Abstract
The capture of attention by stimuli previously associated with reward has been demonstrated across a wide range of studies. Such value-based attentional priority appears to be robust, and cases where reward feedback fails to modulate subsequent attention have not been reported. However, individuals differ in their sensitivity to external rewards, and such sensitivity is abnormally blunted in depression. Here, we show that depressive symptomology is accompanied by insensitivity to value-based attentional bias. We replicate attentional capture by stimuli previously associated with reward in a control sample and show that these same reward-related stimuli do not capture attention in individuals experiencing symptoms of depression. This sharp contrast in performance indicates that value-based attentional biases depend on the normal functioning of the brain's reward system and suggests that a failure to preferentially attend to reward-related information may play a role in the experience of depression.
Keywords: Depression, selective attention, reward learning
Attention guides thought and behavior. Information that is attended becomes available to higher-order cognitive processes such as working memory and decision-making (e.g., Desimone & Duncan, 1995). Therefore, in order to promote well-being, it is important that attention select stimuli associated with rewarding outcomes (Anderson, 2013). Consistent with this idea, stimuli associated with high reward are preferentially attended in healthy individuals (e.g., Della Libera & Chelazzi, 2006, 2009; Hickey, Chelazzi, & Theeuwes, 2010; Kiss, Driver, & Eimer, 2009; Raymond & O'Brien, 2009; Serences, 2008). Such value-based attentional selection becomes automatic and persistent following associative learning between a stimulus and reward outcome, suggesting that the reward history of a stimulus can modify its attentional priority (e.g., Anderson, Laurent, & Yantis, 2011b; Anderson & Yantis, 2012, 2013).
The involuntary capture of attention by stimuli previously associated with reward has been demonstrated across a wide range of studies and appears to be robust (Anderson, Laurent, & Yantis, 2011a, 2011b, 2012, 2013; Anderson, Faulkner, Rilee, Yantis, & Marvel, 2013; Anderson & Yantis, 2012, 2013; Qi, Zeng, Ding, & Li, 2013; Theeuwes & Belopolsky, 2012; Wang, Yu, & Zhou, 2013). Although it has been assumed that the establishment of such value-based attentional biases critically depends on a normally functioning reward processing system, consistent with a distinctly value-driven mechanism of attentional control (Anderson, 2013), there is currently no direct evidence to support this. To the contrary, value-driven attentional capture has only been assessed in healthy individuals obtained through general recruitment methods, with the exception of one study showing elevated value-driven attentional capture in a drug-dependent sample (Anderson et al., 2013). It is unclear whether reward-associated stimuli can fail to acquire value-based attentional priority for certain types of individuals, and identifying the characteristics of such individuals would provide insights into the psychological and underlying neurobiological processes that play an important role in value-driven attention.
One case in which there appears to be a deficit in the processing of reward information is depression. Clinically, depression often presents with decreased pleasure from and reduced interest in activities that were previously experienced as enjoyable, such as hobbies and sex (e.g., Eshel & Roiser, 2010; MacPhillamy & Lewinsohn, 1974). Depression is also associated with increased focus on negative thoughts and events, at the expense of more positive alternatives that tend to be ignored or overlooked (e.g., Mathews & MacLeod, 2005). Experimental evidence demonstrates decreased sensitivity to reward information in depression both behaviorally and neurobiologically (e.g., Foti & Hajcak, 2009; Henriques & Davidson, 2000; Shankman, Klein, Tenke, & Bruder, 2007).
To the extent that value-driven attentional capture depends on the ability to robustly represent the experience of receiving a reward, depressed individuals should show little or no attentional capture by previously high-value stimuli. We hypothesized that symptoms of depression are accompanied by a blunted influence of prior reward on attentional selection compared to that previously observed in non-depressed individuals, such that previously high-value stimuli that are normally attention capturing are more readily ignored in depression.
