Abstract
A ketogenic diet is an important therapy used in the control of drug-refractory seizures. Many studies have shown that children and adolescents following ketogenic diets exhibit an over 50% reduction in seizure frequency, which is considered to be clinically relevant. These benefits are based on a diet containing high fat (approximately 90% fat) for 24 months. This dietary model was proposed in the 1920s and has produced variable clinical responses. Previous studies have shown that the mechanisms underlying seizure control involve ketone bodies, which are produced by fatty acid oxidation. Although the pathways involved in the ketogenic diet are not entirely clear, the main effects of the production of ketone bodies appear to be neurotransmitter modulation and antioxidant effects on the brain. This review highlights the impacts of the ketogenic diet on the modulation of neurotransmitters, levels of biogenic monoamines and protective antioxidant mechanisms of neurons. In addition, future perspectives are proposed.
Keywords: Ketogenic Diet, Ketone Bodies, Refractory Epilepsy
INTRODUCTION
The ketogenic diet (KD) is particularly aimed at treating children and adolescents with refractory epilepsy (drug-refractory seizures), regardless of the etiology 1. Although refractory epilepsy is the initial focus of this treatment, clinical and epidemiologic studies indicate that chronic epilepsy is followed by long-term behavioral changes and cognitive degeneration even in an optimal state of antiepileptic drug therapy 2,3. Consequently, some authors that the KD may be an early option for the treatment of patients with epilepsy instead of the last choice. The KD is also an important coadjuvant treatment for most refractory and generalized epilepsies, such as Dravet, Doose, Lennox-Gastaut and West syndromes 4.
The KD was developed in 1920 by Wilder 5 and many studies have shown its positive benefits, including an over 50% reduction in seizures, which is considered to be clinically relevant 6,7. The average time of treatment with the KD is two years, after which it should be discontinued 1.
Recently, Hirano et al. 8 reported the positive effects of the KD in children with West syndrome who were resistant to adrenocorticotropic hormone (ACTH) therapy, which is a first-line treatment for children with this syndrome. Among the main effects observed in five out of six children in this study included the disappearance of spasms in two children and a decrease in their frequency by 80% in the other three children. Similar positive effects of the KD were observed in a study of 41 children with refractory epilepsy, in which the number of seizures was reduced by 90% in 10.53% of the children and by at least 50% in 36.84% of the children and the seizures disappeared in 5.26% of the children 9.
The KD is based on high fat, low carbohydrate and moderate protein levels and the production of ketone bodies (KBs) from the oxidation of fat as the primary source of metabolic energy, which appears to be involved in the control of seizures 10.
The modified Atkins diet (MAD) is also used in the treatment of patients with refractory epilepsy 11. As opposed to the KD, there is no restriction on protein or daily calorie intake in the MAD. This diet is composed of 60% fat, 30% protein and 10% carbohydrates 12. Although the MAD is more palatable than the KD, its efficacy in relation to the KD is unclear 11. In children with Lennox-Gastaut syndrome, the MAD was effective and well tolerated and the nine children on the diet showed an over 50% reduction in seizure frequency after one year of treatment 13. However, a previous review showed that 37% of patients who were fed the KD had an additional decrease (≥10%) in seizures compared with those who were fed the MAD 14.
Regardless of the use of the MAD or the KD, in some clinical situations, such as those involving patients with glucose transporter 1 deficiency syndrome (GLUT1-DS), these dietary treatments can be used as differentiating tools for identifying patients with metabolic diseases because these patients are generally seizure-free after the introduction of the diet 15.
Previously, the KD protocol recommended that the diet be initiated after a fasting period of 12-48 h, during which the child must stay at a hospital 1. As described in the references, many centers begin the diet without fasting because several studies have found no difference in the use of fasting versus non-fasting in clinical practice 5. The introduction of the KD following specific requirements (fat-to-carbohydrate and -protein ratios) and the subsequent control of seizures usually occur when these ratios are 3:1 or 4:1, which are the most commonly used proportions 16. Diets containing lower proportions (2:1) are normally used when the treatment is introduced 10.
