Abstract
The history of Minimally Invasive Surgery in the thorax is one of evolution, not revolution. The concept of video-assisted thoracic surgery (VATS) to greatly reduce the trauma of chest operations was born over two decades ago. Since then, it has undergone a series of step-wise modifications and improvement. The original practice of three access ports in a ‘baseball diamond’ pattern was modified to suit operational needs, and gradually developed into ‘next generation’ approaches, including Needlescopic and 2-port VATS. The logical, incremental progression has culminated in the Uniportal VATS approach which has stirred considerable interest within the field of Thoracic Surgery in recent years. This measured, evolutionary process has significant implications on how the surgeon should approach, master and realize the full potential of the Uniportal technique. This article gives a précis of the evolutionary history of minimally invasive thoracic surgery, and highlights the lessons it provides about its future.
Keywords: Single-port VATS, thoracoscopic surgery, video assisted thoracic surgery (VATS), Uniportal VATS
Evolution, not revolution
Without doubt, the single greatest advance in Thoracic Surgery of this generation has been the advent of video assisted thoracic surgery (VATS) (1,2). Over the past 20 years since its birth, VATS has been demonstrated to significantly reduce pain, hasten recovery, minimize complications, and improve post-operative quality of life for patients requiring Thoracic Surgery when compared to open thoracotomy (3,4). VATS is now so well established around the world that it is no longer correct to describe it as an ‘emerging’ or ‘new’ approach. It is in fact now the ‘conventional’ approach for almost every common thoracic operation in a number of centers around the world.
Since the birth of VATS, however, the pace of progress appears to have slowed (4). Although much fine work has produced incremental improvement in surgery in the chest, the search for the ‘next big breakthrough’ of the scale of VATS has been in vain for many years. It was wondered whether minimally invasive thoracic surgery had reached a zenith beyond which no further great advance was possible.
The recent emergence of Uniportal VATS has now promised a breath of fresh air to purge the stagnation (5,6). The change from conventional multi-port VATS to the use of just a single port seems like such a radical step that many have viewed it as perhaps the single greatest leap forward in minimally invasive thoracic surgery since the birth of VATS itself. Indeed, many have described it as ‘revolutionary’.
The truth is, though, that this description is wrong. Far from being a sudden revolution, Uniportal VATS is actually simply the next step in the evolution of minimally invasive thoracic surgery itself. When viewed in the context of the history of VATS over the last two decades, Uniportal VATS is technically still just another step forwards—though a very exciting step forwards at that.
The use of the word ‘evolution’ has become very much clichéd in the medical literature in recent years. However, the distinction between revolution and evolution is far more than an issue of pedantic semantics. The fact that this is a process of evolution has very important implications for the practice of Uniportal VATS. This article aims to summarize the evolutionary history of minimally invasive thoracic surgery culminating in Uniportal VATS, and to demonstrate how the lessons from that evolution should guide surgeons learning this technique.
A brief history of minimally invasive thoracic surgery
To operate in the human thorax, a surgeon must place three things into it: a right hand; a left hand; and a pair of eyes to look inside (4). To place these in between the tight intercostal spaces, one must forcibly retract the ribs for up to several hours for a major operation. This causes significant trauma, pain and potential peri-operative morbidity. VATS actually does not deviate from the principle of placing these same three things into the chest. However, surgical instruments are used to replace the right and left hands, and a video-thoracoscope is used to replace direct vision through the wound. All of this is done using three small ports without rib-spreading. VATS therefore allows the same complex operations to be performed, but the avoidance of forcible rib-spreading means that surgical trauma is greatly reduced (1,2). In this way, VATS achieves the good post-operative outcomes so well documented in the literature (1-3).
Early 3-port VATS
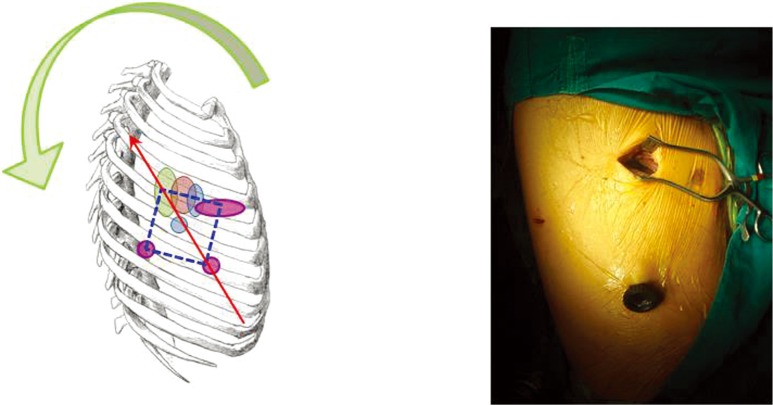
When VATS was first described some 20 years ago, the approach typically used three small ports without rib-spreading (1,2). For a VATS lobectomy, this typically meant two 10 mm ports plus one 3-6 cm ‘utility’ port for delivery of the resected lobe of lung. The strategy for ports placement was described in the early literature as the ‘baseball diamond’ (Figures 1,2). The surgeon typically stands at the ‘home base’ like a baseball batter looking out towards the pitcher and the baseball field—and therefore the camera port representing the surgeon’s eyes are placed at the ‘home base’ position of the diamond. The target lesion being faced by the surgeon is at ‘second base’ opposite the surgeon. The other two ports are placed at the ‘first base’ and ‘third base’ positions to allow the right and left hand instruments to be placed and triangulated forwards towards the target at ‘second base’. Using this strategy, the camera port was typically in about the 7th or 8th intercostal space in the mid-axillary line, and the posterior port just anterior the tip of the scapula. The utility port was usually placed in the anterior axillary line to take advantage of the naturally wider intercostal space towards the front of the chest to facilitate specimen retrieval. The utility port was typically sited in the 4th intercostal space for an upper lobectomy or 5th intercostal space for a lower lobectomy, and was at the ‘first base’ for right-side operations and at ‘third base’ for left side operations.
Figure 1.