In the present study, college undergraduates experiencing symptoms of depression performed a visual search task involving a training phase and a test phase that was identical to the task originally used to demonstrate value-driven attentional capture (Anderson et al., 2011b, Experiment 3). Performance for this depressed sample was compared to that of a control sample drawn from the general undergraduate population. Depressive symptomology was quantified for all participants using the Beck Depression Inventory (BDI-II) on the day of testing. In the training phase, each of two color-defined targets was associated with a monetary reward when correctly reported, with one target color yielding higher reward than the other on average. In the test phase, targets were defined by shape while color was irrelevant to the task; on a subset of the trials, one of the nontargets was rendered in the color of a previously reward-associated target. We have demonstrated in several prior studies that such previously reward-associated distractors robustly capture attention in healthy individuals, as reflected by a slowing of response time particularly on trials containing a high-value distractor (Anderson et al., 2011a, 2011b, 2012, 2013; Anderson & Yantis, 2012, 2013). Of interest was whether such distractors would similarly capture attention in individuals experiencing symptoms of depression. Because visual working memory (VWM) capacity has been shown to be negatively correlated with the magnitude of attentional capture by previously reward-associated stimuli (Anderson et al., 2011b; Anderson & Yantis, 2012), we also compared the VWM capacity of depressed and control participants as measured using a color change detection task.
Methods
Participants
Twenty-eight participants experiencing symptoms of depression (mean age = 22.0y, 6 male) and thirty control participants (mean age = 20.3y, 9 male) were recruited from the Johns Hopkins University undergraduate student population. The depressed participants were recruited through electronic announcements as well as flyers posted on the campus and counseling center that were specifically targeted toward individuals who were feeling depressed. Participants were considered eligible for the depressed group if they scored a 16 or above on the BDI-II (Beck, Steer, & Brown, 1996), were not being treated with psychotropic medications (assessed via self-report), and were not in treatment for or diagnosed with any other psychiatric or neurological condition (assessed via self-report). Participants in the control group were obtained through general recruitment methods targeted toward all undergraduate students and were also assessed using the BDI-II. All participants reported normal or corrected-to-normal visual acuity and normal color vision. The two samples did not differ in either age (p = .112) or sex (p = .456).
Apparatus
A Mac Mini equipped with Matlab software and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P991 monitor. The participants viewed the monitor from a distance of approximately 50 cm in a dimly lit room. Manual responses were entered using a standard keyboard.
Beck Depression Inventory
All participants completed the BDI-II (Beck et al., 1996) immediately prior to testing.
Visual Working Memory Task
After completing the BDI-II, participants completed a 120 trial implementation of a change detection task that has been used in previous studies of value-driven attentional capture (Anderson et al., 2011b; Anderson & Yantis, 2012). Participants were shown a memory array of 4, 6, or 8 differently colored squares for 100 ms. Following a 900 ms retention interval, a single colored square appeared in a position previously occupied by a square in the memory array. Participants indicated whether this colored square was the same or different in color from the square in that position in the memory array via a keypress, without time pressure. Accuracy was measured and VWM capacity was estimated as the number of items remembered using a standard formula that corrects for the probability of guessing correctly (see Cowen, 2001).
Training Phase
Stimuli
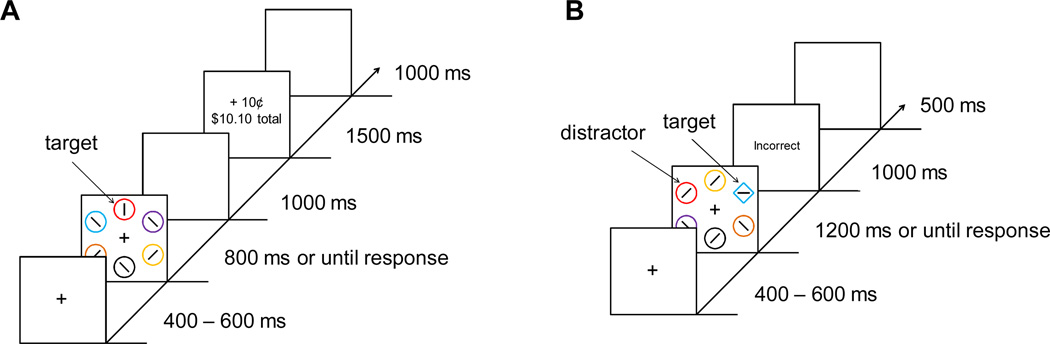
Each trial consisted of a fixation display, a search array, and a feedback display (Figure 1A). The fixation display contained a white fixation cross (.5°×.5° visual angle) presented in the center of the screen against a black background, and the search array consisted of the fixation cross surrounded by six colored circles (each 2.3° × 2.3°) placed at equal intervals on an imaginary circle with a radius of 5°. The target was defined as the red or green circle, exactly one of which was presented on each trial; the color of each nontarget circle was drawn from the set {blue, cyan, pink, orange, yellow, white} without replacement. Inside the target circle, a white bar was oriented either vertically or horizontally, and inside each of the nontargets, a white bar was tilted at 45° to the left or to the right (randomly determined for each nontarget). The feedback display indicated the amount of monetary reward earned on the current trial, as well as the total accumulated reward.