The KD is usually well tolerated and increasing numbers of studies in the literature are reporting its benefits. However, the metabolic pathways involved in the production of KBs have not been well established despite nearly one century of research. This review highlights the main neurobiochemical mechanisms that have been studied over the past 15 years according to original and review studies indexed in the MedLine/PubMed database.
Anticonvulsant mechanisms and ketone bodies
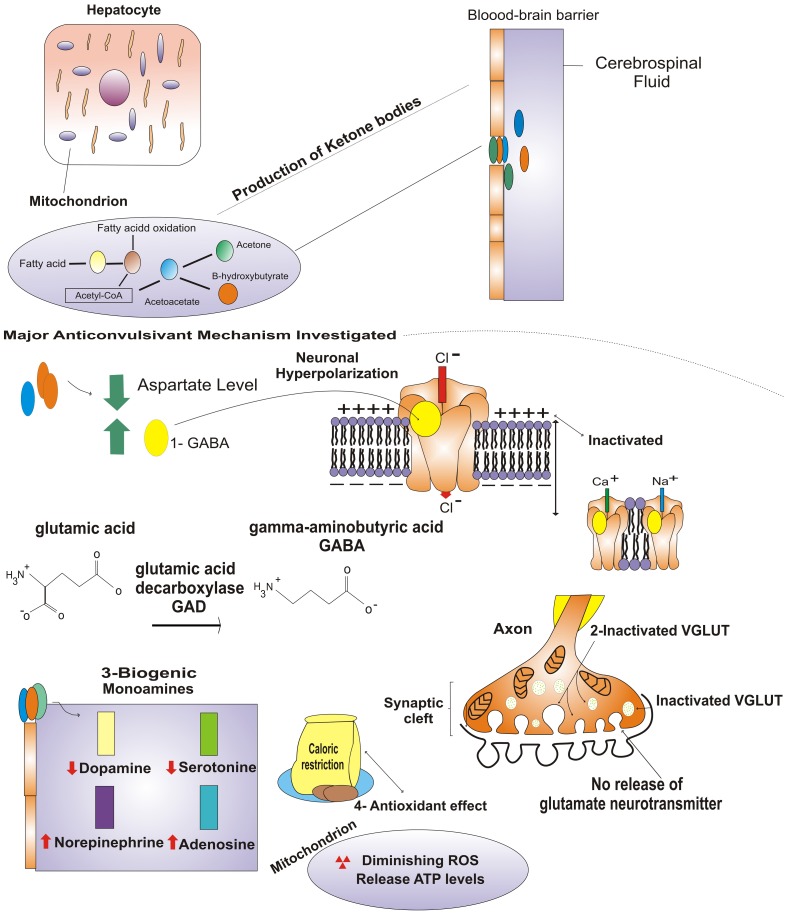
There are many hypotheses regarding the antiepileptic mechanisms of the KD. The early hypotheses regarding its activities were focused on the concepts of acidosis, dehydration and increased ketone concentrations 17. Other factors, such as γ-aminobutyric acid (GABA) and glutamate, membrane potentials, ion channels, biogenic monoamines and neuroprotective activities (Figure 1), have been studied in experimental models (in vivo or in vitro).
Figure 1.
Production of ketone bodies and potential primary anticonvulsant mechanisms: 1 GABA neurotransmitter (neuronal hyperpolarization and membrane channels; (2) inactivation of VGLUT and inhibition of glutamate neurotransmitter; 3 modified concentrations of biogenic monoamines; and 4 antioxidant mechanism of diminishing reactive oxygen species. For more information, please see text.
Although the mechanism by which the KD exerts its anticonvulsant effects is unclear, these effects are often associated with important metabolic changes that induce increased levels of KBs, mainly β-hydroxybutyrate and acetoacetate 18,19.
Energy metabolism in the brain involves distinct and complex pathways. Under physiological conditions, most precursors of KBs are long-chain fatty acids. They are released from adipose tissue in response to a decrease in blood glucose, such as that which occurs during fasting 20.