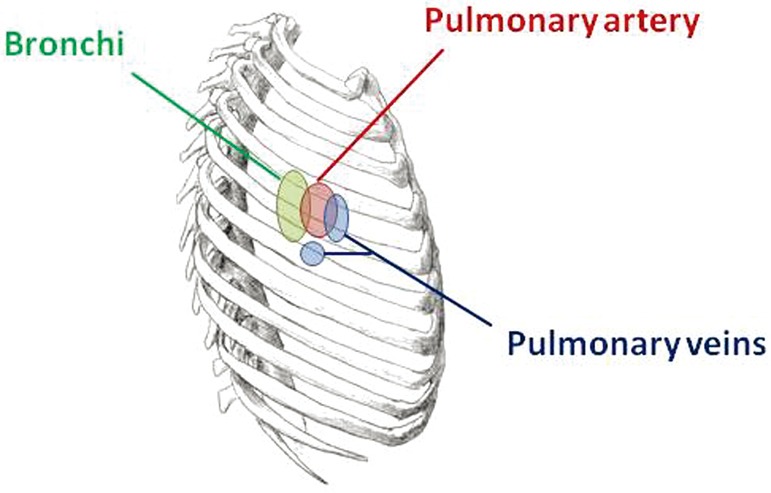
Schematic of the right thorax, showing approximate relative locations of the main hilar structures.
Figure 2.
The ‘classic’ 3-port VATS lobectomy. In a right-side operation, the ‘baseball diamond’ (dotted blue line) has a 10 cmm camera port at ‘home base’, a 3-5 cm utility port at ‘first base’, and a 10 mm posterior port at ‘third base’. The axis of the operation (red arrow) is a straight line from ‘home base’ through the ‘second base’—and in this classic early VATS approach the axis is essentially in a hip-to-head direction along the patient’s body’s longitudinal axis. VATS, video-assisted thoracic surgery.
This strategy of port placement allowed the ‘axis’ of the operation—a straight line from the ‘home base’ through the ‘second base’—to follow the natural longitudinal axis of the patient from feet towards the head. The right and left hands (‘first and third bases’) straddled this axis on both sides and reduced fencing between instruments and camera.
The above reflects the early approach of conventional VATS in Hong Kong, and there are of course many variations described (4).
Modified 3-port VATS
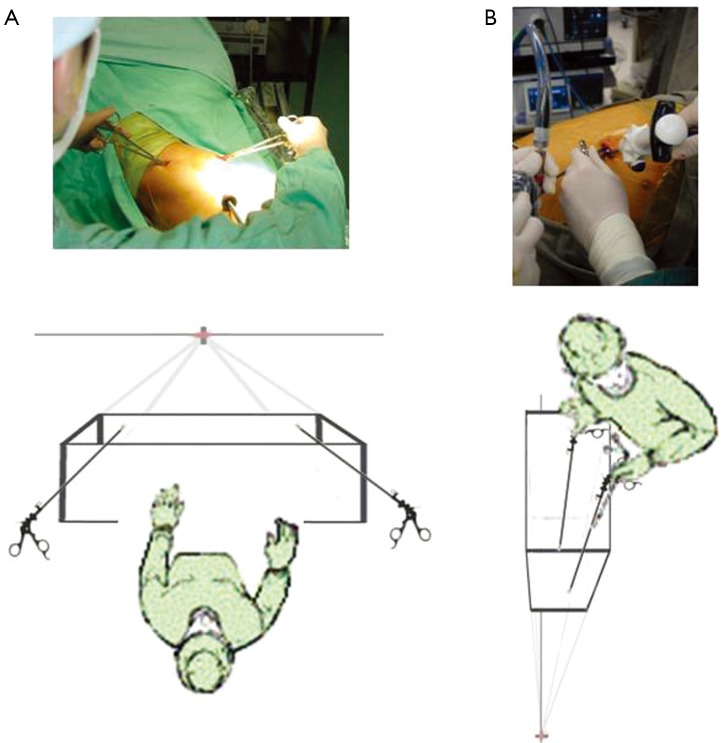
The problem with the conventional 3-port VATS approach as described above was that the port placement did not reflect the reality of how surgeons and assistants stood around the operating table. No surgeon can really stand at the patient’s feet (or hip) where the ‘home base’ is. In reality, to facilitate principal instrumentation via the utility port, many surgeons would stand anterior to the lateral-lying patient. The actual axis of the operation is actually not from the hip to the head of the patient, but from the umbilicus towards the back of the shoulder (i.e., from an anterior-to-posterior as well as inferior-to-superior direction). Adhering to the port placement in the classic ‘baseball diamond’ strategy above would therefore mean the posterior port would be too far ‘superior’ along the axis and the surgeon would have to reach uncomfortably far to effect instrumentation there. In addition, if the camera-holding assistant stands on the opposite side of the operating table from the surgeon (as per classic open surgery), the assistant’s visual axis would be completely different to the surgeon’s—running from the patient’s sacrum towards the chin. This is one of the key reasons for ‘mirror imaging’ and fencing between camera and the surgeon’s instruments commonly noted in the early experience with VATS.
To remedy this, the 3-port VATS port placement strategy was modified slightly (Figure 3). The camera port was brought more anterior to the anterior axillary line. The posterior port was lowered from anterior to the scapula tip to a lower intercostal level. The utility port position is unchanged. The end result of this modification was a posterior rotation of the ‘baseball diamond’ (4). Although the diamond shape was preserved, the axis now reflected the umbilicus-towards-shoulder direction and was more comfortable for the surgeon. The camera-holding assistant now stands on the same side of the operating table as the surgeon and slightly behind. The assistant thus shares the same axis as the surgeon, improving surgeon-assistant co-ordination. The lower posterior port also means that for upper lobectomies, a stapling device placed via that port approaches hilar vessels from a slightly posterior-to-anterior direction and can more easily negotiate around those vessels without the anvil being impeded by other structures behind.
Figure 3.
The ‘modified’ 3-port VATS lobectomy. The ‘baseball diamond’ (dotted blue line) and axis of the operation (red arrow) have essentially been rotated in a posterior direction—and the axis direction is now umbilicus-to-shoulder, better reflecting the fact that in reality the surgeon stands anterior to the patient (rather than sits on the patient’s hip!). VATS, video-assisted thoracic surgery.
For the author, this has become the standard port placement strategy for ‘conventional’ 3-port VATS lobectomy in Hong Kong. Again, it is acknowledged that myriad detail differences in technique exist in different centers around the world, but the basic principles remain.
However, although conventional multi-port VATS greatly reduces morbidity, it does not completely eliminate it. Studies have shown that even with conventional VATS, up to 32% of patients still have some residual discomfort for up to years after surgery (7). We have also found that 53% of patients still feel chest wall paresthesia distinct from nociceptive wound pain at 19 months after VATS (8). Even though such complaints cannot detract from the need to perform curative surgery for lung cancer, there is clearly room for improvement to improve the lot for our patients.
