Abstract
After initial response to androgen receptor targeting drugs abiraterone or enzalutamide, most patients develop progressive disease and therefore, castration resistant prostate cancer (CRPC) remains a terminal disease. Multiple mechanisms underlying acquired resistance have been postulated. Intratumoral androgen synthesis may resume after abiraterone treatment. A point mutation in the ligand binding domain of androgen receptor may confer resistance to enzalutamide. Emergence of androgen receptor splice variants lacking the ligand binding domain may mediate resistance to abiraterone and enzalutamide. Steroid receptors such as glucocorticoid receptor may substitute for androgen receptor. Drugs with novel mechanisms of action or combination therapy, along with biomarkers for patient selection, may be needed to improve the therapy of CRPC.
Keywords: Prostate cancer, androgen receptor, abiraterone, enzalutamide, acquired drug resistance, androgen receptor splice variants, intratumoral androgen synthesis, glucocorticoid receptor
Introduction
In the United States and Europe, prostate cancer has the highest incidence of malignancy and is the second or third leading cause of cancer-related death in men [1,2]. Dr. Charles Huggins demonstrated that androgen deprivation therapy (ADT) through surgical castration led to dramatic palliation of symptoms of metastatic prostate cancer and this seminal discovery ushered a new era of treatment in the 1940's [3]. These treatment principles were furthered cemented by Dr. Andrew Schally, who discovered the structure of luteinizing hormone-releasing hormone (LH-RH) [4]. This work led to development of LH-RH agonists in the 1980's. These agents and newer LH-RH antagonists are still the mainstay of advanced prostate cancer treatment. Unfortunately, virtually all patients develop castrate resistant prostate cancer (CRPC) while on ADT. Metastatic CRPC (mCRPC) remains a terminal disease, and until recently, available treatment options of cytotoxic chemotherapy such as mitoxantrone or docetaxel. Mitoxantrone was approved in 1996 for its benefit in quality of life and bone pain without increasing survival. Doxetaxel was approved in 2004 based on two large randomized phase III trials. The TAX 327 study by Tannock et al demonstrated a 2.9 month survival benefit and improvement in pain for mCRPC patients treated with docetaxel every 3 weeks and prednisone versus mitoxantrone and prednisone in the final analysis [5]. Similarly, the SWOG-9916 trial by Petrylak et al showed a 1.9 month survival benefit for mCRPC patient treated with docetaxel and estramustine versus mitoxantrone and prednisone [6]. In 2010, sipuleucel-T and cabazitaxel were approved for mCRPC. For asymptomatic or minimally symptomatic mCRPC patients, sipuleucel-T (which consists of autologous peripheral-blood mononuclear cells activated ex vivo with a prostatic acid phosphatase-granulocyte macrophage colony stimulating factor recombinant fusion protein and subsequent infusion of the cells into the patient) was approved on the basis of a clinical trial demonstrating a 4.1 month survival advantage (25.8 months vs 21.7 months) [7]. The semi-synthetic taxane-derivative cabazitaxel was shown to prolong survival by 2.4 months compared to mitoxantrone (15.1 months vs 12.7 months) in mCRPC patients who had progressed after docetaxel treatment [8]. The most recently approved agent is the α-emitting radiopharmaceutical Radium-223 chloride for use in mCRPC patients with symptomatic bone metastases and no visceral metastasis [9].
Previously, the role of the androgen receptor (AR) in progression to CRPC was less well appreciated and hence, disease progressing on ADT was termed “androgen-independent”, and this generated controversies on the necessity of continuing LH-RH agents. However, the recent development of two novel AR targeting drugs, abiraterone acetate (an oral androgen biosynthesis inhibitor) and enzalutamide (an oral antagonist of androgen receptor) provided firm evidence that the AR signaling axis remains an important driver of CRPC tumor progression. Despite meaningful clinical benefit of these agents, most patients will eventually succumb to CRPC because of acquired resistance to these drugs. This review article will highlight the potential mechanisms of resistance to androgen receptor targeting drugs and their implications for continued drug development in prostate cancer.