Similar mechanisms are involved in the KD, during which long-chain fatty acids are metabolized in the liver and converted into KBs. These fatty acids are oxidized in the mitochondria, producing high levels of acetyl-CoA, which cannot be oxidized in the Krebs cycle. The excess acetyl-CoA is converted to acetoacetate and subsequently to acetone and β-hydroxybutyrate 21. The KBs cross the blood-brain barrier and are transported by monocarboxylic acid transporters to the brain interstitial space, the glia and the neurons. In these tissues, the KBs act as substrates in the Krebs cycle and respiratory chain, contributing to brain energy metabolism 21.
Currently, there is no evidence that dehydration or fluid restriction is necessary for the clinical efficacy of the KD 17. Furthermore, this diet has been associated with pH changes that directly influence the behaviors of ion channels and neurotransmitter receptors 17.
Some studies have suggested that the KD is more effective in children than in adults. There are high levels of ketone-metabolizing enzymes in the brain and their capacities for taking up ketone bodies are higher in infancy than in adulthood 18,21. The number of monocarboxylic acid transporters decreases with cerebral maturation and they are present at low levels in adulthood 21. Despite these differences, adaptive cerebral metabolic changes occur in adults who are exposed to stress situations, such as ischemia, trauma and sepsis 22. As shown in the literature, there are increases in the concentrations of ketone-dependent monocarboxylic acid transporters in these situations, indicating that KD treatment in adults is feasible 22,23.
Several studies of the mechanisms of action of the KD have been based on animal models, allowing for the investigators to examine the anatomical, chemical, cellular, molecular and functional changes that occur following seizures 24,25. Different animal models have been used that have been exposed to electrical and chemical stimulation and physical, genetic and spontaneous seizure models have been employed that simulate different types of epileptic seizures 17,21,26. Table 1 shows the main outcomes reported in recent years.
Table 1.
Neuroprotective effects of ketone bodies.
| Species | Injury models | Intervention times | Treatments | Effect on seizures | Mechanisms | References |
| Rats | Maximal electroshock or subcutaneous pentylenetetrazol or amygdala kindling or AY-9944 | 1- 6 days | Acetone injection | ↓ | Anticonvulsant effect of acetonea | 27 |
| Mice | Pentylenetetrazol, 4-aminopyridine | 15-240 min | Acetone injection | ↓ | Anticonvulsant effect of acetonea | 26 |
| Mice | - | 3 days | KD | Not rated | ↑ GABA | 32 |
| Rats (cultured astrocytes) | - | 5 days | β-hydroxybutyrate | Not rated | ↓ GABA transaminase mRNA | 33 |
| Humans (children with refractory epilepsy) | - | 3-6 months | KD | ↓ | ↑ GABA | 30 |
| Rats (hippocampal slices) | Antidromic stimulation | 40 min | β-hydroxybutyrate | ↓ | ↑ KATP channels | 34 |
| Rats | - | 3 weeks | KD | ↓ | ↑ Brain KBs and ↓ glucose uptake | 29 |
| In vitro (proteoliposomes) | - | n.d. | Acetoacetate | Not rated | ↓ Glutamate | 36 |
| Mice (norepinephrine transporter knockouts) | Maximal electroshock | n.d. | KD | ↓ | ↑ Norepinephrineb | 38 |
| Humans (children with refractory epilepsy) | - | 3 months | KD | ↓ | ↓ Dopamine and serotonin | 19 |
| Mice (with adenosine deficiency) | Kainic acid | 4-6 weeks | No therapy | ↑ | ↓ Adenosine | 40 |
| Mice (transgenic models) | - | 3 weeks | KD | ↓ | ↑ A1R | 41 |
| Mice (hippocampal slices) | - | 3 weeks | KD | ↓ | ↑ Number of mitochondria | 43 |
| Mice (hippocampal mitochondria) | - | 10-12 days | KD | Not rated | ↑ UCP levels and ↓ ROS | 44 |
| Rats (hippocampal mitochondria) | - | 3 weeks | KD | Not rated | ↑GSH and ↓ mitochondrial H2O2 | 45 |
GSH: glutathione; KD: ketogenic diet, ROS: reactive oxygen species; UCP: uncoupling protein; -: without seizure-inducing substance; n.d.: not described; a: very high doses of acetone may have contributed to motor impairment; b: norepinephrine transporter knockout mice and mice fed the KD showed similar reductions in seizure severity.
