Abstract
Patients with relapsed chronic lymphocytic leukemia (CLL) often achieve response with chemoimmunotherapy but have short remission durations. Studies have shown that patients with CLL have increased angiogenesis in the microenvironment; levels of pro-angiogenic growth factors such as VEGF and/or angiopoietin-2 (Ang-2) are also elevated. Increased angiogenesis correlates with poor outcome in CLL. Bevacizumab (B) is a humanized monoclonal antibody targeting VEGF-A. In this study, we analysed whether a combination of bevacizumab (B) with FCR chemoimmunotherapy (FCR-B) could improve outcomes in patients with relapsed CLL. Sixty-two patients were enrolled. The median age of the patients was 60 years (range, 31–84 years) and 40% had received >1 prior therapy for CLL. Sixty-one patients were evaluable for toxicity and 57 were evaluable for response. Six cycles were planned; 36 (59%) patients completed ≥ 4–6 cycles of the regimen. The overall response rate (ORR) was 79% with 13 (23%) complete remissions (CR), 8 (14%) nodular partial remissions (nPR) and 24 (43%) partial remissions (PR). The median progression free survival (PFS) and overall survival (OS) rates were 13.5 and 45 months, respectively. Grade 3 or 4 toxicities included febrile neutropenia (n=40), infections (n=21), thrombocytopenia (n=18) and anemia (n=9). Results with FCR-B were similar to those observed with an historical cohort of relapsed patients treated with FCR.
Introduction
Chronic Lymphocytic Leukemia (CLL) is a clonal B cell malignancy with a heterogeneous disease course. Chemoimmunotherapy is frequently used as frontline therapy in patients requiring treatment and produces high response rates and durable remissions. However, in relapsed patients subsequent response to chemotherapy-based regimens is less and response duration is significantly shorter.1,2 Advances in the understanding of the CLL microenvironment, cell signaling, and molecular biology have helped in identifying novel therapeutic targets.3, 4 Angiogenesis is one of the main mechanisms promoting the growth and metastasis of cancer cells.5, 6 Studies have demonstrated evidence of active angiogenesis, elevated angiogenic factors such as VEGF (vascular endothelial growth factor), angiopoietin-2 (Ang-2)7, overexpression of VEGF receptors and increased bone marrow microvessel density (MVD) in CLL.8–13 Interaction of microvascular endothelial cells with CLL cells promotes the proliferation of CLL cells in the lymph nodes and the bone marrow14, enhances their survival by NF-kappaB activation and protects CLL cells from fludarabine-induced apoptosis.15, 16 Furthermore, an increased degree of angiogenesis as documented by high bone marrow microvessel density and elevated levels of angiogenic factors in the blood is associated with advanced clinical stage, shorter time to first treatment (TTFT) and shorter progression free survival (PFS) in patients with CLL.17, 18 CLL cells express VEGF receptors and they can also produce VEGF under the influence of microenvironmental factors; in turn, CLL cells can promote endothelial cell proliferation.19–21 In experiments where CLL cells were cultured with exogenous VEGF, VEGF was shown to promote the levels of antiapoptotic proteins such as MCL-1.22
Bevacizumab is a recombinant humanized monoclonal antibody (mAb) targeting VEGF. Clinical trials in patients with metastatic solid tumors have shown that addition of bevacizumab to chemotherapy can prolong the progression free survival (PFS).23–30 In a randomized clinical trial, 402 patients with metastatic colorectal cancer were treated with irinotecan, 5-fluorouracil, leucovorin in combination with bevacizumab. The addition of bevacizumab to chemotherapy significantly improved the progression free survival, overall survival and duration of remission. 24 Similar results were seen when bevacizumab was combined with chemotherapy in patients with non-small cell lung cancer, cervical cancer, ovarian cancer and metastatic breast cancer. 25, 28, 31, 32
Robak et al 33 reported on the use of fludarabine, cyclophosphamide and rituximab (FCR) as a treatment for relapsed CLL in a randomized study which compared FCR to FC. Eighty two percent of these patients had been previously treated with an alkylating agent and only 17% had received fludarabine. The FCR regimen demonstrated an overall response rate (ORR) of 70%, complete remission (CR) rate of 24% and a median PFS of 30.6 months with a median follow up time of 25 months. We have earlier reported on the efficacy of FCR in relapsed patients with CLL.34 Seventy percent of patients had received prior fludarabine based therapy, including four patients who had been treated with FCR previously. The ORR was 74%, the CR rate was 30%, and the median PFS was 21 months with a median follow up time of 43 months.
Given the correlation with increased angiogenesis and disease progression in CLL, we tested whether the addition of bevacizumab to FCR could improve PFS in patients with relapsed CLL.