Androgen receptor and prostate cancer
The human AR gene is located on chromosome Xq11-12. AR consists of an N-terminal transactivation domain (encoded in exon 1), a DNA binding domain (DBD) (exon 2-3), a hinge region (exon 4), and a C-terminal ligand-binding domain (exon 5-8)(Figure 1A)[10]. 5α-dihydrotestosterone (DHT), converted from testosterone by 5α-reductase, is the most potent ligand for AR. In the absence of ligand, AR is located in the cytoplasm in an inactive conformation bound by chaperone proteins such as heat shock proteins. Binding of androgen ligands to the ligand-binding domain of AR results in the translocation of AR from the cytoplasm to the nucleus. In the nucleus, AR binds androgen-response element DNA sequences located in the regulatory regions of its target genes, such as prostate specific antigen (PSA), and regulates their transcription. Prostate tumor cells are dependent on continued activation of AR for viability and proliferation. When gonadal testosterone production is inhibited by initiation of ADT and serum testosterone decreases to the castrate level, AR without ligand is no longer bound to the DNA and loses its transcriptional activity in tumor cells. ADT is initially effective in palliating cancer-related symptoms such as bone pain and is associated with tumor regression. However, efficacy of ADT is short-lived. Patients with metastatic prostate cancer treated with ADT develop progressive disease after an average of approximately 24 months. This stage of disease, termed CRPC, is initially marked by rising levels of prostate specific antigen (PSA) as a harbinger of worsening symptoms and eventual death. Extensive evidence now supports the principle that reactivation of AR signaling drives CRPC progression. Multiple mechanisms underlying continued activation of AR in CRPC tumors include AR gene amplification, increased AR expression, AR point mutations, expression of AR splice variants, and intratumoral production of androgen [11]. Overexpression of AR, frequently due to genomic amplification of the AR gene, enhances transcriptional activation of AR to low levels of androgen in the castrate host [12]. In addition, CRPC tumors were found to contain unexpectedly high levels of testosterone and DHT and overexpress enzymes involved in androgen biosynthetic pathway [13,14]. Cytochrome P450 17A1 (CYP17A1) is a key enzyme in androgen synthesis via its 17α-hydroxylase/C17, 20-lyase activity. CYP17A1 catalyzes the conversion of pregnenolone to 17-hydroxypregnenolone, then to the main androgen precursor dehydroepiandrosterone (DHEA). Although DHEA and its subsequent metabolite androstenedione are considered weak androgens, they play an important role in intratumoral synthesis of androgens in CRPC.
Figure 1.
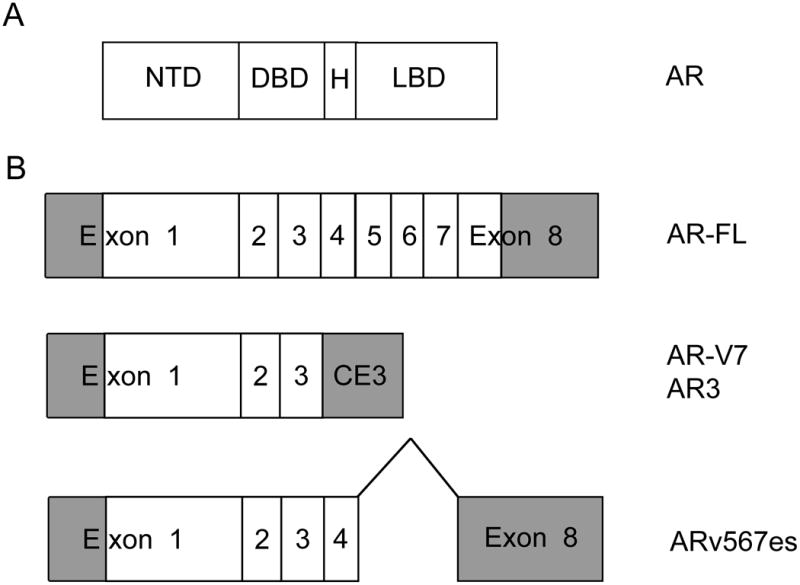
Schematic of androgen receptor and major splice variants. (A) Functional domains of full length androgen receptor protein. NTD=N-terminal transactivation domain, DBD=DNA binding domain, H=hinge region, LBD=ligand binding domain. (B) Organization of the mRNA species encoding androgen receptor and the major splice variants. Exons 1-8 or cryptic exon 3 contained in the transcript are indicated. Shaded areas represent untranslated regions. FL=full length, CE3=cryptic exon 3, V7=variant 7, v567es=variant 5, 6, 7 exons skipped. Data for AR-V7 is from ref. [36]. Data for AR3 is from ref. [35]. Data for ARv567es is from ref. [37].
Clinical efficacy of new-generation AR targeting drugs
Abiraterone acetate
Abiraterone is a potent and specific inhibitor of CYP17A1 [15]. Inhibition of CYP17A1 should decrease adrenal and intratumoral androgen production and thereby reduce the availability of androgen ligands for AR in tumor cells. Based on the results of a phase III clinical trial, FDA approved abiraterone plus prednisone in April, 2011, for treatment of CRPC patients who received prior docetaxel chemotherapy. Prednisone is added to abiraterone in an attempt to minimize the production of the excess mineralicorticoid hormones that may result from CYP17 inhibition. The COU-AA-301 trial randomized 1,195 CRPC patients in a 2:1 ratio to either 1,000 mg of abiraterone plus prednisone 5 mg twice daily or placebo plus prednisone [16]. After a median follow-up of 12.8 months, the abiraterone plus prednisone group had a longer OS of 14.8 months vs 10.9 months for placebo (hazard ratio (HR) of 0.65). The abiraterone plus prednisone group was also favored for secondary outcome measures, including time to PSA progression (10.2 vs 6.6 months), progression free survival (5.6 months vs 3.6 months), and PSA response rate (29% vs. 6%). Adverse events that were associated with higher mineralocorticoid levels due to CYP17 blockage such as fluid retention, edema, hypokalemia, and hypertension were more common in the abiraterone plus prednisone group (55% vs 43%). Final analysis of the study confirmed the OS benefit for the abiraterone plus prednisone group (15.8 months vs 11.2 months; HR of 0.74) [17]. Results of COU-AA-301 provided an fundamentally important insight by demonstrating that AR targeting is an effective strategy in the most advanced stage of prostate cancer that progressed after chemotherapy.