The in vivo and in vitro models have revealed the different anticonvulsant properties and antiepileptic effects of the KD. These aspects have been studied primarily in models of non-epileptic rodents receiving the KD that are later exposed to proconvulsant agents or electrical stimuli 18. However, the levels of therapeutic KBs and the specific effects of each ketone body have not been clearly elucidated.
In 2003, Likhodii et al. 27 administered intraperitoneal injection of acute acetone to rats in increasing doses from 2 to 32 mmol/kg. These authors observed an increase in the protective effect of acetone against seizures as the dose increased in four different models: maximal electroshock, subcutaneous pentylenetetrazol, amygdala kindling and AY-9944 27. Gaisor et al. 26 showed similar results following the administration of acetone (1-32 mmol/kg) to juvenile mice, which was shown to protect them from seizures induced by pentylenetetrazol and 4-aminopyridine. However, acetone doses of ≥10 mmol/kg promoted toxic effects in the pentylenetetrazol model, generating motor impairment in the mice.
Modulation of neurotransmitters
The major mechanisms proposed to explain the increased inhibition and/or decreased excitation that are induced by the KD involve the neurotransmitters GABA and glutamate 28. KBs act not only as energy sources but also contribute to reducing glucose consumption in the brain by modulating the activities of neurotransmitters 29.
Changes in the levels of glutamate and GABA, which are the major excitatory and inhibitory neurotransmitters, respectively and their receptors have been proposed as the possible mechanisms of action of the KD 30. GABA is an intermediate of α-ketoglutarate, which is synthesized in the Krebs cycle (via glutamate) and converted into GABA by glutamate decarboxylase 21. Moreover, KBs inhibit glutamate decarboxylase and decreased levels stimulate the synthesis of GABA, thus contributing to seizure control 31.
In previous experimental studies, animals were fed the KD and were observed to have higher concentrations of β-hydroxybutyrate in the forebrain and cerebellum, indicating increased GABA levels 32. Astrocytes and neuroglial cells, which are also enriched with this enzyme during ketone metabolism, utilize KBs as energy sources 23,33. Suzuki et al. 33 suggested that the inhibition of GABA-transaminase mRNA expression was mainly dependent on β-hydroxybutyrate in astrocytes following the presence of increased GABA levels in the brain 33. This allows glutamate to be more available for GABA synthesis, favoring the hypothesis that β-hydroxybutyrate leads to the inhibition of neuronal firing following recurrent neuronal activity 34.
Similar results were observed in a clinical study in which the GABA levels of responders were higher compared with those of non-responders following treatment with the KD 30. However, an evaluation of the dependence of this response on the levels of β-hydroxybutyrate was not performed.
Increased inhibition or decreased excitability, if sufficiently intense, may influence the normal functioning of the brain in addition to controlling seizures 28. Furthermore, high GABA levels appear to stimulate chloride channel receptors, increasing the influx of negatively charged ions and consequently inducing neuronal hyperpolarization 32. This event is responsible for inhibiting the activation of sodium and calcium channels, the activities of which are required for neuronal excitation. KBs possibly contribute to the activities of KATP channels, which experience activity-dependent opening and could partially explain the reduced numbers of epileptic seizures 34.
In contrast to the high levels of GABA, the glutamate-to-ketone ratio can modulate glutamate physiological functioning through VGLUT, which is responsible for filling presynaptic vesicles with glutamate in a Cl--dependent manner 35. An in vitro study showed that Cl- is an allosteric activator of VGLUT, which is competitively inhibited by KBs (more often by acetoacetate than by β-hydroxybutyrate) 36.