Figure 1.
Sequence of events and time course for a trial during the training phase (A) and test phase (B).
Design
One of the two color targets (counterbalanced across participants) was followed by a high reward of 10¢ on 80% of correct trials and a low reward of 2¢ on the remaining 20% (high-reward target); for the other color target, these percentages were reversed (low-reward target). Each color target appeared in each location equally often, and trials were presented in a random order.
Procedure
The training phase consisted of 240 trials, which were preceded by 50 practice trials. Each trial began with the presentation of the fixation display for a randomly varying interval of 400, 500, or 600 ms. The search array then appeared and remained on screen until a response was made or 800 ms had elapsed, after which the trial timed out. The search array was followed by a blank screen for 1000 ms, the reward feedback display for 1500 ms, and a 1000 ms inter-trial interval (ITI).
Participants made a forced-choice target identification by pressing the "z" and the "m" keys for the vertically- and horizontally-orientated bars within the targets, respectively. They were instructed to respond both quickly and accurately. Correct responses were followed by monetary reward feedback in which a small amount of money was added to the participant's total earnings. Incorrect responses or responses that were too slow were followed by feedback indicating 0¢ had been earned. If the trial timed out, the computer emitted a 500 ms 1000 Hz tone.
Test Phase
Stimuli
Each trial consisted of a fixation display, a search array, and a feedback display (Figure 1B). The six shapes now consisted of either a diamond among circles or a circle among diamonds, and the target was defined as the unique shape. On a subset of the trials, one of the nontarget shapes was rendered in the color of a formerly reward-associated target from the training phase (referred to as the valuable distractor); the target was never red or green. The feedback display only informed participants if their prior response was correct or not.
Design
Target identity, target location, distractor identity, and distractor location were fully crossed and counterbalanced, and trials were presented in a random order. Valuable distractors were presented on 50% of the trials, half of which were high-value distractors and half of which were low-value distractors (high- and low-reward color from the training phase, respectively).
Procedure
Participants were instructed to ignore the color of the shapes and to focus on identifying the unique shape both quickly and accurately, using the same orientation-to-response mapping. The test phase consisted of 240 trials, which were preceded by 20 practice (distractor absent) trials. The search array was followed immediately by non-reward feedback for 1000 ms in the event of an incorrect response (this display was omitted following a correct response) and then by a 500 ms ITI; no monetary rewards were given. Trials timed out after 1200 ms. As in the training phase, if the trial timed out, the computer emitted a 500 ms 1000 Hz tone. Upon completion of the experiment, participants were paid the cumulative reward they had earned in the training phase.
Data Analysis
Only correct responses were included in all analyses of RT, and RTs more than three SDs above or below the mean of their respective condition for each participant were trimmed.
Results
Descriptive Measures
Mean BDI-II score was 29.3 ± 1.8 SEM for the depressed group and 5.4 ± 1.0 SEM for the control group [t(56) = 12.23, p < .001, d = 3.21]. While the mean BDI-II score for the control group fell well within the bottom range defined as minimal depression by the measure, the mean for the depressed group fell within the range of severe depression. There was no overlap in depression scores between the depressed (range: 16–51) and control (range: 0–15) group. Mean VWM capacity was 2.24 ± 0.14 SEM for the depressed group and 2.51 ± 0.20 SEM for the control group and did not significantly differ [t(56) = 1.10, p = .278, d = .29].