The COU-AA-302 phase III randomized trial focused treatment of mCRPC in patients who have not yet received docetaxel chemotherapy. In this study, 1,088 patients were randomized to receive abiraterone plus prednisone or placebo plus prednisone with the two primary endpoints: OS and radiographic PFS. With a median follow-up of 22.2 months, there was a strong trend toward improved OS with abiraterone (median not reached vs 27.2 months for placebo plus prednisone; HR, 0.75) as well as improvement in radiographic PFS (16.5 months for abiraterone vs. 8.3 months with placebo; HR, 0.53) [18]. Pre-specified secondary and exploratory efficacy endpoints, including risk of decline in performance-status (12.3 vs 10.9 months; HR, 0.82), median time to the initiation of cytotoxic chemotherapy (25.2 months vs. 16.8 months; HR, 0.58), a significant delay in the time to opiate use for cancer-related pain (not reached vs. 23.7 months; HR, 0.69) and median time to PSA progression (11.1 months vs. 5.6 months; HR 0.49,) favored the abiraterone group. Based on these results, abiraterone plus prednisone received FDA approval in December 2012 for treatment of mCRPC in the pre-chemotherapy setting. In addition, Basch et al reported that in this trial, the abiraterone group had a delay in patient-reported pain progression (26.7 months vs 18.4 months; HR, 0.82) and health-related quality of life deterioration (12.7 months vs 8.3 months; HR, 0.78) [19]. Abiraterone has become widely accepted as a treatment for CRPC with a favorable efficacy/toxicity profile.
Enzalutamide
First-generation antiandrogens such as flutamide and bicalutamide are antagonists of AR binding to ligands and inhibit AR activity. However, their clinical activity as a treatment of CRPC is modest and transient. At the molecular level, first-generation antiandrogens may act to stimulate AR activity in CRPC tumor cells that overexpress AR rather than antagonize AR [12]. Enzalutamide (also known as MDV3100) was designed to be a more potent antagonist of AR without agonist effects in tumor cells that overexpress AR [20]. Scher et al studied enzalutamide in a phase III placebo-controlled trial called AFFIRM [21]. 1,199 men with mCRPC progressing after chemotherapy were randomly assigned in a 2:1 ratio to receive oral enzalutamide at a dose of 160 mg per day or placebo. The primary endpoint was OS. The enzalutamide group showed a median OS of 18.4 months versus 13.6 months in the placebo group (HR, 0.63). Enzalutamide was favored over placebo with other secondary end points, including the PSA response rate (54 vs. 2%), time to PSA progression (8.3 months vs. 3 months; HR, 0.25), radiographic PFS (8.3 months vs. 2.9 months; HR, 0.40), and time to the first skeletal-related event (16.7 months vs. 13.3 months; HR). Side-effects including fatigue, diarrhea, hot flashes, and rarely seizures (0.6%) were noted in the enzalutamide groups. Based on the results of this trial, enzalutamide received FDA approval in August, 2012 as a second line treatment for mCRPC after docetaxel-based chemotherapy. Results from a phase III trial called the PREVAIL study (NCT01212991) that investigated the efficacy of enzalutamide in chemotherapy-naïve patients with mCRPC were recently reported in an abstract [22]. After randomizing 1,717 men, an interim analysis after 539 deaths demonstrated that in the enzalutamide group, there was a 30% reduction in the risk of death with the median OS of 32.4 months vs 30.2 months in the placebo group. For the other co-primary endpoint, the enzalutamide group also showed an 81% reduction in risk of radiographic progression or death. These studies demonstrating clinical efficacy of enzalutamide further highlight the pivotal role AR-signaling pathway plays in CRPC.
Potential Mechanisms of Acquired Drug Resistance
Abiraterone and enzalutamide represent a paradigm shift in our approach to CRPC treatment by refocusing on AR as the critical therapeutic target in CRPC. However, most patients treated with these agents will die from their disease and prolongation of survival by only a few months in the post-chemotherapy setting represents a modest improvement. Understanding the mechanisms involved in progression of tumor after initially responding to drug treatment is necessary for improvement in therapy outcome. Studies of preclinical models as well as analysis of primary tumor specimens have yielded valuable insights into the tumor biology of CRPC that led to development of new agents and will likely be important in characterizing the nature of acquired resistance to novel AR targeting agents. In clinical trials of abiraterone and enzalutamide, progression of CRPC is accompanied by the rise in PSA in most patients and this observation suggests that mechanisms of acquired resistance to these agents may predominantly involve reactivation of androgen signaling pathways since PSA expression is driven by AR. Potential mechanisms derived from preclinical studies and emerging from clinical specimens will be reviewed (Table 1).
Table 1. Proposed mechanisms of acquired resistance to novel agents.