Biogenic monoamines
The modulation of biogenic monoamine levels was proposed as a plausible mechanism for explaining the anticonvulsant effects of the KD. However, the specific mechanisms underlying such activities remain unclear 17,19,37,38.
In animal models, norepinephrine levels have been shown to increase in rats receiving the KD 37. This beneficial effect of the KD was not observed when norepinephrine transport was inhibited, suggesting that the noradrenergic system is required for the neuroprotective effects of the KD to occur. A similar profile was observed in norepinephrine transporter knockout mice fed normal diets 38.
A clinical study on biogenic monoamines in the cerebrospinal fluid of children treated with the KD showed that their dopamine and serotonin levels were significantly reduced [from 410 to 342 and from 158 to 137 nmol/L (16.6 and 13.3% reductions), respectively] after a three-month treatment, whereas their norepinephrine levels [from 51.7 to 51.0 nmol/L (1.4% reduction)] remained unchanged 19. These authors proposed that changes in monoamine levels are also dependent upon whether children are respondent or non-respondent to the KD.
Some authors have suggested that adenosine is the major seizure inhibitory neuromodulator and that the KD exerts a regulatory role in relation to this monoamine 39. This hypothesis was reinforced by Fedele et al. 40, who used transgenic mice for adenosine A1 receptors (A1Rs) and revealed the presence of spontaneous hippocampal electrographic seizures due to the overexpression of adenosine. Recently, the positive impact of the KD was assessed in transgenic mice with or without adenosine A1Rs. In the mice with A1Rs that were fed the KD, seizures were nearly abolished after four weeks of treatment. In contrast, these effects were not observed in the mice lacking these receptors 41.
Thus, the KD increases adenosine levels. However, its efficiency in the control of seizures depends on the expression of the A1Rs 39.
Neuroprotective mechanisms
Many studies have shown that the epileptogenic state involves complex molecular pathways in which oxidative stress and mitochondrial dysfunction may exert important roles in neuronal programmed/controlled (apoptosis) or uncontrolled/passive (necrosis) cell death 42. Thus, investigators have given particular emphasis to the modulation of the mitochondrial biogenesis of neurons by the KD and caloric restriction, highlighting the neuroprotective role of the mitochondria as the primary key to the control of apoptosis and cell death 43,44,45,46,47,48.
Mitochondria are intracellular organelles that primarily function in the production of cellular energy in the form of adenosine triphosphate (ATP). This nucleotide is produced by the mitochondrial respiratory chain through oxidative phosphorylation, which is performed by five multienzyme complexes (complexes I-V). The dysfunction of complex I may lead to decreased ATP production, which is commonly observed in neuronal diseases 42,49. In prolonged seizures, a temporary reduction in ATP levels can contribute to cell death 50.
Bought et al. 43 showed that mice that were fed the KD for at least three weeks showed a 46% increase in the hippocampal biogenesis of mitochondria compared with the control animals. In addition, these authors observed that 39 out of 42 regulated transcripts encoding mitochondrial proteins were up-regulated, implying increased ATP production and the capacity of this diet to stabilize neuronal membrane potentials 43.
The mitochondria are the major organelles that are responsible for reducing O2 to non-oxidative substances. However, when the mitochondrial respiratory chain is deregulated (the dysfunctioning of calcium homeostasis and imbalances of membrane potentials), decreased rates of ATP generation and the overproduction of reactive oxygen species (ROS) occur 49,51. In normal conditions, 1-5% of O2 in the mitochondrial electron transport chain is not reduced to H2O, CO2 and ATP, stimulating the generation of ROS [H2O2, O2•-, nitric oxide (NO) and peroxinitrite] 52.
Regarding coupled changes that occur during ROS production, other authors 44 have observed that rats that were fed the KD for 10-12 days showed significant increases in uncoupling protein (UCPs) levels in their hippocampal mitochondria. These responses were related to the 15% decrease in ROS levels in the hippocampi of these animals 44. Both effects were associated with mitochondrial biogenesis and the maintenance of calcium homeostasis 43,44.