Training Phase
An analysis of variance (ANOVA) on mean RTs with target value (high vs low) as a within-subjects factor and depressed status (depressed vs control) as a between-subjects factor revealed no main effects or interaction [Table 1, F's < 0.61, p's > .44, η2p's < .02]. This is generally consistent with the pattern observed in previous studies showing similar performance for high- and low-reward targets during training in this task (e.g., Anderson et al., 2011a, 2012, 2013), and suggests that in general, all participants searched for each color target with roughly equal priority. The same ANOVA on accuracy also revealed no main effects [F's < 0.25, p's > .61, η2p's < .01] or interaction [F(1,56) = 1.94, p = .170, η2p = .033].
Table 1.
Mean response time and accuracy as a function of target value in the training phase, separately for depressed and control participants. Standard deviations are in parentheses.
| Depressed | Control | |||
|---|---|---|---|---|
| Low-Reward | High-Reward | Low-Reward | High-Reward | |
| Response Time (ms) | 534 (49) | 531 (51) | 531 (41) | 534 (39) |
| Accuracy | 86.5% (8.6) | 88.0% (8.0) | 89.0% (9.0) | 87.6% (9.9) |
Test Phase
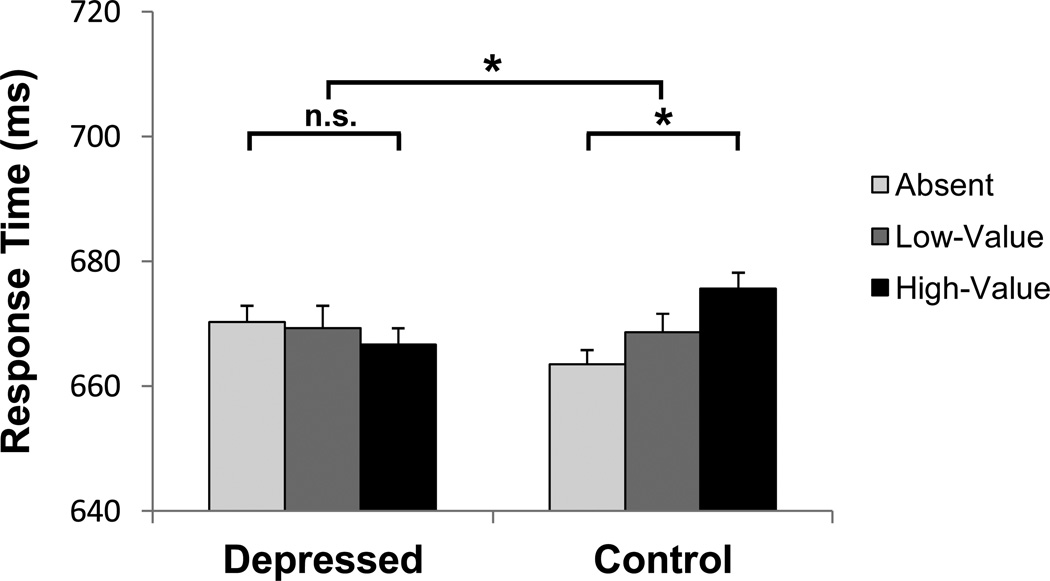
An ANOVA on mean RTs with distractor condition (absent, low-value, high-value) as a within-subjects factor and depressed status (depressed vs control) as a between-subjects factor revealed no main effect of either variable [F's < 0.78, p's > .45, η2p's < .02]. However, distractor condition interacted linearly with depressed status [F(1,56) = 8.26, p = .006, η2p = .129]. We defined value-driven attentional capture as the difference in response time on high-value distractor trials compared to distractor absent trials, as we have done in prior studies (Anderson et al., 2011b, 2013; Anderson & Yantis, 2012, 2013). While value-driven attentional capture was evident in the control participants [t(29) = 3.05, p = .005, d = .56], replicating previous findings (Anderson et al., 2011b, 2013; Anderson & Yantis, 2012, 2013; Qi et al., 2013; Theeuwes & Belopolsky, 2012; Wang et al., 2013), the depressed participants showed no evidence of value-driven attentional capture [t(27) = −0.97, p = .342, d = .18] (see Figure 2).
Figure 2.
