Abstract
Background
Data on advantages of video-assisted thoracoscopic surgery (VATS) lobectomies has been accumulated during the last 10 years then number of thoracoscopic anatomic pulmonary resections rapidly increased. But still there is no agreement about limitations of the method. The most popular “technical contraindications” for VATS anatomic pulmonary resections are: dense pleural adhesions, incompleteness of interlobar fissure, previous chemo- or/and radiotherapy, perivascular or/and peribronchial fibrosis, tumor larger than 5 cm, chest wall involvement, centrally located tumor, severe comorbidity, advanced age, severe COPD and emphysema. Extending of indications for the VATS anatomic pulmonary resection and its influence on the immediate outcomes was investigated.
Methods
Ninety two consecutive cases of VATS anatomic pulmonary resection performed by the single surgeon from January 2012 till December 2013 at the Federal University Hospital #122 in Saint Petersburg, Russia were retrospectively analyzed. Forty three males and 49 females at the age from 21 to 87 years old (mean age 59±7.2). The most of the cases were comprised by lung cancer of I-III stage together with bronchiectasis and tuberculomas. Conversion rate was 3.2% mostly due to perivascular calcification and/or fibrosis. There were no cases of 30-days mortality and readmission. All those patients retrospectively divided into two groups: with standard and extended indications for the VATS lobectomy. Inclusion in “extended” group was made if patients had one or more technical challenges among following: size of the lesion 5 cm and more; strong pleural adhesions and/or “bad fissure”; adjacent structures involvement; hilar or mediastinal lymph nodes enlargement or involvement; centrally located tumors; previous chemo- or chemoradiotherapy or previous thoracic surgery.
Results
According to these criteria, 45 standard (S) and 47 extended (E) patients were pair-matched with no statistically significant differences between the groups in common patients’ characteristics. Postoperative comparison of “standard” and “expanded” groups revealed some differences in average operation time (152 vs. 189 min), in number of resected mediastinal lymph nodes (10.2 vs. 13.1), and in the mean time before removal of the chest tube (3.9 vs. 5.2 days). But the blood loss, morbidity and the length of hospital stay were almost the same in the two groups.
Conclusions
Extension of indications to VATS lobectomy does not compromise the short-term results. Incompleteness of interlobar fissures, pleural adhesions, preoperative chemotherapy, big size of lesion, and some cases of centrally located tumors are not supposed to be the contraindications for VATS lobectomy. Peribronchial and perivascular lymph node calcification may complicate and even preclude lobectomy by VATS.
Keywords: Video-assisted thoracoscopic surgery (VATS), lobectomy, indications, contraindications, matched-pair comparison
Despite a wide acceptance of the video-assisted thoracoscopic surgery (VATS) lobectomies as the standard treatment for early stage of lung cancer (1,2), there are still quite a lot of uncertainty in relation to the potential difficulties and contraindications for the thoracoscopic major pulmonary resections. At the moment there is no agreement about limitations of the method. We have decided to investigate how the expanding of indications for the VATS anatomic pulmonary resection can affect the immediate outcomes.
Methods and materials
Retrospectively analyzed all consecutive 92 cases of VATS anatomic pulmonary resection (segmentectomies, lobectomies, bilobectomies and pneumonectomies) performed by single consultant surgeon at the single institution (Federal University Hospital #122 in Saint Petersburg, Russia) from January 2012 till December 2013. During this period all the patients required anatomic pulmonary resections were elected for VATS, except those with multiple N2 disease, or with involvements of two and more ribs, or with involvements of hilar vessels and main bronchus.
Preoperative workup included contrasted chest and abdominal CT, bronchoscopy, PFT, 18-FDG PET-scan. All procedures were performed under the general anesthesia with double-lumen tube intubation and single lung ventilation. The procedures fulfilled the requirements for “VATS lobectomy” based on the international consensus criteria (3,4):
Visualization by monitor;
No use of rib spreaders;
Separate isolation of hilar structures;
Lymph node dissection in case of malignancy;
Utility incision up to 6 sm. (mean 4.2±0.8 sm.);
-
Number of ports from 1 to 4:
Single port—3 procedures;
Double ports—33 procedures;
Three ports—49 procedures;
Four ports—7 procedures.