The protective effects of the KD on oxidative stress have also been observed in the antioxidant system, particularly involving glutathione (GSH), which exhibits an increased capacity for peroxide detoxification within the cell 47. In juvenile rats that were fed the KD for three weeks, increased levels of mitochondrial-reduced GSH and an increased ratio of GSH to oxidized glutathione (GSSG) were observed, suggesting that the KD improves hippocampal redox statuses and protects mitochondrial DNA from oxidative stress. During seizures, these antioxidants are depleted and oxidative stress is stimulated 45.
Recently, some studies 46,48 have reviewed these mechanisms, emphasizing that the beneficial effects of the KD also involve caloric restriction. In addition to the increased levels of UCPs and the decreased production of ROS, these authors also reported other mechanisms involved in the control of seizures, such as decreases in both insulin-like growth factor 1 (IGF-1) and the mammalian target of rapamycin (mTOR) and increases in both sirtuins and adenosine monophosphate-activated protein kinase (AMPK).
Sirtuins are deacetylases with multiple functions related to fat oxidation and increased mitochondrial size and number. The increased expression of sirtuins may be associated with the inhibition of IGF-1 after caloric restriction 46,48. In addition, the increase in AMPK is directly related to ATP production 48.
The mTOR protein kinase is involved in multiple and complex activities in the body, participating in specific mechanisms in the nervous system. Thus, it is an exciting target for new horizons in drug discovery 53. Brain abnormalities are associated with the hyperactivation of the mTOR pathway and the KD may play an important role in inhibiting this pathway, thus conferring anticonvulsant effects. However, the underlying mechanisms are still unknown and require further exploration 48,53.
It is important to recognize that seizures stimulate the production of free radicals and mitochondrial dysfunction, resulting in a chronic redox state, neuronal changes and an increased susceptibility to seizures, leading to epilepsy 42. As a result, the KD improves the stability of the mitochondrial membrane and increases the efficiency of O2 consumption, stimulating the generation of ATP and minimizing the oxidative stress-induced epileptogenic state and mitochondrial dysfunction.
Future perspectives
Considering the aforementioned studies, we have verified that the mechanisms of action of KBs, which are involved in the reduction of epileptic seizures, are distinct and complex. In addition, the major mechanisms proposed to date are based on experimental models and few clinical studies, which have small sample sizes and uncontrolled designs. Furthermore, the multiple etiologies of epilepsy represent an important limitation to the understanding of the relationships between the KD, KBs and neuronal mechanisms in the control of seizures. Thus, we propose the following: I - that physical or chemical mechanisms employed to induce seizures should follow standardized protocols; II - that the physiological levels of KBs should be more frequently considered in experimental treatments; III - that the etiologies of epilepsy are better characterized in future clinical trials; IV - that biomarkers of treatment efficacies (levels of KBs, GABA and monoamines) are evaluated; V - that the potential side effects of treatments are systematically monitored; and VI - that novel mechanisms of action of KBs are evaluated. In consideration of these proposals, positive clinical responses to the KD remain the principal goal of this treatment. Thus, given the current state of the research, we also propose that KD intervention should be included early in clinical protocols for the treatment of children and adolescents with refractory epilepsy and not only as the last therapeutic option.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from the State of São Paulo Research Foundation (FAPESP # 12/03775-0), National Institute for Science and Technology of Complex Fluids (INCT-FCx-USP) and Group for Research on Complex Fluids (NAP-FCx-USP).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist A, Blackford R, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50(2):304–17. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 2.Cendes F. Progressive hippocampal and extrahippocampal atrophy in drug resistant epilepsy. Curr Opin Neurol. 2005;18(2):173–7. doi: 10.1097/01.wco.0000162860.49842.90. [DOI] [PubMed] [Google Scholar]