Methods
This study was approved by the Institutional Review Board (IRB) of the MD Anderson Cancer Center (MDACC) and registered on (www.clinicaltrials.gov) with a trial identifier number -NCT00448019. All patients provided informed consent as per institutional guidelines and in accordance with the Declaration of Helsinki. A total of (n=62) patients were enrolled in this open-label, phase 2 trial from May 2007 through February 2010. All the patients had progressive CLL with an indication for treatment as per NCI-WG criteria. Patients had relapsed, fludarabine sensitive (duration of response >6 months) or fludarabine naïve CLL. Refractoriness to alkylating agent was defined as failure to achieve at least a partial remission (PR) with the last alkylating agent-based treatment or progression within 6 months of treatment. Patients were required to have an adequate performance status (WHO/Eastern Cooperative Oncology Group [ECOG] performance status ≤2) and adequate organ function (serum creatinine ≤2 mg/dL, total bilirubin ≤2 mg/dL, AST (SGOT) and ALT (SGPT) ≤2 times the upper limit of normal). Prognostic markers including CD38 status, presence or absence of Zap-70, immunoglobulin heavy chain variable gene (IGHV) mutation status, standard metaphase cytogenetic analysis and FISH- fluorescent in-situ hybridization was performed on bone marrow at baseline in the majority of patients.
FCR comparison group
Patients enrolled at MDACC on a previously published clinical trial of FCR as a therapy for relapsed CLL were used as a historical comparison group (n= 205). 34 The median follow up time for the FCR cohort was 120 months (95% CI, 116 – 128 months).
Treatment Plan
FCR consisted of fludarabine (F) 25 mg/m2 IV and cyclophosphamide (C) 250 mg/m2 IV on days 2–4 for course 1 and days 1–3 for courses 2–6. Rituximab (R) was administered on day 1 at 375 mg/m2 for course 1 and 500 mg/m2 for courses 2–6. Bevacizumab (B) 10 mg/kg IV was given on day 3 of course 1 and day 2 of courses 2–6. The basis for choosing bevacizumab 10mg/kg every 4 weeks was based on 10mg/kg being the most commonly used dose as well as 4 weeks being the established schedule of FCR administration. Courses were repeated every 4 weeks or longer depending on the recovery of neutrophil and platelet counts. Dose reductions for FC, but not R or bevacizumab, occurred if the patients experienced prolonged grade 3 or 4 hematologic toxicity or infections. Prophylaxis against tumor lysis syndrome, herpes virus reactivation, Pneumocystis jiroveci pneumonia, and the use of myeloid growth factors were at the discretion of the treating physician.
Response assessment
Patients were evaluated for response according to 1996 NCI-WG response criteria at least 2 months after their last course. 35 Computerized tomography (CT) scans were not routinely performed for response assessment. Adverse events (AEs) and serious adverse events (SAEs) were documented according to the Common Toxicity Criteria (CTC) Version 2.0 (National Cancer Institute).
Statistical considerations
The primary objective was to determine PFS and duration of response to FCR-B in previously treated patients with CLL. Secondary objectives were to assess the response rates, CR rate, ORR and safety of FCR-B. PFS was defined as the time from the start of treatment to progression which included treatment failure, relapse or death. OS was defined as the time from the start of treatment to the last follow-up date or death. The distribution of continuous variables was summarized by mean, standard deviation and range. The distribution of each categorical variable was summarized in terms of its frequencies and percentages. Continuous variables were compared between treatment groups by Wilcoxon rank sum test, and categorical variables were compared between treatment groups by Fisher exact test. The Kaplan-Meier curve was used to estimate OS and PFS. Log rank tests were used to compare time-to-event variables between groups. The Cox proportional hazard model was used to evaluate the ability of the covariates to predict PFS or OS. All computations were carried out in SAS version 9.1 and S-Plus version 8. Kaplan-Meier curves were prepared by graph pad prism version 6.0.
Results
Patient Characteristics
62 patients with relapsed CLL were enrolled in this trial. There were 49 (79%) males and 13 (21%) female patients. The median age was 60 years (31–84 years). The median number of prior regimens was 2 (range 1–5). Five patients (8%) were previously treated with FCR. Of the total of 62 patients, 11(18%) were fludarabine and alkylating agent refractory, one (2%) was fludarabine refractory and 3 (5%) were alkylating agent refractory. Rai stage 3–4 disease was present in 58% of patients. Unmutated IGHV was observed in 83% of patients. Deletion 17p or deletion11q FISH abnormality was detected in 16% and 44% patients respectively. Among the 62 patients, 28 (45%) patients have died with a median follow-up time of 40 months (95% CI 33 – 45 months). A total of 5 patients were not evaluable (NE) in the FCR-B cohort, one patient went to hospice after one cycle due to metastatic squamous cell cancer, one patient went home after one cycle and did not return by choice, one patient declined further treatment due to grade 3–4 nausea and vomiting during first course and two patients died, one due to cardiac arrest another of an unknown cause. Two patients who died early in the course of FCR-B had preexisting cardiac co-morbidities. In one patient who died of cardiac arrest, there was a history of coronary artery disease, atrial fibrillation and anti-anginal medications. Another patient whose cause of death was unknown was a heavy smoker and had a past history of coronary artery bypass surgery. Table -1 shows the characteristics of the patients enrolled in the FCR-B trial as compared to patients treated with FCR. A significantly higher proportions of patients in FCR-B exhibited an unmutated IGHV gene, Zap-70 positivity and deletion17p or deletion11q by FISH analysis as compared to those patients in FCR cohort (P<0.05). The median number of cycles completed by patients in the FCR-B and the FCR trials was 4 and 5 respectively. Among the patients in the FCR-B group, 27 (44%) completed all 6 cycles. In the FCR group 91 (44%) patients completed all 6 cycles. In relapsed patients previously treated with FCR, there were 8 early deaths– 4 after cycle one, 2 after cycle two and 2 after cycle three. The median follow-up for patients in the FCR-B and FCR trials is 40 months and 120 months respectively.