Postoperative management protocol included: antibiotics, respiratory therapy and physiotherapy, continuous paravertebral injection of Bupivacaine with catheter and elastic pomp, and non-steroid anti-inflammatory medications. Chest drain removed if an air leak completely stopped at least for 24 hours and amount of fluid for the previous day was less than 100 mL.
From 92 patients were 43 males and 49 females at the age from 21 to 87 years old (mean age 59±7.2). Thirty three percent of patients were older than 70 years. The patients had different co-morbidities with Charlson co-morbidity index (CCI) ranging from 1 to 9 points (mean CCI 3.6±2.1). Forty five percent of patients had CCI of 5 points and more. FEV1 was used as a universal COPD-marker ranged from 35% to 90% of predicted value.
Patients’ diagnoses are presented on the Figure 1. As you can see from the diagram, most of operated patients suffered from pulmonary malignancies and 76% of patients underwent the VATS lobectomy for non-small cell lung cancer (NSCLC). NSCLC morphology and stage are shown in the Table 1.
Figure 1.
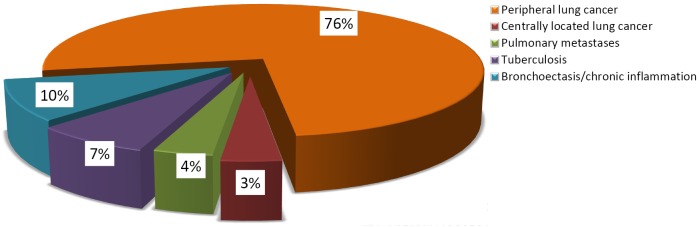
Distribution of the diagnoses in patients with VATS major pulmonary resection. VATS, video-assisted thoracoscopic surgery.
Table 1. Morphological characteristics and stage of NSCLC in patients who underwent major pulmonary resection by VATS.
| Cancer morphology |
| Adenocarcinoma: 55 (75.4%) |
| Squamous cell: 16 (21.7%) |
| Neuroendocrine: 3 (2.9%) |
| Cancer stage |
| Ia: 37 (52.2%), |
| Ib: 14 (17.4%) |
| IIa: 9 (11.6%), |
| IIb: 6 (7.2%) |
| IIIa: 7 (8.7%) |
| IV: 3 (2.9%) |
NSCLC, non-small cell lung cancer; VATS, video-assisted thoracoscopic surgery.
Figure 2 shows a full list of surgical procedures performed.
Figure 2.
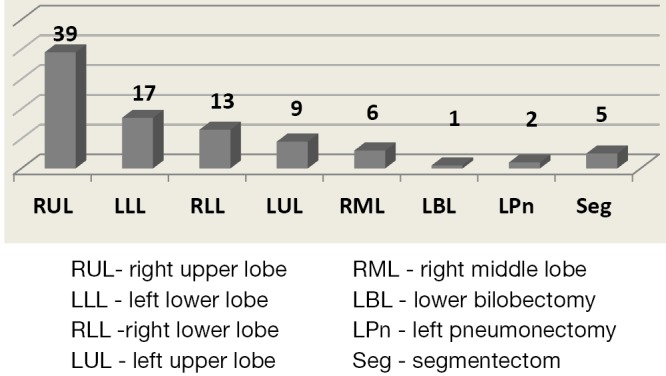
Diagram with distribution of VATS major pulmonary resection according to particular side and lobes resected. VATS, video-assisted thoracoscopic surgery.
The right upper lobectomy (RUL) was the major procedure. Among these operations there were two VATS right upper bronchoplasties and two chest wall resections.
During the investigated period of time, there were 95 intentions to perform the VATS lobectomy with three conversions to open surgery (conversion rate 3.2%). The reasons for conversion were unsafe feelings of the surgeon in one case, and the tear of an anterior trunk without bleeding due to severe perivascular fibrosis and calcification, in another case. In the third case, there was a severe bleeding due to the stapler malfunction.