- 3.Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain. Curr Opin Neurol. 2003;16(2):189–95. doi: 10.1097/01.wco.0000063770.15877.bc. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Lin K. Ketogenic diet: An early option for epilepsy treatment, instead of a last choice only. Biomedical Journal. 2013;36(1):16. doi: 10.4103/2319-4170.107155. [DOI] [PubMed] [Google Scholar]
- 5.Wilder R. The effect of ketonemia on the course of epilepsy. Mayo Clinic Bulletin. 1921;2:307. [Google Scholar]
- 6.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurology. 2008;7(6):500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 7.De Kinderen RJ, Lambrechts DA, Postulart D, Kessels AG, Hendriksen JG, Aldenkamp AP, et al. Research into the (Cost-) effectiveness of the ketogenic diet among children and adolescents with intractable epilepsy: design of a randomized controlled trial. BMC Neurology. 2011;11(1):10. doi: 10.1186/1471-2377-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano Y, Oguni H, Shiota M, Nishikawa A, Osawa M. Ketogenic diet therapy can improve ACTH-resistant West syndrome in Japan. Brain Dev. 2014 doi: 10.1016/j.braindev.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Pablos-Sánchez T, Oliveros-Leal L, Núñez-Enamorado N, Camacho-Salas A, Moreno-Villares JM, Simón-De las Heras R. The use of the ketogenic diet as treatment for refractory epilepsy in the paediatric age. Rev Neurol. 2014;58(2):55–62. [PubMed] [Google Scholar]
- 10.Lee PR, Kossoff EH. Dietary treatments for epilepsy: management guidelines for the general practitioner. Epilepsy & Behavior. 2011;21(2):115–21. doi: 10.1016/j.yebeh.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Jain P. The Modified Atkins Diet in Refractory Epilepsy. Epilepsy Res Treat. 2014;2014:404202. doi: 10.1155/2014/404202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kossoff EH. More fat and fewer seizures: dietary therapies for epilepsy. Lancet Neurol. 2004;3(7):415–20. doi: 10.1016/S1474-4422(04)00807-5. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Jain P, Gulati S, Sankhyan N, Agarwala A. Use of the Modified Atkins Diet in Lennox Gastaut Syndrome. J Child Neurol. 2014 doi: 10.1177/0883073814527162. [DOI] [PubMed] [Google Scholar]
- 14.Kossoff EH, Bosarge JL, Miranda MJ, Wiemer-Kruel A, Kang HC, Kim HD. Will seizure control improve by switching from the modified Atkins diet to the traditional ketogenic diet. Epilepsia. 2010;51(12):2496–9. doi: 10.1111/j.1528-1167.2010.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramm-Pettersen A, Nakken KO, Haavardsholm KC, Selmer KK. Occurrence of GLUT1 deficiency syndrome in patients treated with ketogenic diet. Epilepsy Behav. 2014;32:76–8. doi: 10.1016/j.yebeh.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim DW, Kang HC, Park JC, Kim HD. Benefits of the nonfasting ketogenic diet compared with the initial fasting ketogenic diet. Pediatrics. 2004;114(6):1627–30. doi: 10.1542/peds.2004-1001. [DOI] [PubMed] [Google Scholar]
- 17. Masino SA, Rho JM. Noebls J L, Avoli M, Rogawski M A, Olsen R W, Delgado-Escueta A V, edMechanisms of ketogenic diet action Jasper's Basic Mechanisms of the Epilepsies. 4th edition 2012Bethesda (MD)National Center for Biotechnology Information (US)In: [PubMed] [Google Scholar]
- 18.Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119(3):535–43. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- 19.Dahlin M, Månsson J-E, Åmark P. CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy. Epilepsy Research. 2012;99(1):132–8. doi: 10.1016/j.eplepsyres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kossoff EH, Hartman AL. Ketogenic diets: new advances for metabolism-based therapies. Current Opinion in Neurology. 2012;25(2):173–8. doi: 10.1097/WCO.0b013e3283515e4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally MA, Hartman AL. Ketone bodies in epilepsy. Journal of Neurochemistry. 2012;121(1):28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. Journal of Cerebral Blood Flow & Metabolism. 2008;28(1):1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein P, Janousek J, Barber A, Weissberger R. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav. 2010;19(4):575–79. doi: 10.1016/j.yebeh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Fisher RS. Animal models of the epilepsies. Brain Res Brain Res Rev. 1989;14(3):245–78. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 25.Löscher W. Animal models of intractable epilepsy. Prog Neurobiol. 1997;53(2):239–58. doi: 10.1016/s0301-0082(97)00035-x. [DOI] [PubMed] [Google Scholar]