Robot-assisted thoracic surgery
One of the developments causing the most excitement soon after the turn of the Century was the introduction of robotic surgical systems to Thoracic Surgery. Initially, the robot was used to help perform simple mediastinal operations, but today some are routinely using it even for lung cancer resections (9,10). Nonetheless, the overall narrative of the robot story in the last 10 years has been one of relatively slow and limited acceptance globally.
There are a number of reasons for the missed opportunity for the robot system to become established in Thoracic Surgery. The upfront costs of purchasing the system and—more importantly—the costs of the surgical consumables remains daunting, often prohibitively so in the many countries. The cost in terms of prolonged preparation times for each operation taking up valuable operating theatre time is another important cost issue. The robotic system’s promises of 3D vision, greater intra-thoracic dexterity and steadier instrumentation have also not fully compensated for the loss of tactile feedback so crucial to the thoracic surgeon (11). In terms of wounds, the robot required the same number and sizes of wounds as conventional 3-port VATS, and indeed sometimes required an extra fourth one.
There is no doubt that robotic system has a niche role for delicate mediastinal surgery, but for most Thoracic Surgeons its place in mainstream practice remains limited. Instead, trends over the last several years have showed clearly that the evolution of minimally invasive thoracic surgery has taken a different direction: towards an upgrading of fine surgical technique over the influx of expensive technology.
Needlescopic VATS
Needlescopic VATS is the use of very fine thoracoscopes (2-3 mm diameter) and instruments (3-5 mm diameter) to replace the 10mm versions used in conventional VATS equipment (12). Needlescopic VATS was first used for sympathectomy surgery to treat palmar hyperhidrosis and sympathectomy disorders (13). The small wounds ensured not only reduced pain, but excellent cosmesis with the incisions becoming virtually completely invisible within a few weeks after surgery. From this, we have further extended its use to treating pneumothorax with considerable success (14).
The next step was of course to apply the Needlescopic VATS approach to lung cancer surgery (4). Using the same ports positions as the modified 3-port strategy above, the posterior port is reduced from 10 to 3 mm, although the utility port has to remain at 3-5 cm purely for the purposes of extracting the resected lobe of lung (Figure 4). The camera port is made by using a No. 11 scalpel blade to stab and create a 3 mm skin puncture. A 3 mm trocar is pushed through and the 3 mm 30° video-thoracoscope placed through that. Alternatively, a tract is created into the pleural space by pushing a small mosquito forceps through the skin stab incision, and a 5 mm 30° video-thoracoscope is placed directly through this tract without a trocoar. The lens tip can be wiped within the chest using a pledget held on a Roberts forceps, lightly soaked with anti-fogging solution and inserted via the chest tube thoracostomy wound. The reduction in ports sizes may not sound like much, but in reality the difference is noticeable. Given the narrowness of the human intercostal space, a conventional 10 mm thoracoscope, for example, can lever against and cause blunt trauma to the intercostal bundles during manipulations to look up and down during the operation. By using a much finer thoracoscope and instruments, this torquing at the wound is intuitively reduced. Cosmesis is of course much better. At the same time, because three ports are still being used, the conduct of the operation is essentially the same as with conventional VATS—making it much easier for the experienced VATS surgeon to master. Complete lymph node dissection is also eminently feasible.
Figure 4.
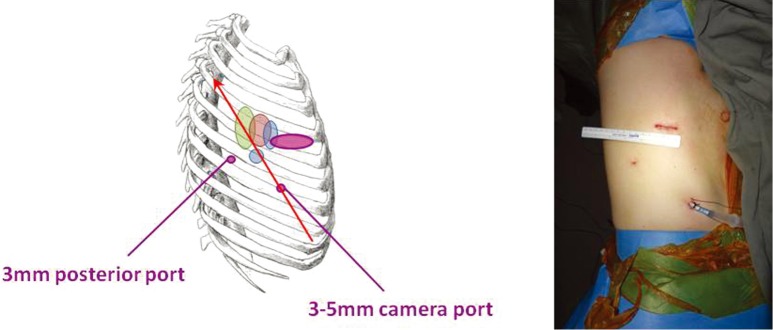
The Needlescopic VATS lobectomy. The ports positions (purple) and axis of the operation (red arrow) are the same as for the ‘modified’ 3-port VATS approach. However, the posterior and camera ports have been reduced in size to 3 mm in diameter only. VATS, video-assisted thoracic surgery.
The author routinely uses a 3 mm (or sometimes a 5 mm) 30° video-thoracoscope. A commonly voiced concern about the use of such fine thoracosocopes is regarding the brightness and resolution of the video image produced. Thankfully, modern high-definition surgical video cameras have such good light sensitivity and superb resolution that this is in practice never a noticeable problem.
2-port VATS
After gaining experience with a 3-port Needlescopic VATS approach, it was soon realized that the posterior 3 mm port was not always essential. The added retraction using a 3 mm instrument through that port did not contribute greatly, and in fact it was possible to deliver such surgical retraction and manipulation using another instrument via the utility port. The natural progression was therefore to omit that posterior port altogether—resulting in a 2-port VATS technique (Figure 5). This delivers all the advantages of Needlescopic VATS, but with one fewer port. If the patient’s lung has no air leak at the end of a lobectomy operation, sometimes a chest tube as small as 16F can be placed via the camera port, further reducing post-operative discomfort and enhancing the cosmetic appeal. The downside is that using only one utility port for all the instrumentation during 2-port VATS requires considerably more VATS experience on the part of the surgeon. Having said that, this approach is now rapidly gaining in popularity, and many large centers in mainland China are already using this technique routinely.
Figure 5.
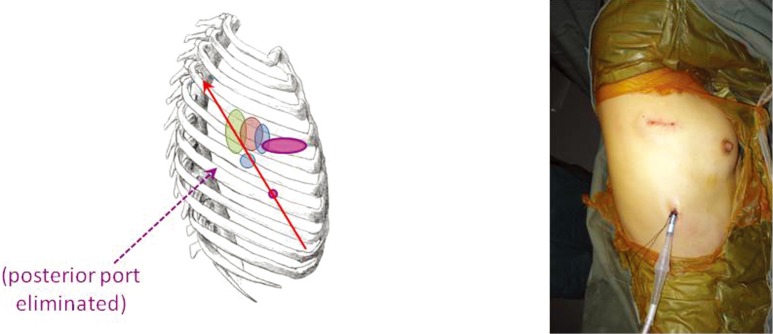
The 2-port VATS lobectomy. The utility and camera ports are identical to the Needlescopic approach, but the posterior port has been eliminated. VATS, video-assisted thoracic surgery.