Table 1.
Patient characteristics by treatment – FCR vs FCR-B
| Variable | FCR (n=205) Median (range) |
FCR-B (n=62) Median (range) |
P-value | |
|---|---|---|---|---|
| WBC (K/uL) | 46 (1,583) | 42(4, 326) | 0.77 | |
| Hemoglobin (gm/dL) | 12 (7,17) | 12 (9,15) | 0.92 | |
| Platelet (K/uL) | 127 (6,391) | 110 (50,338) | 0.22 | |
| Variable | Status | N (%) | N (%) | |
| Age (years) | <65 | 137(67) | 35(56) | 0.17 |
| ≥65 | 68(33) | 27(43) | ||
| Sex | F | 57(28) | 13(21) | 0.33 |
| M | 148(72) | 49(79) | ||
| Prior number of Therapies | 1 | 82(40) | 25(40) | 0.93 |
| 2 | 53(26) | 14(23) | ||
| 3 | 37(18) | 13(21) | ||
| >3 | 33(16) | 10(16) | ||
| Rai Stage | 0,1,2 | 113(55) | 26(42) | 0.08 |
| 3,4 | 92(45) | 36(58) | ||
| IGHV Mutation | Mutated | 19(30) | 6(17) | <0.001 |
| Unmutated | 45(70) | 29(83) | ||
| Zap-70 | Negative | 24(46) | 11(24) | 0.02 |
| Positive | 28(54) | 34(76) | ||
| β2 microglobulin (mg/L) | Low (<4) | 78(39) | 28(49) | 0.17 |
| High (≥4) | 123(61) | 29(51) | ||
| *LDH 618 IU/L (ULN) | <618 | 115(58) | 31(50) | 0.31 |
| ≥618 | 84(42) | 31(50) | ||
| ALC (K/uL) | <100 | 156(76) | 45(73) | 0.62 |
| ≥100 | 49(24) | 17(27) | ||
| Nodes | <10cm | 184(91) | 61(98) | 0.08 |
| ≥10cm | 17(8) | 1(2) | ||
| Conventional Karyotype | Del13q | 6 (3) | 1 (2) | 0.07 |
| Diploid | 108 (54) | 27 (66) | ||
| Del11q | 17 (8) | 6 (15) | ||
| Trisomy 12 | 18 (9) | 1 (2) | ||
| Del17p | 6 (3) | 2 (5) | ||
| Complex | 23 (11) | 6 (15) | ||
| Others | 23 (11) | 0 (0) | ||
| FISH Categories** | Del13q | 5 (16) | 15 (24) | 0.02 |
| Trisomy 12 | 8 (26) | 3 (5) | ||
| Negative | 7 (23) | 7 (11) | ||
| Del11q | 8 (26) | 27 (44) | ||
| Del17p | 3 (10) | 10 (16) | ||
| Alkylating agent, fludarabine refractory | No | 176(86) | 51(82) | 0.48 |
| Yes | 29 (14) | 11 (18) | ||
| Fludarabine refractory | No | 176(86) | 61(99) | 0.09 |
| Yes | 0(0) | 1 (2) | ||
| Alkylating agent refractory | No | 151(74) | 59(96) | 0.04 |
| Yes | 25(13) | 3(5) |
ULN-Upper limit of normal level of LDH (Lactate dehydrogenase),
Only 31 patients treated with FCR had FISH analysis before treatment
Responses
Fifty-seven of 62 patients were evaluable for response assessment to FCR-B. Among the patients evaluable for response - 8 (14%) were fludarabine and alkylating agent refractory (AFR) and 2 (4%) were alkylating agent refractory. Responses were evaluated at least 2 months after completion of therapy to allow for recovery of peripheral blood counts. Table 2 shows the overall response rate by pretreatment characteristics. The response rates with FCR-B included CR in 23% (13/57), nodular partial response (nPR) in 13% (8/14) and PR in 43% (24/43) of patients for an ORR of 79% (45/57). Five (8%) inevaluable patients came off the study early in the course of the treatment. Two patients died after cycle 2 - one patient died of cardiac arrest, and one patient died of an unknown cause. Three patients came off study after the first course, one went to hospice due to metastatic squamous cell cancer, one patient withdrew consent after a significant infusion reaction to rituximab and grade 3–4 nausea and vomiting during the first cycle and one patient went home and came off study by choice.