Intratumoral androgen synthesis
Abiraterone treatment results in marked reduction in testosterone and DHT levels in blood and tumor-infiltrated bone marrow aspirates of CRPC patients [23]. This finding supports the concept that the clinical efficacy of abiraterone derives from inhibition of intratumoral production of androgen. In CRPC xenograft tumors, abiraterone treatment reduced testosterone and DHT levels in tumor tissues [24]. CYP17 inhibition (by abiraterone or by ketoconazole, a less potent inhibitor) leads to increased expression of CYP17A1 transcripts and other transcripts encoding enzymes involved in steroid synthesis in tumor cells [24,25]. These results suggest that CYP17 inhibition may select for tumor cells that reactivate the androgen synthesis pathway and thereby become resistant to abiraterone. However, in one of two xenograft models, androgen levels in abiraterone-resistant tumors remained suppressed [24] and this suggests alternate mechanisms of resistance to abiraterone, as detailed in subsequent sections. There may be multiple pathways for CRPC tumors to produce DHT. 3β-hydroxysteroid dehydrogenase 1 (3βHSD1) is involved in synthesis of DHT from adrenal androgen DHEA and may allow increased accumulation of DHT in tumor tissue in a synthetic pathway that bypasses testosterone. A gain-of-function point mutation in 3βHSD1 was found to be associated with CRPC [26]. Development of abiraterone resistance in xenograft tumors was associated with acquisition of this mutation. This suggests the possibility that CRPC tumors may synthesize DHT through alternate pathways in the setting of continuing CYP17 inhibition by abiraterone. Several plausible alterations in intratumoral steroid metabolism that may lead to abiraterone resistance have been suggested by preclinical models. More extensive studies of tumor specimens collected from patients treated with abiraterone will be required to determine if any of proposed mechanisms contributes to clinical resistance to abiraterone.
AR structural alterations: point mutations
One of the most frequent genomic alterations in CRPC is high level gene amplification of AR found in ∼30% of CRPC tumors[27]. AR gene amplification is rarely found in tumors prior to ADT. Increased AR expression resulting from high copy numbers of the AR gene leads to constitutive activation of AR in the castrate environment and resistance to first-generation antiandrogens such as bicalutamide[12]. The AR gene may acquire a gain-of-function point mutation in prostate tumor cells [28], as opposed to loss of function mutations associated with congenital androgen insensitivity syndrome. The AR point mutation usually affects the ligand binding domain and broadens the specificity of ligands that are capable of activating the receptor. Mutant AR may respond to adrenal androgens and steroid hormones such as corticosteroids, progesterone, estrogens, and even antiandrogens as agonists. 10-20% of CRPC tumors may have a mutation in AR and it is most frequently found in CRPC tumors exposed to ADT and antiandrogen for a prolonged period [29]. This data suggests that AR mutations develop under a strong selective pressure of antiandrogen use. Since enzalutamide acts as a competitive inhibitor of binding between AR and DHT [20], one may postulate that a point mutation in the critical residue on the AR protein that mediates binding to enzalutamide would confer resistance to enzalutamide. Balbas et al performed mutagenesis screen for AR resistant to enzalutamide and found the AR mutation in which phenylalanine at amino acid 876, located in the ligand binding domain, is substituted with leucine (referred to as F876L mutation) [30]. This F876L mutation converted enzalutamide into an agonist and expression of the AR F876L mutant in prostate cancer cells led to resistance to growth inhibition by enzalutamide. This mutant also conferred resistance to ARN-509, a novel potent AR antagonist in clinical development [31]. Two independent studies also identified the F876L mutation by deriving prostate cancer cell lines that became resistant to enzalutamide or ARN-509 [32,33]. The AR F876L mutant encoding DNA (presumably associated with circulating tumor cells shed from prostate cancer) was detected in the plasma of patients with progressive disease on ARN-509 treatment, but not in pre-treatment specimens [32]. This finding is consistent with the hypothesis that acquired resistance to enzalutamide or ARN-509 is mediated by emergence of AR F876L mutation. Point mutations in many codons in the ligand binding domain of AR are associated with development of resistance to first-generation antiandrogens[29]. It is unclear presently whether there are additional residues in AR protein that could be mutated to confer resistance to second-generation antiandrogens. If the F876L mutation is the only target of mutation for resistance, the development of mutation may be expected to be less frequent than it would be the case if multiple residues could be altered for resistance. Also, the spectrum of mutations leading to resistance would have implications for designing new generations of antiandrogens and strategies for overcoming or preventing resistance.