At the University of Hong Kong, the author has switched completely from using conventional 3-port VATS for routine lung cancer surgery to using these ‘Next Generation’ approaches of Needlescopic and 2-port VATS. The median length of stay after lobectomy is now 3 days. Although in Asia there is less pressure for early discharge home after surgery, unlike in many Western countries, this outcome serves as a useful indicator of the excellent recovery experienced by patients using these ‘Next Generation’ techniques.
Compared to robot assisted surgery, these newer VATS evolutions require no expensive equipment (most hospitals already have needlescopic instruments), take no longer than conventional VATS, and can be quickly learned by experienced VATS surgeons by further honing their skills.
Uniportal VATS
From the above progression from conventional VATS to Needlescopic VATS to 2-port VATS, it was merely logical to try to simply forego the separate camera port altogether and have the video-thoracoscope placed through the utility port as well (5). The concept of Uniportal VATS was actually first pioneered by Dr Gaetano Rocco for simpler intra-thoracic procedures over a decade ago (15,16). However, as with so many innovative ideas in surgery, the gestation period of Uniportal VATS prior to global acceptance has been a long one. It was eventually developed in more recent years to allow major lung resections by Dr. Diego Gonzalez-Rivas of A Coruna, Spain (5,6). His extensive experience now includes a few hundred lobectomies, and has extended to complex procedures such as sleeve lobectomies and pulmonary artery reconstructions.
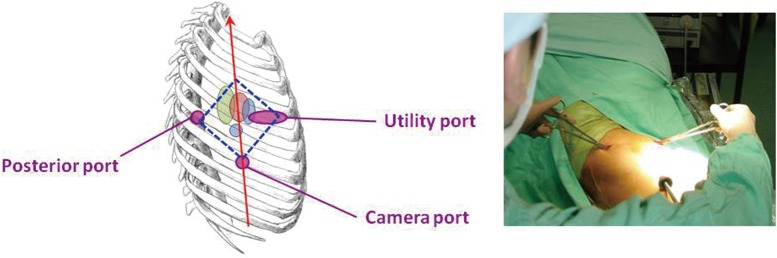
The author typically uses a single 3-5 cm incision in the anterior axillary line for Uniportal VATS major lung resections, essentially in the same place as with Needlescopic and 2-port VATS (Figure 6). The only minor difference in port strategy is that the 5th intercostal space is preferred for both upper and lower lobectomies. A 5 mm diameter 30° video-thoracoscope is placed alongside the instruments used by the surgeon’s right and left hands. This ‘shared port’ technique makes for a very ‘cosy’ operating environment, and requires a degree of skill not only from the surgeon but from the assistant.
Figure 6.
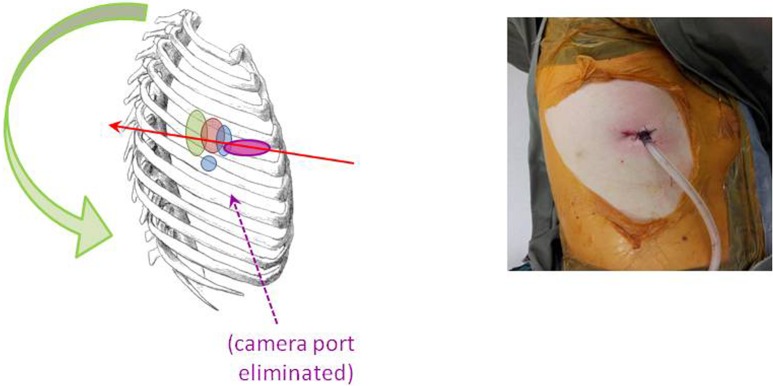
The Uniportal VATS lobectomy. Compared to the 2-port approach, the camera port has been eliminated. The Uniport is in the 5th intercostal space and is largely unchanged from the utility port of all previous iterations of VATS lobectomies. VATS, video-assisted thoracic surgery.
Another significant challenge for the conventional VATS surgeon converting to the Uniportal approach is the further rotation of the whole axis of the operation towards a posterior direction. With Needlescopic and 2-port VATS, the visual axis is the same as with the modified 3-port VATS approach, and hence very easy to get used to. But with the Uniportal approach, the axis is changed from an umbilicus-to-shoulder direction to a nipple-to-scapula tip direction. Furthermore, instead of the ‘looking across a baseball field’ horizontal perspective offered by multi-port, Needlescopic and 2-port VATS, the perspective in Uniportal VATS is more vertical and more like looking down a tunnel. Consequently, the surgeon and the assistant must to some extent re-learn the hand-eye co-ordination.
Nevertheless, potential benefits for patients are promised by Uniportal VATS. In the author’s experience, the safety profile has been excellent and the conversion rate has been less than 5%. Patients have had a median length of stay post-operatively of 3 days. Critics are not incorrectly in pointing out that there has so far been no evidence to unequivocally prove the superiority of Uniportal VATS over other forms of minimally invasive Thoracic Surgery. However, there has been enough clinical data to show that the approach can be performed with equal levels of safety and oncological adequacy as conventional VATS. It is therefore not unreasonable to further develop and accumulate experience with it in the hope that patients may ultimately benefit.
Lessons from history
What is clear from the above history is that Uniportal VATS is a product of a gradual evolution of minimally invasive thoracic surgery: from classical 3-port VATS, through Needlescopic and 2-port VATS, to eventually Uniportal VATS. The evolution has primarily involved a gradual rotation of the axis of the operation as well as a step-wise reduction in the size and number of the incisions.
This evolution has taught the author a number of very important lessons that should be shared with any surgeon approaching the Uniportal VATS approach. These lessons can be summarized thusly:
Single-port instrumentation;
Coping without the posterior port;
Axis and perspective;
Troubleshooting;
Peri-operative care;
Dealing with the rookie.
Single-port instrumentation
To the beginner starting to learn Uniportal VATS, it may feel very uncomfortable having to place the instruments from both right and left hands through the same port, and furthermore having to share that port with a video-thoracoscope. One would think that this is a technique that required considerable time to master. However, in reality, the author’s learning curve was surprisingly short. Table 1 summarizes only the very first 15 consecutive lobectomies performed by the author using the Uniportal VATS approach. Although the operations in the latter 10 patients were technically more challenging than in the first 5 patients (poorer lung function, more upper lobectomies), results in terms of operation times, blood loss and post-operative recovery were no worse. In all of these outcome measures, the results achieved even with these first 15 operations were already equivalent to those being obtained with conventional VATS. The key to obtaining good results so quickly was not in the personal skill of the author, but in the fact that Uniportal VATS was indeed simply a natural evolution of minimally invasive thoracic surgery.
