopolysaccharidosis IV with lower leg paresis due to thoraco-lumbar kyphoscoliosis. J Clin Neurosci. 2003;13:358–361. doi: 10.1016/S0967-5868(03)00033-X. [DOI] [PubMed] [Google Scholar]
- Feillet F, Journeau P, Straczek J, Vidailhet M (2006) Mucopolysaccharidoses in EMC Pédiatrie. Elsevier SAS, Paris
- Kelly AS, Metzig AM, Steinberger J, Braunlin EA. Endothelial function in children and adolescents with mucopolysaccharidosis. J Inherit Metab Dis. 2013;36:221–225. doi: 10.1007/s10545-011-9438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleigman RM, Muenzer JL. Mucopolysaccharidosis. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson textbook of pediatrics. 17. Philadelphia: WB Saunders; 2004. pp. 482–486. [Google Scholar]
- Mut M, Cila A, Varli K, Akalan N. Multilevel myelopathy in Maroteaux–Lamy syndrome and review of the literature. Clin Neurol Neurosurg. 2005;107:230–235. doi: 10.1016/j.clineuro.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Sheridan M, Chaseling R, Johnston IH. Hydrocephalus, lumbar canal stenosis and Maroteaux–Lamy syndrome (mucopolysaccharidosis type VI) J Neurosurg Sci. 1992;36:215–217. [PubMed] [Google Scholar]
- Tong CK, Chen JC, Cochrane DD. Spinal cord infarction remote from maximal compression in a patient with Morquio syndrome. Neurosurg Pediatr. 2012;9:608–612. doi: 10.3171/2012.2.PEDS11522. [DOI] [PubMed] [Google Scholar]
- White KK, Harmatzc P. Orthopedic management of mucopolysaccharidosis disease. J Pediatr Rehabil Med. 2010;3:47–56. doi: 10.3233/PRM-2010-0102. [DOI] [PubMed] [Google Scholar]