AR structural alterations: splice variants lacking the ligand binding domain
Several groups independently characterized the occurrence of splice variants of AR initially in prostate cancer cell lines and xenograft tumors and in clinical specimens [34-37]. More than 11 isoforms of AR have been characterized [38] and most of splice variants share the common structural motifs of the N-terminal transactivation domain and the central DNA binding domain. However, these truncated AR splice variants lack the ligand binding domain. AR splice variant AR-V7 (also named AR3) contains exon 1 (encoding the N-terminal transactivation domain), exon 2-3 (encoding the DNA binding domain), and a terminal cryptic exon (Figure 1B)[35,36]. Other AR splice variants typically contain exon 1-3, but differ in the terminal cryptic exons [38]. Another major variant, ARv567es (exons skipped), contains exons 1–4 and because of a frame-shift from loss of exons 5–7, exon 8 has a stop codon generated resulting in truncated AR protein lacking the ligand binding domain[37]. Investigators, by using a PCR assay for the mRNA or an antibody directed against the unique C-terminal peptide of the AR-V7/AR3, demonstrated that expression of AR variants in clinical specimens increased with progression to CRPC and increased expression correlated with the risk of recurrence of cancer after prostatectomy or shortened survival of CRPC patients [35,36,39]. AR splice variants, since they do not contain the ligand-binding domain, are constitutively active without the need for ligands. They are constitutively localized to the nucleus and activate AR target gene expression in the androgen depleted environment [40,41]. The AR splice variants induce a distinct set of genes associated with the cell cycle while full length AR activation is associated with genes involved in biosynthesis, metabolism and differentiation [41]. Truncated AR splice variant proteins interact with and activates full length AR protein in an androgen-independent manner [37,42]. AR splice variant proteins also interact with other transcription factors and coactivators such as FOXO1, NF-κB2/p52, and Vav3, and these interactions regulate the functional activity of AR splice variants through multiple signaling pathways [43-45]. In preclinical models, expression of AR splice variants promotes castration resistant growth of xenograft tumors [35]. Since the activity of AR splice variants is not expected to be inhibited by abiraterone and enzalutamide, one may hypothesize that treatment with abiraterone or enzalutamide would select for tumor cells that express AR splice variants and thereby reactivate AR target genes and resume growth. In xenograft models, treatment with abiraterone and enzalutamide induced the expression of AR variants AR-V7 and ARv567es [24,41]. Mechanisms underlying a shift to expression of AR splice variants were suggested by the finding of genomic rearrangement (focal deletion or duplication) within the AR gene locus that occurs in cell lines and in primary CPRC tumors [46-48]. In the heterogeneous cell population with cells containing intact or rearranged AR gene locus, androgen deprivation selected for clones containing AR gene rearrangement and increased expression of truncated AR variants [46]. Cells with the rearranged AR gene locus were resistant to enzalutamide [47]. In many tumor types, gene amplification and rearrangement occur together, presumably due to common underlying mechanisms. Therefore, the potential hypothesis is that in prostate cancer cells, gene amplification and rearrangement in the AR locus may be selected during tumor evolution as a result of selective pressure put on by androgen deprivation, then antiandrogen treatment. Emergence of truncated AR splice variants will allow continued growth of prostate cancer cells that require expression of AR target genes. Recent characterization of the prostate cancer genome demonstrated that there are extensive genomic rearrangement and translocations in a process termed “chromoplexy” [49]. However, expression of AR splice variants from genomic rearrangement has not been confirmed in clinical specimens yet and the extent to which AR splice variants and AR gene rearrangement contribute to clinical resistance to abiraterone and enzalutamide remains to be characterized.
Glucocorticoid receptor that may bypass AR
New data suggests that glucocorticoid receptor (GR)-mediated expression of a subset of AR target genes in the presence of AR inhibition by potent antiandrogen therapy is an alternate mechanism of resistance [50]. Gene expression analysis of harvested tumors resistant to antiandrogen treatment demonstrated high GR expression. Moreover, cells derived from enzalutamide-resistant xenografts require GR expression for enzalutamide-resistant tumor growth. Furthermore, GR has the ability to drive expression of a subset of AR target genes in enzalutamide-resistant cell lines. Elevated expression of GR in clinical specimens of mCRPC predicted for poor response to enzalutamide. Treatment of cells with dexamethasone (GR agonist) induced resistance to enzalutamide. Thus, the loss or inhibition of AR transcriptional activity could be bypassed by the expression of other steroid receptors that may activate a subset of AR target genes required for growth. These data raises a possibility that improving outcome of antiandrogen treatment may require combined inhibition of AR and GR. Indeed, the post-hoc analysis of the phase III AFFIRM trial of enzalutamide showing that patients with concomitant use of corticosteroids had worse outcome with enzalutamide is consistent with this hypothesis that GR may bypass the AR inhibition to drive prostate cancer progression, but confounding variables preclude definitive conclusions [51].
In summary, multiple independent mechanisms have been proposed after studies of preclinical models and there is emerging evidence supporting these mechanisms from clinical studies. However, the relative importance of each mechanism remains unclear at this time.
Novel AR targeting drugs in clinical development
Novel AR antagonists
Similar to enzalutamide, ARN-509 is a competitive AR inhibitor that fully antagonizes overexpressed AR without agonist activity. ARN-509 exhibited greater activity in preclinical models than bicalutamide and enzalutamide [31]. Rathkopf et al examined thirty patients with progressive mCRPC who received continuous oral doses between 30 mg and 480 mg daily and were monitored by positron emission tomography/computed tomography (PET/CT) imaging for binding of [(18)F] fluoro-α -dihydrotestosterone (FDHT) to tumors before and during treatment [52]. PSA declines greater than 50% at 12 weeks was observed in 46.7% of patients. Reduction in FDHT uptake was observed at all doses with a plateau at 120 mg or higher. Adverse events observed most frequently was fatigue (47%). Based on this overall safety of this study, phase II clinical trials using ARN-509 at 240 mg daily are in progress (NCT01171898). In addition, ARN-509 is being investigated in combination with abiraterone plus prednisone through a phase Ib study in mCRPC patients (NCT01792687). ODM-201 is another AR antagonist in early phase clinical development [53,54].