Table 1. Outcomes for the author’s first 15 consecutive patients receiving Uniportal VATS lobectomy.
| Characteristic/Outcome | First 5 patients | Next 10 patients | P value |
|---|---|---|---|
| Mean pre-op predicted FEV1 (%) | 110.0±11.0 | 88.0±11.0 | <0.01 |
| Upper lobectomy | 1.0 (20%) | 8.0 (80%) | 0.03 |
| Mean operation time (min) | 161.0±32.0 | 204.0±62.0 | 0.1 |
| Mean blood loss (mL) | 101.0±122.0 | 161.0±152.0 | 0.43 |
| Mean tumor diameter (mm) | 16.0 (range, 9-35) | 25.0 (range, 13-40) | 0.15 |
| Mean NO. of lymph node stations dissected | 4.4±1.8 | 5.4±1.0 | 0.3 |
| Mean chest drain duration (days) | 2.4±0.5 | 2.4±0.9 | 0.89 |
| Mean length of stay (days) | 3.4±0.5 | 3.7±1.3 | 0.45 |
| Post-op minor complications | 1.0 (20%) | 2.0 (20%) | 1 |
As the author progressed from conventional and Needlescopic 3-port VATS to 2-port VATS, the lesson learned was that all instrumentation could be accomplished readily via a single utility port. Maintaining the camera in the classic lower position of conventional and Needlescopic 3-port VATS made it easy for the assistant to provide a familiar, orthodox view of the operative field while the surgeon experimented with placing right and left hand instruments via the utility port only during 2-port VATS. During this time, a greater appreciation of using curved or right-angled instruments (such as long curved ring forceps, or simply Roberts and Rumel forceps) to facilitate dissection through a shared port is quickly gained. Because the utility port is in the same place as with conventional and Needlescopic 3-port VATS, most of the very same instruments could also be used—including standard Metzenbaum scissors, Debakey forceps, hand-control diathermy devices with long-tip extensions, and so on. This proved invaluable by allowing a familiar set of instruments to be maintained without the surgeon having to learn to use new ones alongside learning a new technique.
Once one has mastered using right and left hand instruments via the same shared utility port, proceeding to a Uniportal VATS approach merely becomes the transferral of the video-thoracoscope to the same port. By breaking down VATS into the manual and visual elements, and then learning the manual element before the visual element, it was quite easy for the author to acquire proficiency of Uniportal VATS. As the video-thoracoscope is brought up to the utility port, the same right and left hand instrumentation is preserved as before with 2-port VATS, and that allows the surgeon the comfort of a familiar manual element whilst only having to focus on learning the visual element.
This author highly recommends that learning Uniportal VATS should take this step-wise approach. For the surgeon familiar only with open surgery, it is advised that experience first be acquired with 3-port VATS. After mastering that, it is advised that some time is spent with 2-port VATS. Only when that has been accomplished should the surgeon proceed to Uniportal VATS. It is understandable that the number of operations performed using 3-ports and then 2-ports before going on to Uniportal VATS will vary greatly from surgeon-to-surgeon depending on prior experience with conventional VATS and other factors. The author appreciates that there are a number of surgeons who have successfully gone from performing open surgery straight to Uniportal VATS. Nonetheless, in general, the technical challenges posed by Uniportal VATS should never be underestimated, and the safety of the patient must come first. A step-wise approach to acquiring the manual and visual skills is intuitively more cautious and theoretically safer. It should never be considered (at present anyway) that Needlescopic, 2-port or even 3-port VATS is ‘inferior’ to Uniportal VATS. Evidence for that does not currently exist. Hence, the surgeon should rest assured that when providing these other forms of VATS he/she is giving the patient virtually equally good care–even as he/she is learning to eventually perform Uniportal VATS. Furthermore and by the same logic, Needlescopic and 2-port VATS need not be merely considered ‘stepping stones’ along the path to Uniportal VATS, but as the destination in their own right. If a surgeon feels that Uniportal VATS is not the right approach for him/her for any reason, there is nothing wrong with sticking to Needlescopic and 2-port VATS.
Another point to make about the evolution of sharing a port is the fact that using familiar instruments and techniques is important. When changing from 3-ports to 2-ports, the instruments via the utility port can stay the same as with 3-ports. When changing from 2-ports to Uniportal and only the video-thoracoscope position is changed, again the instruments used in 2-ports VATS can be kept unchanged. The implication of this is that it is not necessary to purchase any expensive new instruments to ‘allow’ one to start performing Uniportal VATS. Instead, it is better to approach the new technique using familiar instruments. Not only will this make it easier to learn, but it will allow the surgeon to gradually understand exactly where the old instruments may or may not be deficient when performing Uniportal VATS. There are many so-called ‘dedicated for Uniportal’ instruments available, some of which are very good but many of which are quite expensive. It is advised that the surgeon should gain some experience with the technique and understand the specific areas where an expensive new instrument may help before splurging on a new purchase.
Coping without the posterior port
When transitioning from a conventional 3-port VATS approach to a Needlescopic and then a 2-port approach, it becomes evident what the posterior port is used for. Primarily, it is a port for retraction. A grasping instrument (such as a Rampley forceps) is used to distract the lung allowing instrument(s) from the anterior utility port to approach the targets for dissection. The second most common use is for introduction of the staple-resection device (or ‘stapler’ for short). As mentioned above in relation to the modified 3-port VATS approach, introduction of the stapler from a posterior-to-anterior and inferior-to-superior direction has advantages, particularly in avoiding impingement of the anvil against other hilar structures as the stapler is passed around vessels. A third (less common) use is placement of the video-thoracoscope to look behind or above the lung hilum during certain points of the dissection. Therefore, when progressing to 2-port and Uniportal VATS, the surgeon must compensate for the loss of these three uses of the posterior port.
Distracting the lung from the area of dissection can be accomplished to some degree by greater use of rotation of the operating table. Approaching the anterior hilum can be facilitated by tilting the table posteriorly, and approaching the upper mediastinum by tilting it ‘head up’, for example. More importantly, experience with 2-port VATS shows that effective retraction can be easily provided using curved lung clamps—such as curved ring forceps or Harken clamps. These are usually completely sufficient, and there is no need for more fanciful endoscopic rectractors. However, the surgeon may find that an ability to hold more than one retractor with the fingers of the non-dominant hand while performing intricate dissection with the dominant hand is an invaluable skill. This is because with a 2-port or Uniportal VATS technique, there is little or no room for an extra assistant to reach in and help with retraction.