Mean response time by distractor condition in the test phase, separately for depressed and control participants. Error bars reflect the within-subjects SEM for each participant group. *p < .01, n.s. non-significant
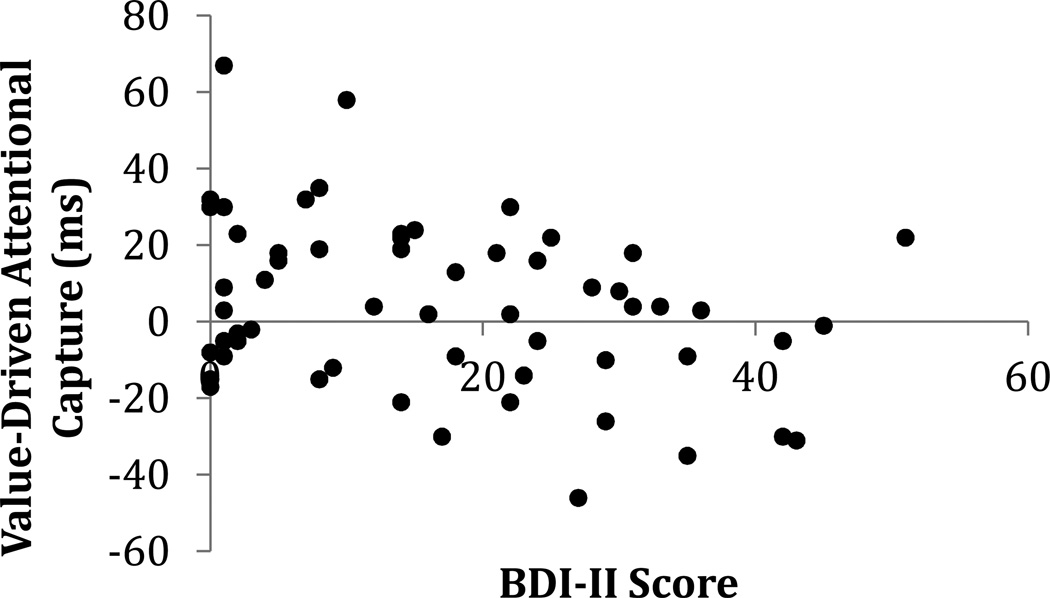
To more fully characterize the relationship between depressive symptoms and value-driven attentional capture, Figure 3 depicts value-driven capture across the range of BDI-II scores obtained in our sample. BDI-II score was significantly correlated with the magnitude of value-driven capture (r = −.311, p = .018). Qualitatively identical results were obtained using the anhedonic subscore of the BDI-II (Leventhal, Chasson, Tapia, Miller, & Pettit, 2006) instead of total BDI-II score (r = −.313, p = .017).
Figure 3.
Relationship between value-driven attentional capture (slowing of RT on high-value distractor compared to distractor absent trials) and BDI-II score across all participants (r = −.311, p = .018).
Value-driven attentional capture did not differ between male and female participants in either the depressed group or the control group [t's < 0.53, p's > .60, d's < .25]. Response time did not differ between depressed and control participants on distractor absent trials [t(56) = 0.36, p = .719, d = .09], indicating that depression was not associated with generally slower information processing in this task. An ANOVA on accuracy with distractor condition and depressed status as factors revealed no main effects or interaction [Table 2, F's < 1.09, p's > .34, η2p's < .02], and the interaction between depressed status and distractor condition for RT remains significant when accuracy is partialled out as a covariate [F(1,55) = 7.84, p = .007, η2p = .125].
Table 2.
Accuracy as a function of distractor condition in the test phase, separately for depressed and control participants. Standard deviations are in parentheses.