Table 2.
Overall response rate (ORR) after FCR-B by patient characteristics
| Variable | Subcategory | Number | OR (%) | P value |
|---|---|---|---|---|
| Age | <65 | 32 | 28(88) | 0.12 |
| ≥65 | 24 | 17(71) | ||
| Rai Stage | 0–2 | 26 | 22(85) | 0.33 |
| 3–4 | 31 | 23(75) | ||
| Bulky Disease | No | 56 | 44(79) | 1.0 |
| Yes | * NA | 1(2) | ||
| β2-microglobulin, mg/L | <4.0 | 27 | 23(86) | 0.16 |
| ≥4.0 | 26 | 18(70) | ||
| Prior treatment | ≤2 | 36 | 31(87) | 0.08 |
| >2 | 21 | 14(67) | ||
| ZAP 70 | Negative | 10 | 9(90) | 0.27 |
| Positive | 30 | 22(74) | ||
| IGHV Mutation Status | Unmutated | 25 | 18(72) | 0.56 |
| Mutated | 6 | 5(84) | ||
| Alkylating agent, Fludarabine refractory | No | 49 | 40(82) | 0.21 |
| Yes | 8 | 5(63) | ||
| Alkylating agent refractory | No | 47 | 39(80) | 0.23 |
| Yes | 2 | 1(2) | ||
| FISH Category | Del13q | 15 | 13(87) | 0.05 |
| Trisomy 12 | 3 | 3(100) | ||
| Negative | 6 | 3(50) | ||
| Del11q | 25 | 22(88) | ||
| Del17p | 8 | 4(50) |
NA – Not assessable
Patients with deletion17p by FISH also had a lower ORR as did the patients without del17p - 7% (4/57) vs 72% (41/57) respectively (P=0.03).
Response rates with FCR-B were similar to those observed with FCR in relapsed patients with CLL, CR rate (23 vs 30 %) and ORR (79 vs 78 %) respectively.
Toxicities
Sixty-two patients were evaluable for toxicity. A summary of toxicities is presented in Table 3. The most common side effects were myelosuppression, infection, nausea and vomiting. Grade 3 or 4 toxicities included febrile neutropenia (n=40), infections (n=21), thrombocytopenia (n=14), anemia (n=9) and nausea and vomiting (n=5). Grade 3–4 toxicities observed in FCR-B were similar to those seen in the historical cohort of patients treated with FCR. In the historical cohort treated with FCR, grade 3–4 pneumonia and sepsis were the most common serious infections n=46 (16%) and in patients with FCR-B, grade 3–4 pneumonia and sepsis were observed in 11 patients (18%). Potential bevacizumab associated toxicities36 included hypertension in one patient, gastrointestinal hemorrhage in two (without perforation) and rash in two patients. All of these toxicities were grade 1–2.
Table 3.
Toxicities among the patients who received FCR-B
| Toxicity | All Grades N (%) | Grades 3–4 N (%) |
|---|---|---|
| Neutropenia | 41 (67) | 40 (65) |
| Thrombocytopenia | 18 (29) | 14 (23) |
| Infection | 30 (49) | 21 (35) |
| Nausea and vomiting | 22 (36) | 5 (8) |
| Fatigue | 16 (26) | 0 |
| Anemia | 9 (15) | 9 (15) |
| GI bleeding | 2 (4) | 0 |
| Rash | 2 (4) | 0 |
| Blurred vision | 2 (4) | 0 |
| Syncope | 1 (2) | 0 |
| Hypertension | 1 (2) | 0 |
Progression free survival (PFS)
PFS was analysed among patients receiving FCR-B and compared to that of the historical cohort of patients who were treated with FCR. The Kaplan-Meier estimates of PFS according to treatments (FCR-B vs. FCR) are shown in Figure 1. The median PFS was 13.5 months with FCR-B versus 21 months with the FCR regimen (P=0.05). Of note is that the FCR-B trial included a higher proportion of patients with poor prognostic characteristics, including complex karyotype, del17p or del11q, and unmutated IGHV as compared to those in the FCR trial (Table -1). These characteristics are known to shorten the PFS seen with FCR. The median PFS by response (CR, nPR and PR) in FCR-B was 42, 22 and 12 months respectively while in the historical cohort the corresponding values were 49,29 and 15 months respectively. Multivariate analysis showing factors correlating with PFS are shown in Table -4. Factors in the multivariate model predicting for shorter PFS with FCR or FCR-B included ≤ 3 cycles of therapy, alkylating agent and fludarabine refractoriness, and deletion 11q or complex karyotype. After accounting for differences in pretreatment characteristics, treatment was not significant in the multivariate analysis.
Figure 1. Progression free survival (PFS) by therapy in patients receiving FCR-B and FCR.