The loss of the posterior port for introduction of the stapler is compensated for by better understanding of how to manoeuvre the lung (Figure 7). Simple ‘pulling up’ retraction of the lung can result in the wrong angle of approach for the stapler, with medial or posterior structures impeding the passage of the anvil. For example, this is often the problem when beginners find it difficult to pass the stapler around a superior pulmonary vein during Uniportal VATS. Because the port is sited immediately over the vein, passing a stapler straight into the wound means that the near-vertical direction causes the anvil to be blocked against the hilum posteriorly. Instead, the retraction should aim to lift the lung in such a way as to allow the stapler to approach a vessel in a perpendicular direction. Using the above example, retracting the lung in a cephalad and slightly anterior direction allows the stapler placed via the uniport to approach the superior vein in a more horizontal direction without hitting the hilar structures behind the vein. It goes without saying that a reticulating stapler is a must to facilitate stapling during Uniportal VATS. The use of curved tip reloads also greatly helps to negotiate vessels in the absence of the posterior port for staler introduction. It should also be noted that the use of the 5th intercostal space for upper lobectomies is also because it allows a more horizontal angle for the stapler to approach the superior pulmonary vein.
Figure 7.
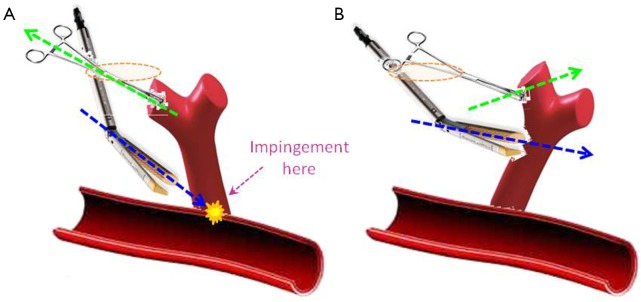
Example of manoeuvring the lung to enable the correct angulation for stapling. (A) If the lung is simply retracted upwards (green arrow) or towards the Uniport (yellow ring), the stapler is inserted downwards near-vertically (blue arrow) and even with reticulation of the stapler head the stapler tip will impinge against the mediastinal or hilar structures, impeding passage of the stapler around the vessel branch; (B) if the lung is instead distracted away (green arrow) from the Uniport (yellow ring), the target vessel branch is better displayed, allowing the reticulated stapler to approach perpendicularly at a ‘flatter’ angle (blue arrow) and avoid impingement against any structures on the far side.
The lack of the posterior port to allow the video-thoracoscope to look ‘behind’ or ‘above’ the hilum is actually not the problem it first appears to be during Uniportal VATS. The simple reason is that the scope is now placed via the utility port anyway, which is already at a higher level than the posterior port. Using a 30° video-thoracoscope via the Uniport already gives at least as good a view over the hilum as would have been obtained via the old posterior port.
Axis and perspective
The evolution of the VATS approaches has highlighted the concepts of the operative axis and of the visual perspective during surgery. The axis is that imaginary straight line from the surgeon (or camera port) through the point of dissection to the video monitor. Using the ‘baseball diamond’ imagery, this is the line from the ‘home base’ to the ‘second base’ and beyond. If the ports at ‘first and third bases’ are on a line perpendicular to this axis, then the left and right hand instruments will triangulate towards ‘second base’ and fencing between instruments with each other and with the camera is theoretically reduced. Also, the closer line between the surgeon and the monitor is to the line between the ‘home base’ camera port and the monitor (‘second base’), less discrepancy there is between the visual axis and the actual operative axis—and consequently the easier the hand-eye co-ordination will be.
Understanding the role of this axis explains why the modification of the classic 3-port VATS ports placement was necessary, and why it is relatively easy transitioning from 3-port VATS to Needlescopic VATS. When changing to a 2-port VATS approach, the axis is maintained as for Needlescopic VATS, but essentially the dissection is now done exclusively from ‘first base’. When changing to Uniportal VATS, the axis is rotated posteriorly as already described earlier. A grasp of this evolution of the axis direction-coupled with knowledge of the hilar anatomy-helps in understanding how to visualize the surgery during Uniportal VATS and to negotiate the dissection.
The concept of perspective is different from that of the axis (Figure 8). With 3-port, Needlescopic and 2-port VATS, the surgeon’s perspective of the operative field is exactly that of the ‘baseball diamond’. Namely, the surgeon’s view is that of a batter standing at ‘home base’ and looking out across a flat horizontal baseball field towards ‘second base’ with ‘first and third base’ on the same horizontal plane to the right and left respectively. Because this same perspective of looking out across a field is the same between these approaches, it is relatively easy to switch between these approaches. It also makes sense that the ‘first and third base’ ports are ‘above’ the level of the camera port. With Uniportal VATS, the camera looks down the same wound as the instruments, and the perspective is instantly changed (17). This calls for a bit of adaptation of the usual hand-eye co-ordination to get used to. It also means that the surgeon looks into the wound from the perspective of a standing human looking down into a mine-shaft instead of across a horizontal field. In this position of a human looking downwards, the eyes are actually ‘above’ the right and left hands. Therefore in the uniport (assuming the surgeon stands anterior to the patient), if the camera is placed at the lower or more anterior part of the wound and the right and left hand instruments enter the wound ‘above’ the camera at the more posterior part of the wound, it becomes disorientating for the surgeon. Instead, to maintain the normal perspective of a human looking down a mine-shaft, the camera should be kept at the posterior end of the wound and the right and left hand instruments should enter anterior (‘below’) to the camera or ‘eyes’. Obviously, this rule may sometimes be overruled for certain situations, but keeping to it makes Uniportal VATS less disorientating for most of the time.
Figure 8.
The issue of perspective. (A) With classic 3-port VATS, the surgeon looks out onto a flat, horizontal baseball field. With the surgeon at ‘home base’, the right and left hand instruments at ‘first and third bases’ are in front of the surgeon and farther along that flat field. This translates into a camera port positioned ‘lower’ (closer to the surgeon) than the right and left ports; (B) with Uniportal VATS, the view is more like looking downwards into a mine shaft. As the surgeon looks into the mine shaft, the eyes are naturally at a higher level than the right and left hands. This translates into the camera placed in the Uniport ‘higher’ (father from the surgeon) than the right and left hand instruments. For a surgeon standing anterior to the patient, this means the camera is placed towards the posterior end of the wound. VATS, video-assisted thoracic surgery.