| Absent | Low-value | High-value | |
|---|---|---|---|
| Depressed | 87.3% (6.7) | 86.8% (7.8) | 86.2% (6.9) |
| Control | 85.8% (9.1) | 84.7% (10.6) | 84.8% (10.2) |
Discussion
Stimuli previously associated with reward have been consistently shown to involuntarily capture attention in healthy individuals (e.g., Anderson et al., 2011a, 2011b; Anderson & Yantis, 2012). The dependence of value-driven attentional capture on the ability to process rewards normally lacks direct experimental evidence, however, as a deficit in value-based attention has never been reported. Depression is associated with an abnormally blunted sensitivity to reward information (e.g., Foti & Hajcak, 2009; Henriques & Davidson, 2000; Shankman et al., 2007) and provides an opportunity to investigate this question. In the present study, we show that individuals experiencing depressive symptoms largely ignore previously high-value stimuli, suggesting that such stimuli are less attention-grabbing in depression. This sharp contrast to the pattern of performance observed in prior studies (e.g., Anderson et al., 2011a, 2011b; Anderson & Yantis, 2012) and replicated here in the control participants indicates that depression is accompanied by changes in how the attention system is shaped by reward information.
The present study provides the first evidence that the attention system of certain individuals can be largely unaffected by reward history. Even though the depressed participants in the present study performed the training phase accurately and were exposed to feedback concerning the receipt of external reward, this feedback did not produce any apparent attentional bias as compared to the robust attentional bias demonstrated in control participants. Our findings demonstrate that deficits in the experienced salience of reward information characteristic of depression impact how the attention system is shaped by those rewards. Given evidence that attentional capture by physically salient but otherwise neutral stimuli is actually elevated in depression (Esterman et al., 2013), it is unlikely that our findings can be explained by a general depression-related insensitivity to attentional capture and are instead specific to attentional capture by reward-associated stimuli.
On a continuous level, the severity of depressive symptoms (as measured using the BDI-II) was significantly negatively correlated with the magnitude of value-driven attentional capture. However, it is worth noting that value-driven attentional capture was very weak to absent across a range of higher BDI-II scores, resulting in a near-zero mean effect in the depressed group. This may simply reflect the nature of the relationship between the distractor and the target in our experimental task, which compete for selection: once the distractor has a lower attentional priority than the target, it can be ignored. Although value-based attentional priority may in fact vary continuously across the entire range of BDI-II scores tested, it will become undetectable in a visual distraction paradigm once it has fallen to a level sufficiently below the attentional priority of the target. As different objects constantly compete for attention in everyday life, the observed non-linear relationship between automatic attentional capture by previously high-value stimuli and depressive symptoms might generalize to other situations and contexts.
The mechanisms by which reward-related stimuli fail to acquire high attentional priority in individuals experiencing depressive symptoms poses an important question for future research. Depression presents with reduced sensitivity to reward information (e.g., Foti & Hajcak, 2009; Henriques & Davidson, 2000; Shankman et al., 2007). One possibility, then, is that the signals elicited by reward feedback in the present sample of depressed individuals were insufficient for learning of the stimulus–reward associations to occur. While participants in the present study were explicitly told that the money earned in the experiment was contingent on correctly identifying red and green targets, the relationship between these colors and monetary reward may have been only weakly represented and not maintained after completion of the training phase. Another possibility is that such stimulus–reward associations are sufficiently learned and represented in depression, but these associations fail to influence attention because reward information is given low priority in the determination of stimulus selection. Of course, both possibilities could be responsible for the deficit in value-driven attentional capture observed in the present study. It would be informative to test whether other effects of prior reward learning on cognition, such as choice preferences, are evident in the absence of value-driven attentional capture in depression.
Reduced automatic attention to previously reward-associated stimuli could play a role in the experience of symptoms of depression. By failing to orient to reward-associated stimuli, potentially enjoyable or otherwise beneficial opportunities may become less salient. This reduced salience could then, in turn, decrease the extent to which an individual pursues rewarding opportunities and thinks about rewarding outcomes, with implications for overall mood. Reduced attention to reward-related stimuli could represent a risk factor for the development of depressive symptoms, or it could reflect depression-related changes in how the brain processes information. To the extent that reduced automatic attention to reward-related stimuli follows the development of depressive symptoms, it could serve to facilitate the maintenance of a depressed state by biasing subsequent information processing. Participants in the present study were screened for the absence of other significant psychological conditions such as an anxiety disorder; however, it is important to note that the absence of value-driven attentional capture observed in the depressed participants might be at least partially accounted for by comorbidities with depression. It should also be noted that depressed participants in the present study were recruited on the basis of depressive symptoms outside of the normal range as defined by the BDI-II (Beck et al., 1996) and did not necessarily meet the diagnostic criteria for clinical depression; thus, caution is warranted in generalizing our findings to clinical samples.