- 26.Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1, 2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia. 2007;48(4):793–800. doi: 10.1111/j.1528-1167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 27.Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC, Burnham WM. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Annals of Neurology. 2003;54(2):219–26. doi: 10.1002/ana.10634. [DOI] [PubMed] [Google Scholar]
- 28.Ruskin DN, Masino SA. The nervous system and metabolic dysregulation: emerging evidence converges on ketogenic diet therapy. Front Neurosci. 2012;6:33. doi: 10.3389/fnins.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, Lee Z. Ketones suppress brain glucose consumption. Adv Exp Med Biol. 2009;645:301–6. doi: 10.1007/978-0-387-85998-9_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahlin M, Elfving Å, Ungerstedt U, Åmark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64(3):115–25. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Neal E, Cross J. Efficacy of dietary treatments for epilepsy. J Hum Nutr Diet. 2010;23(2):113–9. doi: 10.1111/j.1365-277X.2010.01043.x. [DOI] [PubMed] [Google Scholar]
- 32.Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Brain amino acid metabolism and ketosis. J Neurosci Res. 2001;66(2):272–81. doi: 10.1002/jnr.1221. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, Takahashi H, Fukuda M, Hino H, Kobayashi K, Tanaka J, et al. &bgr;-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 2009;1268(1):17–23. doi: 10.1016/j.brainres.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 34.Tanner GR, Lutas A, Martínez-François JR, Yellen G. Single KATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci. 2011;31(23):8689–96. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omote H, Miyaji T, Juge N, Moriyama Y. Vesicular neurotransmitter transporter: bioenergetics and regulation of glutamate transport. Biochemistry. 2011;50(25):5558–65. doi: 10.1021/bi200567k. [DOI] [PubMed] [Google Scholar]
- 36.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinshenker D. The contribution of norepinephrine and orexigenic neuropeptides to the anticonvulsant effect of the ketogenic diet. Epilepsia. 2008;49(s8):104–7. doi: 10.1111/j.1528-1167.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 38.Martillotti J, Weinshenker D, Liles LC, Eagles DA. A ketogenic diet and knockout of the norepinephrine transporter both reduce seizure severity in mice. Epilepsy Res. 2006;68(3):207–11. doi: 10.1016/j.eplepsyres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Masino SA, Kawamura M, Wasser CD, Pomeroy LT, Ruskin DN. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009; 7(3):257–68. doi: 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rülicke T, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128(10):2383–95. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 41.Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121(7):2679–83. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang YC. Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death. Acta Neurol Taiwan. 2010;19(1):3–15. [PubMed] [Google Scholar]
- 43.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60(2):223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55(4):576–80. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 45.Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106(3):1044–51. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- 46.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milder J, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100(3):295–303. doi: 10.1016/j.eplepsyres.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuen AW, Sander JW. Rationale for using intermittent calorie restriction as a dietary treatment for drug resistant epilepsy. Epilepsy Behav. 2014;33C:110–4. doi: 10.1016/j.yebeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3-4):222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 50.Chuang YC, Lin JW, Chen SD, Lin TK, Liou CW, Lu CH, et al. Preservation of mitochondrial integrity and energy metabolism during experimental status epilepticus leads to neuronal apoptotic cell death in the hippocampus of the rat. Seizure. 2009;18(6):420–8. doi: 10.1016/j.seizure.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37(12):1951–62. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 53.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19(1):51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]