No perioperative deaths were registered. No cases of readmission to the hospital were registered as well. Short-term results of the procedures are summarized in the Table 2.
Table 2. Short-term results after VATS major pulmonary resections.
| 30-days mortality: 0% |
| Morbidity: 16 (17.4%) |
| Prolonged air leak in 9 patients |
| Hyperexudation in 3 patients and chylothorax in 2 patients (reVATS in 1) |
| Nonsurgical in 2 patients |
| Operation time |
| From 100 to 290 min (mean time 184±60.5 min) |
| Number of lymph nodes dissected |
| From 7 to 21 (mean number 14.4) |
| Chest drainage duration |
| From day 1 to 24 (mean 4.8±5.4 days) |
| Length of postoperative stay |
| From 3 to 24 days (mean 7.7±4.8 days) |
| 54% of patients discharged before 5 days |
VATS, video-assisted thoracoscopic surgery.
All the patients from described cohort were stratified into two groups depending on standard indications and contraindications for the VATS lobectomy and were compared. For choosing of relative contraindications we based on chapter 63 “VATS lobectomy” from “Adult chest surgery” by Sugarbaker et al. edition [2009] and on table 23-5 relative contraindications for a VATS lobectomy from “Medical management of the thoracic surgery patient” by Lewis and McKenna Jr edition published in 2010.
Finally, the inclusive criteria for the extended indications group were as follow:
Lesion size 5 cm and more;
Strong pleural adhesions (dens and occupied more than 50% of pleural space); fussed fissure and severe emphysema;
Adjacent structures involvement (invasion to a parietal or mediastinal pleura, diaphragm, or a rib, except first);
N1 or N2 (confirmed by PET in case of malignance, or enlarged more than 12 mm, or antraco fibrotic);
Centrally located tumors (endo- or peri-bronchial extension to lobar bronchus orifice);
Previous chemo- or chemoradiotherapy, or previous surgery.
Results
According to these criteria, 45 standard (S) and 47 extended (E) cases were pair-matched. In the extended group 67% of patients had two or more “technical contraindications” each. To confirm homogeneity of the of formed groups by gender, age, diagnosis, stage, CCI etc. we compare these characteristics and found no statistically significant differences between the groups in common anthropometric signs (Table 3).
Table 3. Patients’ characteristics comparison in the groups with standard (S) and extended (E) indications for VATS major pulmonary resection.
| Group S (N45) | Group E [47] | P value | |
|---|---|---|---|
| Age | 58 [34-85] | 56 [23-87] | 0.581 |
| Gender, male | 21 (46.7%) | 21 (44.7%) | 0.923 |
| CCI | 2.6 | 3.4 | 0.097 |
| BMI | 26.6 | 25.6 | 0.449 |
| FEV1, % | 82 | 81 | 0.886 |
| Diagnosis | 0.339 | ||
| Lung cancer | 32 (71.1%) | 35 (74.5%) | |
| Bronchoectasis | 3 (6.7%) | 6 (12.8%) | |
| Tuberculomas | 4 (8.9%) | 2 (4.3%) | |
| Lobe resected | 0.393 | ||
| RUL | 20 (44.4%) | 19 (40.4%) | |
| RML | 4 (8.9%) | 2 (4.3%) | |
| RLL | 3 (6.7%) | 10 (21.3%) | |
| LUL | 7 (15.6%) | 2 (4.3%) | |
| LLL | 5 (11.1%) | 12 (25.5%) | |
| Number of ports | 0.903 | ||
| 1 | 2 (4.4%) | 1 (2.1%) | |
| 2 | 8 (17.8%) | 15 (31.9%) | |
| 3 | 30 (66.7%) | 27 (57.4%) | |
| 4 | 5 (11.1%) | 4 (8.5%) |
CCI, Charlson co-morbidity index; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe;VATS, video-assisted thoracoscopic surgery.