Novel CYP17 inhibitors
Similar to abiraterone, both galeterone (TOK-001, VN/124-1) and orteronel (TAK-700) are novel CYP17 inhibitors in clinical development for treatment of CRPC [55,56]. Galeterone disrupts the AR signaling in three ways: 1) Selec tivelyinhibiting C17, 20 lyase, 2) competitively antagonizing androgen binding to AR, and 3) degrading the AR protein itself [57]. When compared to both abiraterone and orteronel, galeterone was the most potent CYP17 inhibitor with minimal effects on 17α-hydroxylase products, which are responsible for decrease in cortisol and negative feedback increase in ACTH, resulting in mineralcorticoid excess symptoms such as hypokalemia, hypertension, and edema seen with abiraterone. Galeterone would therefore not require concomitant prednisone administration. Currently, a phase II trial evaluating the safety and efficacy of galeterone in CRPC patients is underway (NCT01709734). Orteronel was less potent than galeterone or abiraterone, but more selective for CYP17A1 lyase inhibition with reduced hydroxylase products [58]. Several studies with orteronel are underway or have been completed. The phase III trial of post-docetaxel mCRPC patients assigned to orteronel plus prednisone vs placebo plus prednisone (NCT01193257) was recently reported as failing to meet its primary endpoint of prolonging overall survival (17.0 months vs 15.2 months; HR of 0.886; p=0.1898) [59]. A similarly designed phase III randomized trial with orteronel in chemotherapy-naïve mCRPC patients (NCT01193244) is awaiting completion. A phase II trial comparing orteronel vs bicalutamide in patients with metastatic prostate cancer who failed first line treatment with LH-RH agonists (NCT01658527) is currently under way. A phase III randomized trial to compare ADT plus orteronel 300 mg twice daily versus ADT plus bicalutamide 50 mg daily in patients with newly diagnosed metastatic hormone sensitive prostate cancer (NCT01809691) is also ongoing.
Potential strategies for overcoming acquired resistance
Novel inhibitor of AR transactivation domain
Anderson et al identified EPI-001, a small molecule agent that blocks transactivation of the AR N-terminal domain and demonstrated that EPI-001 inhibited growth of CRPC xenograft tumors in animals [60]. EPI-001 and its structural analogs inhibit the AR transactivation by covalently binding to the N-terminal domain. EPI analogs blocked growth of CRPC xenograft tumors driven by AR splice variants lacking the ligand binding domain [61]. These preclinical data highlights the potential for novel compounds that target the AR function through mechanisms distinct from abiraterone and enzalutamide and thereby overcome resistance to these agents. Future clinical trials that target the AR N-terminal domain are warranted.
Combination therapy
The current practice of sequential monotherapy with targeted agents in diseases such as chronic myelogenous leukemia may lead to acquisition of multidrug resistance and therefore, combination therapy may delay disease progression. and improve clinical outcome [62]. Similarly, combination therapy with abiraterone and enzalutamide may suppress emergence of acquired resistance. A phase 3 trial is being planned to compare the overall survival of mCRPC patients treated with enzalutamide alone vs enzalutamide and abiraterone (NCT01949337). Other approaches may include adding agents with a non-cross resistant mechanism, such as combing AR targeted agents with Radium-223 or cabazatinib (an inhibitor of c-Met and vascular endothelial growth factor receptor 2 tyrosine kinases [63]).
Conclusion
The approval of abiraterone and enzalutamide for treatment of CRPC represents a substantial progress. However, patients with CRPC still succumb to their disease within a few years because of acquired resistance to these agents. Preclinical and clinical data suggest that there are several potential mechanisms involved in development of resistance, such as intratumoral androgen synthesis, structural alterations in AR (i.e. point mutations and splice variants lacking the ligand binding domain), and other steroid hormone receptors that may bypass AR. The relative contribution of these mechanisms is unknown at present. Future drug development will need to address these resistance mechanisms in order to improve the clinical efficacy of novel agents in CRPC patients who have already been treated with abiraterone or enzalutamide.