Troubleshooting
The realization that Uniportal VATS is part of the evolution of minimally invasive thoracic surgery means that whenever difficulties are encountered, the same solutions that are used in other forms of VATS can also be applied just as effectively (1,2). Some examples include:
Bleeding: mild bleeding can be effectively controlled with topical hemostats and compression. More severe bleeding can be amenable to endoscopic suturing. It is again emphasized that experience with 3-port and 2-port VATS may be invaluable in providing proficiency with endoscopic suturing and hemostatic techniques prior to embarking on Uniportal VATS;
Fused interlobar fissure: the ‘fissure-less’ (or ‘fissure last’) approach to a lobectomy is now commonly used in conventional VATS (18), and remains perfectly applicable for dealing with fused fissures during Uniportal VATS;
Air leaks detected on-table: as with conventional VATS, major air leaks can be repaired by endoscopic suturing. Minor air leaks are effectively treated with the application of topical sealants. In the author’s experience, aerosolized fibrin sealant sprayed onto areas of small air leaks can reduce both chest drain durations and lengths of stay after VATS lung surgery. The cost of the sealant is usually more than offset by the reduced costs in post-operative hospital stay. If in doubt, the degree of air leak can be assessed by connecting a portable digital chest drain system whilst the patient is still on-table, and the digital reading of how much air flow is coming out can help guide whether further on-table intervention is required. It is the author’s preference to always deal aggressively with air leaks, because a prolonged air leak can negate the advantages of any form of VATS in reducing patient lengths of stay and allowing faster recovery;
Large tumors: it is frustrating for the surgeon to complete a major resection and then find that the resected specimen is too large to deliver via the patient’s intercostal space. Converting to a thoracotomy or use of forcible rib-spreading would negate the advantages of any form of VATS in reducing pain. In such situations, the author uses a technique of controlled cutting of a rib anteriorly to allow the intercostal space to be widened with minimal force (19). This technique was developed for multi-port VATS, but has proved useful in 2-port and Uniportal VATS.
Peri-operative care
One of the more important lessons learned during the evolution of VATS was that how the patient is managed outside the operating room is just as important as how well he/she is managed inside it. In the early days of VATS, patients receiving a VATS procedure were nursed and rehabilitated exactly the same as a patient who received the same procedure via an open thoracotomy. The result was that such patients recovered or were mobilized so slowly that they did not enjoy the full potential benefits of having received Minimally Invasive Surgery.
With all VATS patients in the author’s institute today, a bespoke Clinical Pathway is used to guide their peri-operative care from all clinical disciplines (4). The Pathway (equivalents are also referred to as ‘fast track’ or ‘expedited recovery’ in other centers) covers every aspect of nursing, physiotherapy, mobilization schedules, peri-operative investigations, pain management, chest drain management, nutrition, communication with the family, and so on. Goals for each day are set and monitored. Using this Pathway has already reaped significant benefits in terms of: pre- and post-operation lengths of stay; morbidity; readmission rates; and so on. Consistent, objective care is also ensured for all patients regardless of which member of the surgical team sees each patient. The Pathway has been updated to complement the increasing use of Needlescopic and 2-port VATS in recent years, and further updating is planned to take advantage of Uniportal VATS. Any center planning to introduce ‘next generation’ VATS approaches is urged to first plan a Clinical Pathway, lest the advantages of good operating become squandered.
Another area where peri-operative care is augmented to complement VATS is the infusion of cost-effective new technology. A prime example is the use of the aforementioned portable digital chest drainage system (20). This system is a small, portable box connected to a patient’s chest tube that has an internal suction mechanism delivering any level of negative pressure set by the clinician. The negative pressure level is regulated very precisely, avoiding variations that may prolong post-operative air leaks. The mechanism is also completely internal (it runs on internal rechargeable batteries like a mobile phone) and does not require connection to any outside contraption such as wall suction. The advantage is that even with negative pressure applied, the patient is not tied down and can freely mobilize even on the day of surgery. this complements VATS—and especially our ‘Next Generation’ and Uniportal VATS—perfectly, allowing the faster physical recovery expected of such techniques. The ‘digital’ part of the system refers to an in-built digital air flow monitor that accurately displays in real-time the flow of air coming out of the chest tube from the patient’s thorax, providing an objective quantification of any air leak after surgery. This avoids the inherent uncertainty in identifying air leaks using a water seal system, which can lead to hesitancy in chest drain removal or air leak interventions, and hence in turn to prolonged lengths of stay. We have previously reported significantly reduced chest drain durations and lengths of stay for our patients using the new digital chest drain systems (20). When developing a Uniportal or ‘next generation’ VATS program, it is therefore advised that one should look out for peri-operative technology that can complement the operation and help it fulfill its potential for patients.
Dealing with the rookie
One lesson from natural history is that evolution does not occur at the same pace for everyone. Even within a surgical team, different individuals may have evolved to different degrees. In the author’s unit, the surgeon may have acquired advanced VATS skills in the step-wise fashion as described above, but the assistants are often very inexperienced—often barely out of internship. The frustration is that regardless of how good a surgeon’s manual dexterity is, if the assistant is unable to deliver a decent view with the video-thoracoscope then visual element of the surgery will impede the performance of the operation. The rookie assistant therefore becomes the rate-limiting step. The evolution of VATS has taught us three simple lessons on how to help the rookie deliver a better performance.
First, the rookie assistant may need to undergo the same step-wise progression through the various incarnations of VATS. The classical 3-port VATS uses a fixed camera port with a trocar and is perhaps the easiest for the beginner camera-assistant to cope with. The fixed port reduces camera wandering and the trocar protects the lens from becoming easily smeared when introducing the video-thoracoscope into the chest. On the other end of the spectrum, Uniportal VATS is the greatest challenge for the camera-assistant. The video-thoracoscope is not held in a snug port but is actually free to wander around the entire 3-5 cm length, and the lack of a trocar means the lens can be easily smeared. This is made even worse by the fact that the wound is only a short distance from the point of dissection with performing Uniportal VATS (as opposed to the camera coming in from a distance via a low camera port with other types of VATS). With inexperienced camera-assistants, therefore, it may always be a good idea to start with 3-port or 2-port VATS before moving on to a Uniportal operation. It does not matter how experienced the surgeon is: if the camera-assistant cannot cope with handling a Uniportal VATS procedure, then for the sake of patient safety, it is best to fall back to 3-port or 2-port VATS without hesitation. The rookie can then be trained up from there.