Postoperative comparison of “standard” and “expanded” groups revealed some differences in the duration of procedures, number of resected mediastinal lymph nodes, and in the mean time before removal of the chest tube (Table 4). In patients with extended indications operations were longer (mean 189 vs. 152 min) as well as chest drainage durations (mean 5.2 vs. 3.9 days). Also in patients with extended indications were extracted more mediastinal lymph nodes (mean 13.1 vs. 10.2). But these differences were not statistically significant.
Table 4. Comparison of perioperative results in the groups with standard (S) and extended (E) indications for VATS major pulmonary resection.
| Group S (n=45) | Group E (n=47) | P value | |
|---|---|---|---|
| Average operation time (minutes) | 152 | 189 | 0.093 |
| Number of mediastinal LN (mean) | 10.2 | 13.1 | 0.068 |
| Blood loss (mean, mL) | 159 | 151 | 0.89 |
| Chest tube duration (mean, days) | 3.9 | 5.2 | 0.08 |
| Amount of drained fluid per day (mean, mL) | 203.5 | 180.5 | 0.638 |
| Total amount of postop. opioids (number of dose) | 0.8 | 1.1 | 0.398 |
| Morbidity (number of patients) | 5 (16.7%) | 7 (21.9%) | 0.443 |
| Length of stay (mean, days) | 7.1 | 7.9 | 0.353 |
VATS, video-assisted thoracoscopic surgery.
As you can see in the Table 4, the blood loss, morbidity and the length of hospital stay were almost the same in the two groups.
Discussion
Beginning of the 21st century was marked by the rapidly growing number of thoracoscopic anatomic pulmonary resections. Data on advantages of VATS lobectomies has been accumulated during this period. These advantages are well known today and they include: low postoperative pain (5,6), better cosmetic results and early postoperative rehabilitation (7), shorter length of stay in the hospital (8,9), better visualization (10), early delivery of postoperative chemotherapy (6), and even lower morbidity and cost (11) comparing to the open procedure.
However, thoracic surgeons intending to start their program for VATS lobectomies are often concerned about rationality and feasibility of this approach in each particular clinical case. Among the most popular “technical contraindications” for VATS anatomic pulmonary resections are dense adhesions, incompleteness of interlobar fissure, previous chemo- or/and radiotherapy, perivascular or/and peribronchial fibrosis, tumor larger than 5 cm, chest wall involvement, centrally located tumor, severe comorbidity, advanced age, severe COPD and emphysema.
We mixed all this potential difficulties or relative contraindications and tried to evaluate if this have any influence on early postoperative results. We accept that there are two potential weak points in this study. First is heterogeneity of so-called “technical contraindication” we have choosing for investigation. Their mixing in one box could be criticized by reviewers. The second weakness is heterogeneity of the comparing diseases. We understand that this could decrease the power of evidence. Nevertheless, we assume the aim of this article to show that nothing is impossible. These “technical contraindications” for VATS lobectomies are using as scary story for the beginners. But each of “contraindication” has its own way to solve, and does not preclude performing VATS lobectomy.
In unison, many publications have been emerging advocating VATS lobectomies after chemoradiation (12), thoracoscopic broncoplasty (13), etc., and some surgeons do not accept the “standard contraindications” as an absolute rule (14). So we will discuss the most “popular” difficulties one by one.
Pleural adhesions and “bad fissures”
Some surgeons consider dense pleural adhesions as a relative contraindication for the VATS lobectomy (15). The authors speculate that pneumolysis can increase the blood loss and obscure the vessels. By contrast, there are experienced surgeons in the field of VATS lobectomies for TB who does not see the adhesions as an obstacle (16). Regarding other inflammatory diseases such as bronchoectasis (BE) one should cite the data of Zhang et al. who found multiple pleural adhesions in 15 out of 52 patients with BE operated by VATS (17).