Expert Commentary
Since 2010, five agents that have demonstrated an increased survival in phase III trials (sipuleucel-T, cabazitaxel, abiraterone, enzalutamide, and Radium-223) have become available to clinicians for treatment of patients with mCRPC. The pace of progress has been unprecedented and these advances are fueled by improved understanding of tumor biology and signaling mechanisms. Before these agents, docetaxel chemotherapy represented the only agent known to increase survival and patients progressing after docetaxel had few treatment options and faced poor prognosis with survival less than 12 months. Therefore, drug development efforts and clinical trials initially focused on patients who already received docetaxel, in part due to the fact that survival endpoints are feasible with a relatively short follow up. In this setting, cabazitaxel, abiraterone, enzalutamide, and Radium-223 received US FDA approval for treatment of mCRPC. However, proliferation of active available agents raises difficult questions. Is there an optimal agent for an individual patient? Is there an optimal sequence of treatment? The patient's co-morbidities (such as heart failure or history of seizures) and preference would make an impact in some cases, but in many cases, there is no currently available evidence or biomarkers to help clinicians and patients make an informed choice. Because of the favorable toxicity profile and oral dosing, abiraterone and enzalutamide are appealing both in the post- and pre-chemotherapy settings. There is now emerging evidence for cross-resistance between abiraterone and enzalutamide. In patients who have previously taken abiraterone, enzalutamide treatment is only modestly active, with a lower PSA response rate and a shorter duration of response, than expected for those who have not received abiraterone [64,65]. In patients who have been exposed to enzalutamide, abiraterone is less active than in those who never took enzalutamide [66,67]. These results suggest common mechanisms of resistance to both of these agents, as discussed above. For example, emergence of AR splice variants without the ligand binding domain will lead to reactivation of AR signaling after abiraterone and enzalutamide treatment. AR point mutation may confer resistance to one or both drugs. Novel drugs in development may be able to address mechanisms of acquired drug resistance. In chronic myelogenous leukemia, the disease that ushered the era of targeted therapy in clinical oncology, more potent kinase inhibitors introduced after imatinib, the first Bcr-Abl kinase inhibitor, appear to produce more durable remission although resistance may eventually develop. It is not yet clear that novel agents such as ARN-509 or galeterone will be more effective than abiraterone or enzalutamide. Further studies will be necessary. Combination therapy with both abiraterone and enzalutamide has the potential to improve the clinical efficacy relative to monotherapy. Novel drugs that inhibit the N-terminal transactivation of AR may inhibit tumors driven by AR splice variants that mediate resistance to abiraterone and enzalutamide. However, if glucorticoid receptor or another steroid hormone receptor or more broadly other pathways drive tumor escaping from complete AR inhibition, these mechanisms of resistance will likely require non-AR targeting drugs. Currently, biomarkers that distinguish between these alternative mechanisms are lacking.
Five-year view
Over next five years, clinical studies of next generation AR antagonists such as ARN-509 and ODM-201 or novel CYP17 inhibitors such as galeterone and orteronel will be completed in an attempt to demonstrate clinical efficacy of these agents in a defined clinical setting (i.e. nonmetastatic CRPC or post-chemotherapy mCRPC). However, the survival endpoint, the “gold standard” of clinical efficacy in oncology, will be increasingly difficult to demonstrate, in part due to availability of multiple agents and increasing survival stemming from stage migration in which CRPC patients are treated earlier in the course of the disease. Given the fact that tumor cells may overcome drugs such as abiraterone and enzalutamide targeting the ligand bind domain of AR through several distinct mechanisms, it remains to be seen whether drugs currently in development with mechanisms similar to available agents (i.e. CYP17 inhibitors or AR antagonists) represent substantial improvement in treatment of mCRPC patients. It is likely that drugs targeting the N-terminal transactivation domain of AR will be developed. Clinical trials will explore the optimal sequencing and combinations of AR targeted agents. Biomarkers that may predict for response to novel therapy will likely contribute greatly to drug development. For example, detection of specific AR point mutants, analogous to Bcr-Abl mutants in chronic myelogenous leukemia, may help in selection of AR antagonists. Expression of AR splice variants or glucocorticoid receptor in tumor may be associated with resistance to certain agents or sensitivity to other agents. High throughput sequencing technology may be another avenue of biomarker development. It is likely that prognosis of patients with CRPC will continue to improve in the near future.
Key Issues.
The recent approval of abiraterone (androgen synthesis inhibitor) and enzalutamide (androgen receptor antagonist), based on randomized clinical trials showing increased survival in pre-chemotherapy and post-chemotherapy mCRPC patients, establishes androgen receptor as an important therapeutic target in CRPC.
After initial response, most patients develop progressive disease, with a rising PSA, the androgen receptor target gene. In many patients, there may be cross resistance to abiraterone and enzalutamide. Understanding mechanisms of resistance is necessary for developing better treatments.
Resistance to abiraterone may include reactivation of intratumoral androgen synthesis through increased expression of CYP17 or other enzymes involved in androgen synthesis.
A point mutation F867L in the ligand binding domain of androgen receptor confers resistance to second-generation antiandrogens enzalutamide and ARN-509.
Emergence of androgen receptor splice variants lacking the ligand binding domain may mediate resistance to abiraterone or enzalutamide. Novel agents such as EPI-001 targeting the N-terminal transactivation domain of androgen receptor may be effective in inhibiting splice variants.
Glucocorticoid receptor in tumor cells may bypass the need for androgen receptor by activating some androgen target genes.
Combination therapy with existing or novel drugs may delay development of resistance.
Acknowledgments
Financial disclosures: This work was supported by grants from NIH T32CA009688 (DC) and T32CA071341 (DD).