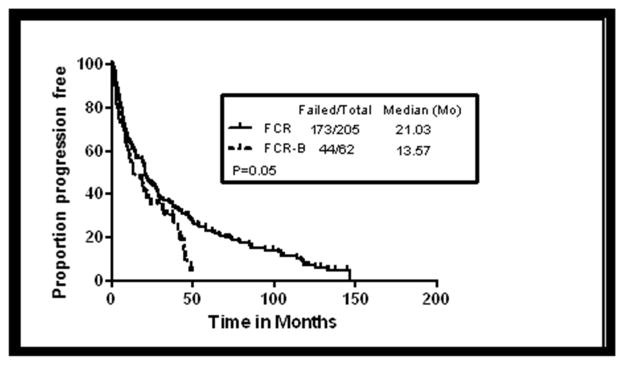
Shows comparison of FCR-B with the historical cohort of patients who were treated with salvage FCR (P=0.05).
Table 4.
Cox proportional hazard model for progression free survival (PFS) for patients who received FCR-B and FCR
| Analysis | HR (95% CI) | P-value |
|---|---|---|
| Cycle (>3 vs.≤ 3) | 0.43 (0.32–0.58) | <0.0001 |
| * AFR (Yes vs. No) | 2.35 (1.56–3.53) | <0.0001 |
| ** Complex vs. Diploid (CG) | 2.92 (1.83–4.65) | <0.0001 |
| Del 11q vs. Diploid | 1.70 (1.04–2.78) | 0.03 |
| Treatment (FCR-B vs. FCR) | 1.28 (0.88–1.86) | 0.19 |
| β2 microglobulin (mg/dL) (≥4 vs. <4) | 1.10 (0.80–1.5) | 0.57 |
| Prior treatment (>2 vs.≤ 2) | 1.16 (0.84–1.59) | 0.37 |
| Rai stage (3,4, vs. 0,1,2) | 1.27 (0.90–1.79) | 0.18 |
| # Del 17p vs. Diploid (FISH) | 1.64 (0.50–5.34) | 0.41 |
| # Trisomy12 vs Diploid (FISH) | 0.74 (0.41–1.31) | 0.30 |
AFR refers to fludarabine and alkylating agent refractory disease,
CG: conventional cytogenetics,
FISH: fluorescent in situ hybridization
The impact of cytogenetics by FISH on PFS in patients who received FCR-B was analysed (Figure -2). The median PFS (months) in patients with del17p, normal FISH, del11q and del13q categories were 3, 10, 17 and 22 months respectively (Log rank p=0.037). There were 3 patients with trisomy 12 and only one has relapsed.
Figure 2.
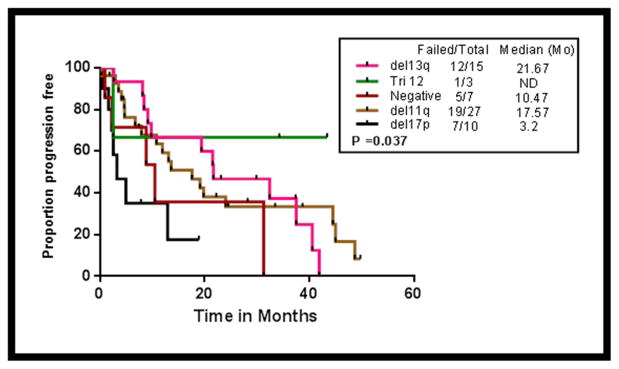
Progression free survival (PFS) by different FISH (Fluorescent in-situ hybridization) cytogenetic categories (del17p, del11q, negative FISH, trisomy 12 and del13q) in patients receiving FCR-B (P=0.037).
Overall survival (OS)
Overall survival (OS) was analysed among the patients receiving FCR-B and compared to that of patients treated with FCR. The Kaplan-Meier estimates of OS according to treatment (FCR-B vs. FCR) are shown in Figure 3. The median OS was 45 months in FCR-B versus 49 months in FCR regimen (P=0.48). The median OS by response (CR, nPR and PR) in patients who received FCR-B was not reached, not reached and 34.3 months respectively while in the historical cohort the corresponding values were 129, 100 and 42 months respectively. Multivariate analysis showing factors predicting for overall survival is summarized in Table -5. Pre-treatment characteristics significantly associated with shorter OS included >2 prior therapies, ≤ 3 cycles of treatment, alkylating agent and fludarabine refractoriness, del17p by FISH and complex karyotype. Of note, treatment with FCR-B or FCR was not predictive of OS in the final Cox regression model (Table 5).
Figure 3. Overall survival (OS) by therapy in patients receiving FCR-B and FCR.