We believe that pleural adhesions of different volume can complicate the procedure but it should not be considered as a contraindication for VATS lobectomy. Some contemporary technical devices can reduce blood loss while performing the adhesiolysis. The one who decides to continue the procedure by VATS despite the adhesions should be encouraged by realizing that usually, in mediastinum, adhesions are less strong. No doubt, the visualization by VATS is much better for the manipulation on diaphragmatic surface and sinuses. Thinking of thoracotomy, one should keep in mind that it wouldn’t necessarily help much to overcome adhesions. The use of ultrasonic devices for dividing the adhesions can be worthwhile for keeping the operative field clear from the blood (Figure 3). In case of dense and wide adhesions, we recommend to use extrapleural technique (Figure 4). The video examples 1 and 2 demonstrate how to deal with adhesions.
Figure 3.

Dividing of adhesions in right pleural cavity with ultrasonic device followed by VATS right upper lobectomy (18). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/318
Figure 4.

The creating the initial space with the fingers and extra-intrapleural isolation of right lung followed by VATS right upper lobectomy (19). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/319
Incompleteness of interlobar fissure or absence of fissure could be a problem for the beginners. Normally the doubts appear regarding the isolation of the pulmonary artery branches. However, this is a problem for the lower lobectomies than you need find the artery in the fissure, and is not a problem for the upper and the middle lobes. In the case of lower lobectomectomy, it is either possible to staple the PA together with parenchyma, or to isolate the PA from the mediastinum with “tunnel” technique by stapling parenchyma over the PA (Figure 5). If one needs to find an interlobar plane in a fissureless patient, it is advised to use the technique “bronchus first” and, after stapling the bronchus, when the anesthesiologist inflates the lung, one can see the plane for the stapler. We agree with Hanna et al. that prior thoracic surgery, incomplete or absent fissures, and pleural adhesions should not be considered contraindications for the VATS major pulmonary resection (21).
Figure 5.

Appearing of left lower lobe artery in the interlobar fissure by stapling parenchyma over the artery with “tunnel” technique (VATS left lower lobectomy) (20). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/320
Centrally located tumors
The centrally located tumor is another issue. The VATS bronchoplastic lobectomy is a difficult task that requires the surgeon to possess a special thoracoscopic skill. Such type of thoracoscopic lobectomies has been criticized in the literature (22). One of the first VATS bronchoplasty published reported left lower lobectomy (LLL) with resection of left main bronchus and anastomosis with upper lobe bronchus, which was performed in Italy (23). Nowadays, we can find several papers on VATS bronchoplasty (13,24-26). The most popular indication for VATS bronchoplastic lobectomy is a tumor of RUL bronchus. Our own experience consists of two procedures of this type. Left upper bronchoplasty is the second popular procedure by VATS (27). Some skilled surgeons perform broncoplasty through two ports (26), or through a single port (24). We would like to pointed out, that anastomosis should be made as last step of procedures in order to preclude any torsions and traction on the sutures line.
The issue is the bronchial anastomosis technique. Some surgeons use interrupted sutures (13), the others combine running and interrupted sutures (24,25). At some point, such a combination makes the procedure less difficult. We performed anastomosis with interrupted sutures and found it more complicated. Predina et al. recommended isolating anastomosis from pulmonary artery with intercostal or pericardial flap to prevent bronchovascular fistula (27). Other authors, however, do not suggest anastomosis isolation as a compulsory step of the VATS bronchoplasty (24).
Chemo-radiotherapy (CRT) and involvement of lymph nodes
Chemotherapy and radiotherapy as well as involvement of adjacent structures are also considered contraindications for the VATS lobectomy by some authors (28). Nevertheless, VATS anatomic pulmonary resection in such cases has been reported (29). It is difficult to understand why previous chemo- or/and radiotherapy is defined as “contraindication” for the VATS lobectomy. In my opinion, the wound healing problems after chemoradiation should be of more concern for thoracothomy than for the VATS. The fact that one has to accept is that chemoradiation is associated with a more pronounced bleeding and can provide pleural adhesions. The means of dealing with this issue have been previously discussed in this article. Our own experience shows that the main point one should take into account is that usually the reason for CRT is LN involvement, so the hilar problems should be expected.
Peribronchial and perivascular fibrosis may be viewed as contraindications for VATS lobectomy (28). However, Yen et al. did not face significant problems with the hilar fibrosis in TB patients who underwent VATS lobectomy (16). Zhang et al. described seven cases of conversion in 52 patients with bronchoectasis due to bleeding in 3 patients, fused fissure in 3 patients and severe mediastinal lymph nodes enlargement in 13 patients (17).