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 4.Schally AV, Arimura A, Kastin AJ, et al. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173(4001):1036–1038. doi: 10.1126/science.173.4001.1036. [DOI] [PubMed] [Google Scholar]
- 5.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 10.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20(13):3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 12.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 13.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 14.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 15.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69(12):4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 18.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basch E, Autio K, Ryan CJ, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013;14(12):1193–1199. doi: 10.1016/S1470-2045(13)70424-8. [DOI] [PubMed] [Google Scholar]
- 20.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 22.Beer TM, Armstrong AJ, Sternberg CN, et al. Enzalutamide in men with chemotherapy-naive metastatic prostate cancer (mCRPC): Results of phase III PREVAIL study. J Clin Oncol. 2014;32(4_suppl) LBA1. [Google Scholar]
- 23.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30(6):637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17(18):5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71(20):6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314–319. [PubMed] [Google Scholar]
- 28.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91(3):483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 29.Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21(14):2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 30*.Balbas MD, Evans MJ, Hosfield DJ, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2:e00499. doi: 10.7554/eLife.00499. This article used the mutagenesis screen to discover the point mutation that confers resistance to second-generation antiandrogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 33*.Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3(9):1030–1043. doi: 10.1158/2159-8290.CD-13-0142. Above two articles demonstrate that the point mutation in the androgen receptor ligand binding domain confers resistance to second-generation antiandrogens. [DOI] [PubMed] [Google Scholar]
- 34.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18(5):R183–196. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6(4):e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan SC, Li Y, Dehm SM. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J Biol Chem. 2012;287(23):19736–19749. doi: 10.1074/jbc.M112.352930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72(14):3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao B, Qi Y, Zhang G, et al. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget. 2014 doi: 10.18632/oncotarget.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohrer LR, Liu P, Zhong J, et al. FOXO1 binds to the TAU5 motif and inhibits constitutively active androgen receptor splice variants. Prostate. 2013;73(10):1017–1027. doi: 10.1002/pros.22649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nadiminty N, Tummala R, Liu C, et al. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12(8):1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peacock SO, Fahrenholtz CD, Burnstein KL. Vav3 enhances androgen receptor splice variant activity and is critical for castration-resistant prostate cancer growth and survival. Mol Endocrinol. 2012;26(12):1967–1979. doi: 10.1210/me.2012-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011;71(6):2108–2117. doi: 10.1158/0008-5472.CAN-10-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–489. doi: 10.1158/0008-5472.CAN-12-3630. This article uses preclinical models to support the concept that androgen receptor splice variants may mediate resistance to enzalutamide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Hwang TH, Oseth LA, et al. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31(45):4759–4767. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309–1322. doi: 10.1016/j.cell.2013.11.012. This article demonstrates that another steroid hormone receptor glucocorticoid receptor may bypass the need for androgen receptor by activating androgen target gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scher HI, Fizazi K, Saad F, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide (ENZA), an androgen receptor inhibitor. J Clin Oncol. 2013;31(6_suppl):6. [Google Scholar]
- 52.Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31(28):3525–3530. doi: 10.1200/JCO.2013.50.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fizazi K, Massard C, James ND, et al. ODM-201, a new generation androgen receptor inhibitor for castration-resistant prostate cancer: Preclinical and phase I data. J Clin Oncol. 2013;31(6_suppl):65. [Google Scholar]
- 54.Massard C, Tammela TLJ, Vjaters E, et al. A study of two ODM-201 formulations with a safety and tolerability extension phase in patients with metastatic chemotherapy-naive castration-resistant prostate cancer (CRPC) J Clin Oncol. 2014;32(4_suppl):115. [Google Scholar]
- 55.Vasaitis TS, Bruno RD, Njar VC. CYP17 inhibitors for prostate cancer therapy. J Steroid Biochem Mol Biol. 2011;125(1-2):23–31. doi: 10.1016/j.jsbmb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin L, Hu Q. CYP17 inhibitors--abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol. 2014;11(1):32–42. doi: 10.1038/nrurol.2013.274. [DOI] [PubMed] [Google Scholar]
- 57.Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7(8):2348–2357. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacoby DB, Williams M. Differential effects of galeterone, abiraterone, orteronel, and ketoconazole on CYP17 and steroidogenesis. J Clin Oncol. 2013;31(6_suppl):184. [Google Scholar]
- 59.Dreicer R, Jones R, Oudard S, et al. Results from a phase 3, randomized, double-blind, multicenter, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel-based therapy (ELM-PC 5 trial) J Clin Oncol. 2014;32(4_suppl):7. doi: 10.1200/JCO.2014.56.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 61*.Myung JK, Banuelos CA, Fernandez JG, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123(7):2948–2960. doi: 10.1172/JCI66398. Two articles above establish novel mechanisms of action for the agent EPI-001 that targets the N-terminal transctivation domain of androgen receptor and can inhibit androgen receptor splice variants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawyers CL. Perspective: combined forces. Nature. 2013;498(7455):S7. doi: 10.1038/498S7a. [DOI] [PubMed] [Google Scholar]
- 63.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120(7):968–975. doi: 10.1002/cncr.28518. [DOI] [PubMed] [Google Scholar]
- 65.Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65(1):30–36. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 66.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24(7):1807–1812. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 67.Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24(7):1802–1807. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]