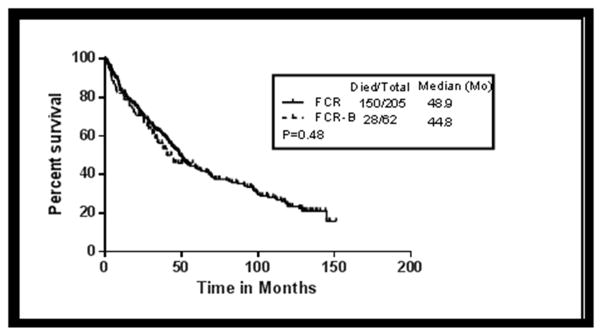
Shows comparison of FCR-B with the historical cohort of patients who were treated with salvage FCR (P=0.48).
Table 5.
Cox proportional hazard model for overall survival (OS) for patients who received FCR-B and FCR
| Analysis | HR (95% CI) | P-value |
|---|---|---|
| Cycle (>3 vs. ≤3) | 0.50 (0.36, 0.70) | <0.0001 |
| # Del 17p vs. Diploid (FISH) | 4.43 (1.74, 11.32) | <0.01 |
| * Complex vs. Diploid (CG) | 2.39 (1.45, 3.94) | <0.01 |
| Prior treat (>2 vs. <=2) | 1.61 (1.13, 2.28) | 0.01 |
| ** AFR (Yes vs. No) | 1.64 (1.06, 2.55) | 0.03 |
| Age 65 Yrs (≥65 vs. <65) | 1.39 (0.99, 1.95) | 0.06 |
| Sex (Male vs. Female) | 1.44 (0.99, 2.11) | 0.06 |
| Treatment (FCR-B vs. FCR) | 1.19 (0.74–1.92) | 0.46 |
| β2 microglobulin (mg/dL) (≥4 vs. <4) | 1.24 (0.83–1.84) | 0.29 |
| Rai stage (3,4, vs. 0,1,2) | 1.27 (0.90–1.79) | 0.18 |
| Bulky nodes (≥10cm vs. <10cm) | 1.24 (0.70–2.19) | 0.47 |
| # Trisomy12 vs Diploid (FISH) | 0.64 (0.32–1.25) | 0.19 |
| # Del 11q vs. Diploid (FISH) | 0.96 (0.55–1.68) | 0.89 |
| LDH (≥618 vs <618 IU/L) | 1.27 (0.90–1.80) | 0.18 |
FISH: fluorescent in situ hybridization,
CG: conventional cytogenetics,
AFR refers to fludarabine and alkylating agent refractory disease,
The impact of FISH categories on OS in patients who received FCR-B was analysed (Figure-4). Patients with del17p had inferior survival. The median OS (months) in patients with del17p, normal FISH, del11q, trisomy 12 and del13q categories were 7.3, 33, not reached, 34.3 and not reached respectively (Log rank P=0.0015).
Figure 4.
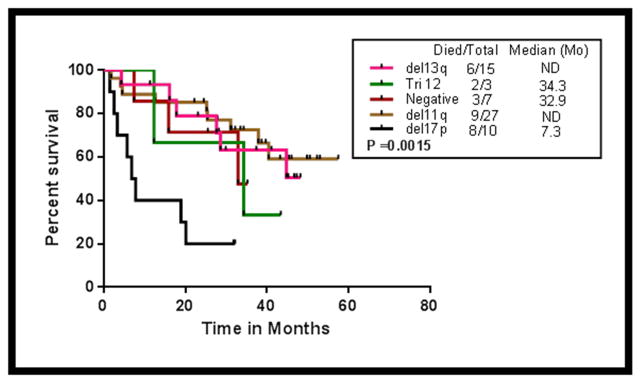
Overall survival (OS) by different FISH (Fluorescent in-situ hybridization) cytogenetic categories (del17p, del11q, negative FISH, trisomy 12 and del13q) in patients receiving FCR-B (P=0.0015).
Discussion
Management of relapsed patients with CLL remains a challenge. Relapse and disease progression in patients with CLL who are treated with chemotherapy can occur due to clonal evolution of CLL cells29, persistence of drug resistant clones, presence of minimal residual disease (MRD) and pro-survival signals to CLL cells from the microenvironment. Antiangiogenic strategies have involved treatment with a monoclonal antibody against VEGF such as bevacizumab or aflibercept, or treatment with tyrosine kinase inhibitors such as sorafenib, sunitinib or pazopanib in the treatment of various solid tumors. Various randomized clinical trials have demonstrated that the combination of bevacizumab with chemotherapy in metastatic solid tumors can improve progression-free and overall survival. 30 The relevance of angiogenesis in CLL progression generated the rationale of adding bevacizumab to chemotherapy. The primary objective of this trial was to assess the outcome after the addition of bevacizumab to FCR in relapsed patients with CLL. The results using FCR-B were compared with the results of our previous trial of FCR in relapsed CLL. The addition of the bevacizumab to FCR did not improve the PFS or OS. The FCR-B regimen was similar to FCR in terms of response rates (ORR and CR - 79% vs 78% and 23% vs 30%) respectively. The FCR-B trial had a higher proportion of patients with adverse prognostic features such as unmutated IGVH, complex karyotype, del11q and del17p. The majority of the patients in the historical cohort did not have FISH data available and only had karyotype analysis performed by conventional cytogenetics. In the multivariate analysis the type of therapy was not significant in predicting PFS or OS. Similar to FCR, FCR-B regimen did not improve PFS or OS in patients with del17p. Bevacizumab has been associated with cardiac and vascular toxicities. These toxicities were observed in 10–35% patients who received bevacizumab in combination with chemotherapy to treat various solid tumors. In this study potential bevacizumab associated toxicities were mild - hypertension in one patient, gastrointestinal hemorrhage in two (without perforation) and rash in two patients. This reduced toxicity in the current trial may be explained by the less frequent dosing of bevacizumab – once every 2 weeks in other studies vs once every 4 weeks in the current trial.