From our point of view, perivascular fibrosis is much more harmful due to obscuring the vessel from visualization and making it difficult to differentiate the layers on the vessel. In addition, LN calcinosis is extremely dangerous as the traction for the calcified node can tear the PA. Patients with evidence of calcifications around the hilar vessels had a 37% risk of conversion to open procedure and it was the only predictor of conversion in multivariable modeling (30). Having scrutinized the unplanned conversion for VATS lobectomy, Park et al. found that 41% of conversions were due to the hilar nodal anthracofibrosis and hilar adhesions, and were associated with increased operative time and length of stay (31). Our data and experience strongly supported this statement, because our conversions and difficulties were mostly related to perivascular lymph nodes anthracofibrosis. So if you feel unsafe to continue with VATS lobectomy you need just to convert to open!
Another potential limitation for VATS-lobectomy suggested involvement of mediastinal LN. There are some authors who propose to convert to open procedure in such cases (32,33), while others still perform VATS-lobectomy (34). Zhong et al. compared 67 VATS and 90 open procedures in patients with N2 and concluded that conversion is an “unnecessary” option. In support of this conclusion, a Japanese group checked the number of extracted mediastinal LN by performing thoracotomy after VATS LN-dissection. They found only 2% to 3% of missed LN that was not statistically significant (35).
Size of the lesion
As of the lesion size, the 5 cm limit as a criterion against VATS lobectomy should be discussed. Shigemura et al. [2006] emphasized serious technical difficulties while performing lobectomy thoracoscopicaly when the tumor was larger (36). Even the founder of the method does not recommend operate big tumors by VATS. We need to recognize some problems with extraction of the specimen and problems with instrumentation performing lobectomy for the tumor larger than 5 cm. However, this is not an absolute contraindication to the procedure. In the series of Liang et al. short-term and long-term results were independent of the tumor size (37). The authors just emphasize that a surgeon needs to have advanced skills in VATS lobectomy and require two surgeon-assistants.
Moreover, the true size of tumor is difficult to measure precisely, especially for tumors with destruction. And if there is no hilar structures involvement, the size of lesion doesn’t matter. The video demonstrates the VATS right lower lobectomy (RLL) for the tumor about 10 cm (Figure 6).
Figure 6.

VATS right lower lobectomy in patient with destructive peripheral lung cancer of 10 cm (38). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/321
Conclusions
Extension of indications to VATS lobectomy does not compromise the short-term results;
Incompleteness of interlobar fissures, pleural adhesions, preoperative chemotherapy, big size of lesion, and some cases of centrally located tumors are not supposed to be the contraindications for VATS lobectomy;
Peribronchial and perivascular lymph node calcification may complicate and even preclude lobectomy by VATS.
Acknowledgements
The author thanks his team of thoracic surgeons (Alexander Kovalenko, Alexander Obornev and Eugeniy Zinchenko) and anesthesiologist (Nazim Shirinbekov) for their support with this project.
Disclosure: The author declares no conflict of interest.