Broadly, our findings support the idea that value-based attention and emotional state are interrelated. Recent evidence from primate neurophysiology demonstrates that the amygdala, which is known to play an important role in the processing of emotional content (e.g., LeDoux, 2003), also plays a role in representing the position and reward value of visual objects (Peck, Lau, & Salzman, 2013). By relating value-based attention to depression, our findings provide converging evidence that attention to reward and the regulation of emotional state are governed by overlapping cognitive and neural mechanisms.
The findings of the present study demonstrate a link between depressive symptoms and value-based attention. Compared to the typical pattern of attention allocation observed in previous studies and replicated in the present control sample, the attention system of depressed individuals exhibits an apparent hyposensitivity to the reward history of visual objects. Our findings provide further insight into how cognitive processes are affected in depression, and demonstrate that the ability to attribute value-based attentional priority to visual stimuli is related to impairments in ability to represent reward information. The latter provides direct evidence for a critical relationship between value-based attention and the brain systems involved in representing reward information, as predicted by a distinctly value-driven mechanism of attentional control (Anderson, 2013).
References
- Anderson BA. A value-driven mechanism of attentional selection. Journal of Vision. 2013;137(3):1–16. doi: 10.1167/13.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Faulkner ML, Rilee JJ, Yantis S, Marvel CL. Attentional bias for non-drug reward is magnified in addiction. Experimental and Clinical Psychopharmacology. 2013;21:499–506. doi: 10.1037/a0034575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PLoS ONE. 2011a;6(11):e27926. doi: 10.1371/journal.pone.0027926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences, USA. 2011b;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Generalization of value-based attentional priority. Visual Cognition. 2012;20:647–658. doi: 10.1080/13506285.2012.679711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Reward predictions bias attentional selection. Frontiers in Human Neuroscience. 2013;7:262. doi: 10.3389/fnhum.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Attention, Perception, and Psychophysics. 2012;74:1644–1653. doi: 10.3758/s13414-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Persistence of value-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance. 2013;39:6–9. doi: 10.1037/a0030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio, Texas: The Psychological Corporation; 1996. [Google Scholar]
- Cowen N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. The Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Visual selective attention and the effects of monetary reward. Psychological Science. 2006;17:222–227. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychological Science. 2009;20:778–784. doi: 10.1111/j.1467-9280.2009.02360.x. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Esterman M, DeGutis J, Mercado R, Rosenblatt A, Vasterling JJ, Milberg W, McGlinchey R. Stress-related psychological symptoms are associated with increased attentional capture by visually salient distractors. Journal of the International Neuropsychological Society. 2013;19:835–840. doi: 10.1017/S135561771300057X. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. Journal of Neuroscience. 2010;30:11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14:711–724. [Google Scholar]
- Kiss M, Driver J, Eimer M. Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychological Science. 2009;20:245–251. doi: 10.1111/j.1467-9280.2009.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- MacPhillamy DJ, Lewinsohn PM. Depression as a function of levels of desired and obtained pleasure. Journal of Abnormal Psychology. 1974;83:651–657. doi: 10.1037/h0037467. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Lau B, Salzman CD. The primate amygdala combines information about space and value. Nature Neuroscience. 2013;16:340–348. doi: 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Zeng Q, Ding C, Li H. Neural correlates of reward-driven attentional capture in visual search. Brain Research. 2013;1532:32–43. doi: 10.1016/j.brainres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Raymond JE, O'Brien JL. Selective visual attention and motivation: The consequences of value learning in an attentional blink task. Psychological Science. 2009;20:981–988. doi: 10.1111/j.1467-9280.2009.02391.x. [DOI] [PubMed] [Google Scholar]
- Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:85–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Belopolsky AV. Reward grabs the eye: oculomotor capture by rewarding stimuli. Vision Research. 2012;74:80–85. doi: 10.1016/j.visres.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu H, Zhou X. Interaction between value and perceptual salience in value-driven attentional capture. Journal of Vision. 2013;13(3):5, 1–13. doi: 10.1167/13.3.5. [DOI] [PubMed] [Google Scholar]