Bevacizumab was also evaluated in other hematologic malignancies. In a randomized phase 2 trial in elderly patients with acute myelogenous leukemia (AML), the addition of bevacizumab to chemotherapy (3+7) did not result in improved clinical benefit.37 In non-Hodgkin’s lymphoma (NHL), the addition of bevacizumab to CHOP chemotherapy alone or to rituximab-CHOP did not result in any benefit.38, 39 In the only prior trial conducted in CLL, 12 patients with relapsed disease were treated with single agent bevacizumab and none responded. 40
A possible explanation for the lack of efficacy of bevacizumab in hematological malignancies as compared to solid tumors could be due to less dependency of hematological malignancies on VEGF signaling and rapid adaptation of malignant cells to VEGF inhibition. Other reasons for the lack of improved PFS with FCR and bevacizumab included the fact that the status of angiogenesis (serum VEGF, Ang-2 levels, amount of bone marrow microvascular density etc.) was not evaluated in patients before therapy. It is possible that the addition of bevacizumab might be selectively useful for patients with increased levels of angiogenesis in the microenvironment.
In summary, adding bevacizumab to FCR in patients with relapsed CLL did not improve outcome and results were similar to those seen previously with FCR.
Acknowledgments
Source of Funding - None
This article was funded by P30 CA016672
Footnotes
Conflicts of Interests - The authors report no competing conflicts of interest.
Authorship Contributions
S.O.B. designed the study.
P.J., M.K., H.L, W.Q., and S.O.B. analyzed results.
P.J., H.L, W.Q, O.B., and S.O.B. wrote the paper.
P.J., M.K., H.L, W.Q., and S.O.B. did the clinical correlation.
M.K., S.O.B., J.B., W.W., A.F., Z.E., contributed patients.
All authors have reviewed and contributed to the manuscript.
References
- 1.Cramer P, Hallek M. Prognostic factors in chronic lymphocytic leukemia-what do we need to know? Nat Rev Clin Oncol. 2011;8:38–47. doi: 10.1038/nrclinonc.2010.167. [DOI] [PubMed] [Google Scholar]
- 2.Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:110–118. doi: 10.1182/asheducation-2011.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Ramsay AD, Rodriguez-Justo M. Chronic lymphocytic leukaemia - the role of the microenvironment pathogenesis and therapy. Br J Haematol. 2013 doi: 10.1111/bjh.12344. [DOI] [PubMed] [Google Scholar]
- 4.Gaidano G, Foa R, Dalla-Favera R. Molecular pathogenesis of chronic lymphocytic leukemia. J Clin Invest. 2012;122:3432–3438. doi: 10.1172/JCI64101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt T, Carmeliet P. Angiogenesis: a target in solid tumors, also in leukemia? Hematology Am Soc Hematol Educ Program. 2011;2011:1–8. doi: 10.1182/asheducation-2011.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Maffei R, Martinelli S, Santachiara R, et al. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood. 2010;116:584–592. doi: 10.1182/blood-2009-11-252494. [DOI] [PubMed] [Google Scholar]
- 8.Frater JL, Kay NE, Goolsby CL, Crawford SE, Dewald GW, Peterson LC. Dysregulated angiogenesis in B-chronic lymphocytic leukemia: morphologic, immunohistochemical, and flow cytometric evidence. Diagn Pathol. 2008;3:16. doi: 10.1186/1746-1596-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kay NE, Bone ND, Tschumper RC, et al. B-CLL cells are capable of synthesis and secretion of both pro- and anti-angiogenic molecules. Leukemia. 2002;16:911–919. doi: 10.1038/sj.leu.2402467. [DOI] [PubMed] [Google Scholar]
- 10.Molica S, Vacca A, Ribatti D, et al. Prognostic value of enhanced bone marrow angiogenesis in early B-cell chronic lymphocytic leukemia. Blood. 2002;100:3344–3351. doi: 10.1182/blood-2002-01-0084. [DOI] [PubMed] [Google Scholar]
- 11.Piechnik A, Dmoszynska A, Omiotek M, et al. The VEGF receptor, neuropilin-1, represents a promising novel target for chronic lymphocytic leukemia patients. Int J Cancer. 2013 doi: 10.1002/ijc.28135. [DOI] [PubMed] [Google Scholar]
- 12.McCabe D, Bacon L, O’Regan K, Condron C, O’Donnell JR, Murphy PT. CD38 expression on B-cell chronic lymphocytic leukemic cells is strongly correlated with vascular endothelial growth factor expression. Leukemia. 2004;18:649–650. doi: 10.1038/sj.leu.2403282. [DOI] [PubMed] [Google Scholar]
- 13.Peterson L, Kini AR. Angiogenesis is increased in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2529. doi: 10.1182/blood.v97.8.2529. [DOI] [PubMed] [Google Scholar]