References
- 1.Hartwig MG, D’Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [DOI] [PubMed] [Google Scholar]
- 2.Weber A, Stammberger U, Inci I, et al. Thoracoscopic lobectomy for benign disease--a single centre study on 64 cases. Eur J Cardiothorac Surg 2001;20:443-8. [DOI] [PubMed] [Google Scholar]
- 3.Yan TD, Cao C, D’Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [DOI] [PubMed] [Google Scholar]
- 4.Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. [DOI] [PubMed] [Google Scholar]
- 5.Swanson SJ, Herndon JE, 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [DOI] [PubMed] [Google Scholar]
- 6.Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [DOI] [PubMed] [Google Scholar]
- 7.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [DOI] [PubMed] [Google Scholar]
- 8.Ueda K, Hayashi M, Tanaka T, et al. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardiothorac Surg 2013;44:225-9; discussion 229. [DOI] [PubMed] [Google Scholar]
- 9.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico TA. Long-term outcomes of thoracoscopic lobectomy. Thorac Surg Clin 2008;18:259-62. [DOI] [PubMed] [Google Scholar]
- 11.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93(4):1027-32. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214-8; discussion 219. [DOI] [PubMed] [Google Scholar]
- 13.Mahtabifard A, Fuller CB, McKenna RJ, Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Rivas D, Delgado M, Fieira E, et al. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg 2013;17:889-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg 2010;34:2368-72. [DOI] [PubMed] [Google Scholar]
- 16.Yen YT, Wu MH, Lai WW, et al. The role of video-assisted thoracoscopic surgery in therapeutic lung resection for pulmonary tuberculosis. Ann Thorac Surg 2013;95:257-63. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Zhang F, Jiang S, et al. Video-assisted thoracic surgery for bronchiectasis. Ann Thorac Surg 2011;91:239-43. [DOI] [PubMed] [Google Scholar]
- 18.Pischik VG. Dividing of adhesions in right pleural cavity with ultrasonic device followed by VATS right upper lobectomy. Asvide 2014;1:305. Available online: http://www.asvide.com/articles/318
- 19.Pischik VG. The creating the initial space with the fingers and extra-intrapleural isolation of right lung followed by VATS right upper lobectomy. Asvide 2014;1:306. Available online: http://www.asvide.com/articles/319
- 20.Pischik VG. Appearing of left lower lobe artery in the interlobar fissure by stapling parenchyma over the artery with “tunnel” technique (VATS left lower lobectomy). Asvide 2014;1:307. Available online: http://www.asvide.com/articles/320
- 21.Hanna JM, Berry MF, D’Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5Suppl 3:S182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi K.Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [DOI] [PubMed] [Google Scholar]
- 23.Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang J.Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 2013;44:1108-12. [DOI] [PubMed] [Google Scholar]
- 26.Jiao W, Zhao Y, Huang T, et al. Two-port approach for fully thoracoscopic right upper lobe sleeve lobectomy. J Cardiothorac Surg 2013;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Predina JD, Kunkala M, Aliperti LA, et al. Sleeve lobectomy: current indications and future directions. Ann Thorac Cardiovasc Surg 2010;16:310-8. [PubMed] [Google Scholar]
- 28.Marty-Ané CH, Canaud L, Solovei L, et al. Video-assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013;17:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [DOI] [PubMed] [Google Scholar]
- 30.Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [DOI] [PubMed] [Google Scholar]
- 32.Shiraishi T, Hiratsuka M, Yoshinaga Y, et al. Thoracoscopic lobectomy with systemic lymph node dissection for lymph node positive non-small cell lung cancer--is thoracoscopic lymph node dissection feasible? Thorac Cardiovasc Surg 2008;56:162-6. [DOI] [PubMed] [Google Scholar]
- 33.Naruke T, Tsuchiya R, Kondo H, et al. Lymph node sampling in lung cancer: how should it be done? Eur J Cardiothorac Surg 1999;16Suppl 1:S17-24. [DOI] [PubMed] [Google Scholar]
- 34.Zhong C, Yao F, Zhao H.Clinical outcomes of thoracoscopic lobectomy for patients with clinical N0 and pathologic N2 non-small cell lung cancer. Ann Thorac Surg 2013;95:987-92. [DOI] [PubMed] [Google Scholar]
- 35.Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg 2002;73:900-4. [DOI] [PubMed] [Google Scholar]
- 36.Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2006;132:507-12. [DOI] [PubMed] [Google Scholar]
- 37.Bu L, Li Y, Yang F, et al. Completely video-assisted thoracoscopic lobectomy versus open lobectomy for non-small cell lung cancer greater than 5 cm: a retrospective study. Chin Med J (Engl) 2012;125:434-9. [PubMed] [Google Scholar]
- 38.Pischik VG. VATS right lower lobectomy in patient with destructive peripheral lung cancer of 10 cm. Asvide 2014;1:308. Available online: http://www.asvide.com/articles/321