Second, a system of effective verbal instructions must be developed. The surgeon’s hands during Uniportal VATS are often ‘busier’ than with 3-port or 2-port VATS, because retraction is so much more critical to exposure of the site of interest or dissection. As said before, each hand may hold more than one retractor in order to adequately expose the site. The surgeon therefore cannot reach out to physically correct a badly positioned video-thoracoscope. Verbal instructions to the rookie are all-important. For example, even seemingly simple commands as ‘higher/lower’ must be clarified before starting: does this mean to look up/down or to go more cephalad/caudal? More importantly when using a 30o video-thoracoscope, the inexperienced camera-assistant needs to be told how to use the angled view. The author uses the clock face to tell the assistant how to do this when using a conventional video-thoracoscope setup with both a video-camera and a separate light cable attached. A ‘12 o’clock’ view means to hold the light cable at the top side of the video-thoracoscope, so that the 30° is from the top looking downwards. A ‘3 o’clock view’ means to hold the light cable at the right side of the video-thoracoscope, so that the 30° is from the right looking leftwards.
Third, the lesson about perspective above is employed: the inexperienced camera-assistant is given a simple instruction to keep the video-thoracoscope lightly pressed at the posterior end of the Uniport throughout the procedure. This allows a steadier view as the scope rests against the posterior edge of the wound, whilst maintaining the ‘eyes-above-hands, looking down into a mine-shaft’ perspective. A simple instruction like this is much easier for the rookie to follow than more complex ones.
What evolution means for VATS
The fact that the development of minimally invasive thoracic surgery is an evolutionary process has implications for those looking to learn and practice Uniportal VATS as discussed above. To summarize, the take home message for surgeons is threefold:
Uniportal VATS is not an ‘all-or-nothing’ proposition. There are many steps between open thoracotomy and Uniportal VATS. These are not only steps along the path of training, but legitimate alternative approaches in their own right;
For the surgeon with experience in conventional VATS, there is no need to be intimidated. Because Uniportal VATS is ‘just’ another step in the evolutionary process, the same basic principles and techniques of conventional VATS are all applicable–including instruments, methods for trouble-shooting, and so on. This reassurance of familiarity should help guide the learning of the Uniportal approach;
The surgeon is not the only one evolving. As with the evolution of any species, a change in one individual can perpetuate if it can be shared with the population. In VATS, a skilled surgeon alone cannot sustain a new approach or technique. It is necessary to train the assistant, involve the multi-disciplinary team in a Clinical Pathway, and introduce appropriate complementary technology, and so on.
Looking ahead, the evolution of VATS holds another message for the future–and that is that evolution never stops. It is an ongoing process. That means that even Uniportal VATS is not the end of the road, and future advances great or small are inevitable. This in turn means that each new surgical technique must not only be mastered, but very well studied and analyzed for its strengths and weaknesses. Just as the analysis of classical 3-port VATS, Needlescopic VATS and 2-port VATS over the years have provided vital lessons about how to perform Uniportal VATS today, a close study of the practice of Uniportal VATS may provide invaluable experience to help nurture future generations of minimally invasive thoracic surgery.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- 1.Sihoe AD, Yim AP. Video-assisted pulmonary resections. In: Patterson FG, Cooper JD, Deslauriers J. eds. Thoracic Surgery (3rd Edition). Philadelphia: Elsevier, 2008:970-88. [Google Scholar]
- 2.Sihoe AD, Yim AP. VATS as a diagnostic tool. In: Shields TW, LoCicero J, Reed CE, et al. eds. General Thoracic Surgery (7th Edition). Philadelphia: Lippincott Williams & Wilkins, 2009:313-32. [Google Scholar]
- 3.Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [DOI] [PubMed] [Google Scholar]
- 4.Sihoe AD. The Evolution of VATS Lobectomy. In: Cardoso P. eds. Topics in Thoracic Surgery. Rijeka: Intech, 2011:181-210. [Google Scholar]
- 5.Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [DOI] [PubMed] [Google Scholar]
- 7.Passlick B, Born C, Sienel W, et al. Incidence of chronic pain after minimal-invasive surgery for spontaneous pneumothorax. Eur J Cardiothorac Surg 2001;19:355-8; discussion 358-9. [DOI] [PubMed] [Google Scholar]
- 8.Sihoe AD, Au SS, Cheung ML, et al. Incidence of chest wall paresthesia after video-assisted thoracic surgery for primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2004;25:1054-8. [DOI] [PubMed] [Google Scholar]
- 9.Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico TA. Robotics in thoracic surgery: applications and outcomes. J Thorac Cardiovasc Surg 2006;131:19-20. [DOI] [PubMed] [Google Scholar]
- 12.Yim AP, Sihoe AD, Lee TW, et al. A simple maneuver to detect air leaks on the operating table after needlescopic video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2002;124:1029-30. [DOI] [PubMed] [Google Scholar]
- 13.Sihoe AD, Au SS, Cheung ML, et al. Incidence of chest wall paresthesia after video-assisted thoracic surgery for primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2004;25:1054-8. [DOI] [PubMed] [Google Scholar]
- 14.Chou SH, Li HP, Lee JY, et al. Needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2009;18:221-4. [DOI] [PubMed] [Google Scholar]
- 15.Rocco G, Khalil M, Jutley R.Uniportal video-assisted thoracoscopic surgery wedge lung biopsy in the diagnosis of interstitial lung diseases. J Thorac Cardiovasc Surg 2005;129:947-8. [DOI] [PubMed] [Google Scholar]
- 16.Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [DOI] [PubMed] [Google Scholar]
- 17.Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5:S214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sihoe AD, Tan HY. Complete Video Assisted Thoracic Surgery Major Lung Resection for Lung Cancer: the impact of Tuberculosis and Post-inflammatory Adhesions on outcomes. Respirology 2011;16:138. 20920144 [Google Scholar]
- 19.Sihoe AD, Chawla S, Paul S, et al. Technique for delivering large tumors in video-assisted thoracoscopic lobectomy. Asian Cardiovasc Thorac Ann 2014;22:319-28. [DOI] [PubMed] [Google Scholar]
- 20.Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter International Randomized Comparison of Objective and Subjective Outcomes Between Electronic and Traditional Chest Drainage Systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [DOI] [PubMed] [Google Scholar]