- 14.Cols M, Barra CM, He B, et al. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. J Immunol. 2012;188:6071–6083. doi: 10.4049/jimmunol.1102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maffei R, Fiorcari S, Bulgarelli J, et al. Physical contact with endothelial cells through beta1- and beta2- integrins rescues chronic lymphocytic leukemia cells from spontaneous and drug-induced apoptosis and induces a peculiar gene expression profile in leukemic cells. Haematologica. 2012;97:952–960. doi: 10.3324/haematol.2011.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buggins AG, Pepper C, Patten PE, et al. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res. 2010;70:7523–7533. doi: 10.1158/0008-5472.CAN-10-1634. [DOI] [PubMed] [Google Scholar]
- 17.Kini AR, Kay NE, Peterson LC. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia. 2000;14:1414–1418. doi: 10.1038/sj.leu.2401825. [DOI] [PubMed] [Google Scholar]
- 18.Molica S, Cutrona G, Vitelli G, et al. Markers of increased angiogenesis and their correlation with biological parameters identifying high-risk patients in early B-cell chronic lymphocytic leukemia. Leuk Res. 2007;31:1575–1578. doi: 10.1016/j.leukres.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Abrams ST, Brown BR, Zuzel M, Slupsky JR. Vascular endothelial growth factor stimulates protein kinase CbetaII expression in chronic lymphocytic leukemia cells. Blood. 2010;115:4447–4454. doi: 10.1182/blood-2009-06-229872. [DOI] [PubMed] [Google Scholar]
- 20.Farahani M, Treweeke AT, Toh CH, et al. Autocrine VEGF mediates the antiapoptotic effect of CD154 on CLL cells. Leukemia. 2005;19:524–530. doi: 10.1038/sj.leu.2403631. [DOI] [PubMed] [Google Scholar]
- 21.Maffei R, Martinelli S, Castelli I, et al. Increased angiogenesis induced by chronic lymphocytic leukemia B cells is mediated by leukemia-derived Ang2 and VEGF. Leuk Res. 2010;34:312–321. doi: 10.1016/j.leukres.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19:513–523. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 23.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 25.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 26.Zahiragic L, Schliemann C, Bieker R, et al. Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity. Leukemia. 2007;21:1310–1312. doi: 10.1038/sj.leu.2404632. [DOI] [PubMed] [Google Scholar]
- 27.Bellou S, Pentheroudakis G, Murphy C, Fotsis T. Anti-angiogenesis in cancer therapy: Hercules and hydra. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 30.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlowicz KA, Ternus MP. Issues influencing psychiatric nurse retention during the first year of employment: a case analysis. J Nurs Manag. 2009;17:49–58. doi: 10.1111/j.1365-2834.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 32.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robak T, Jamroziak K, Gora-Tybor J, et al. Comparison of cladribine plus cyclophosphamide with fludarabine plus cyclophosphamide as first-line therapy for chronic lymphocytic leukemia: a phase III randomized study by the Polish Adult Leukemia Group (PALG-CLL3 Study) J Clin Oncol. 2010;28:1863–1869. doi: 10.1200/JCO.2009.25.9630. [DOI] [PubMed] [Google Scholar]
- 34.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 37.Ossenkoppele GJ, Stussi G, Maertens J, et al. Addition of bevacizumab to chemotherapy in acute myeloid leukemia at older age: a randomized phase 2 trial of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK) Blood. 2012;120:4706–4711. doi: 10.1182/blood-2012-04-420596. [DOI] [PubMed] [Google Scholar]
- 38.Ganjoo K, Hong F, Horning SJ, et al. Bevacizumab and CHOP (A-CHOP) in Combination for Patients with Peripheral T-Cell or Natural Killer Cell Neoplasms: An Eastern Cooperative Group Study (E2404) Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.816700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stopeck AT, Unger JM, Rimsza LM, et al. A phase 2 trial of standard-dose cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) and rituximab plus bevacizumab for patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: SWOG 0515. Blood. 2012;120:1210–1217. doi: 10.1182/blood-2012-04-423079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanafelt T, Zent C, Byrd J, et al. Phase II trials of single-agent anti-VEGF therapy for patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51:2222–2229. doi: 10.3109/10428194.2010.524327. [DOI] [PMC free article] [PubMed] [Google Scholar]


