This retrospective study demonstrates that patients with tumors in the head and neck region have significant sleep-related breathing disorders as judged by sleep-related complaints and polysomnographic data. Symptoms typically include daytime fatigue and sleepiness, and a heightened clinical suspicion is recommended for this cohort.
Keywords: Sleep-related breathing disorder, Obstructive sleep apnea, Cancer, Head and neck tumors, Head and neck squamous cell carcinoma, Polysomnography, Sleep disruption, Radiation therapy
Abstract
Background.
Sleep disturbance is a prominent complaint of cancer patients. Most studies have focused on insomnia and cancer-related fatigue. Obstructive sleep apnea (OSA) has been reported in small studies and case reports.
Methods.
In a retrospective review of patients who underwent formal sleep evaluation and polysomnography (PSG) from 2006 to 2011, 56 patients with tumors in the head and neck region were identified. Clinical characteristics, sleep-related history, and PSG data were reviewed.
Results.
Most patients had active cancer (80%), and the majority had squamous pathology (68%). Prominent symptoms included daytime fatigue (93%), daytime sleepiness (89%), and snoring (82%). Comorbid conditions primarily included hypertension (46%) and hypothyroidism (34%). Significant sleep-related breathing disorder was noted in 93% of patients, and 84% met clinical criteria for OSA. A male predominance (77%) was noted, and patients were not obese (body mass index <30 kg/m2 in 52%). The majority of patients (79%) underwent radiation prior to sleep study, of which 88% had OSA, and in the group without prior radiation, 67% had OSA. Adherence to positive airway pressure (PAP) therapy was slightly better when compared with the general population. A subset of patients with persistent hypoxia despite advanced forms of PAP required tracheostomy. Multivariate analysis revealed that patients with active disease and radiation prior to PSG were more likely to have OSA.
Conclusion.
Sleep-related breathing disorder was common in patients with tumors in the head and neck region referred for evaluation of sleep disruption, and most met clinical criteria for OSA. Daytime fatigue and sleepiness were the most common complaints. OSA was prevalent in male patients, and most with OSA were not obese. Architectural distortion from the malignancy and/or treatment may predispose these patients to OSA by altering anatomic and neural factors. A heightened clinical suspicion for sleep-related breathing disorder and referral to a sleep specialist would be beneficial for patients with these complaints.
Implications for Practice:
Sleep-related breathing disorders are common in patients with tumors in the head and neck region referred for evaluation of sleep disruption, and most met clinical criteria for obstructive sleep apnea (OSA). Daytime fatigue and sleepiness were the most common complaints. OSA was prevalent in male patients, and most were not obese. Adherence to positive airway pressure therapy was slightly better when compared with the general population. Multivariate analysis revealed an increased likelihood of OSA in those with active disease and radiation prior to sleep study. A heightened clinical suspicion for OSA and referral to a sleep specialist would be beneficial for patients with these complaints.
Introduction
Sleep disturbance is a prominent complaint of cancer patients with the most prevalent reported symptoms being fatigue, leg restlessness, insomnia, and excessive sleepiness [1]. However, there have been few systematic studies evaluating the etiology or consequences of poor sleep in this population, and most have focused on insomnia and cancer-related fatigue [2, 3]. Very little is known about potentially treatable sleep disorders, such as sleep-related breathing disorder or, more specifically, obstructive sleep apnea (OSA) in this type of patient, particularly in those with head and neck malignancies that can compromise upper airway patency.
There are many possible causes for sleep disruption in patients with cancer. These include the cancer itself, medical therapy (such as sedatives, hypnotics, narcotics, chemotherapy, steroids, and sympathomimetics), or psychosocial disturbances and comorbid medical issues. In addition, undiagnosed OSA, with or without symptoms, can further complicate their outcome because OSA in the general population has been shown to be independently associated with an increased likelihood of hypertension, cardiovascular disease, stroke, daytime sleepiness, insulin resistance, motor vehicle accidents, and diminished quality of life [4, 5].
The hallmark of OSA is upper airway obstruction, and the pathophysiology is intricate. Narrowing of the upper airway occurs normally during sleep, but superimposed features including anatomic abnormalities (enlarged soft tissues or narrowing of the pharyngeal space) and neural factors (loss of function of the pharyngeal dilator muscles) may result in upper airway obstruction [6, 7]. Intuitively, tumors in the head and neck region may cause anatomic abnormalities of the upper airway. Treatments including radiation and surgery may further engender airway narrowing and architectural distortion. These therapies may also create neurosensory dysfunction affecting feedback from the upper airway to the pharyngeal dilators [8].
Detection and treatment of OSA in patient with tumors in the head and neck regions has not been extensively studied. The first report of OSA and cancer described a patient with newly diagnosed lymphocytic lymphoma in the head and neck region with OSA as the presenting symptom and subsequent improvement after treatment [9]. Other malignancies affecting the upper airway, including sarcoma, pleomorphic adenoma, mycosis fungoides, paraganglioma, and rhabdomyoma, have also been reported to cause or present with OSA [10–14]. Several small case series have described an increased risk of OSA in patients with squamous cell head and neck cancers. Using polysomnography (PSG) as the gold standard, these reports describe an incidence of OSA ranging from 8% to 92% [7, 15–18]. Only one study was in a pretreatment group, and the rest were in head and neck cancer patients post-treatment (radiation or surgery).
The principal aims of our investigation are to describe the characteristics of sleep disorders in patients with tumors located in the head and neck region referred for evaluation based on PSG data and to determine the risk factors and symptoms that suggest underlying sleep-related breathing disorder in these patients.
Materials and Methods
Patients
We retrospectively reviewed the medical records of all patients with tumors in the head and neck region that underwent outpatient PSG at The University of Texas MD Anderson Cancer Center from November 1, 2006 to May 31, 2011. All patients underwent formal sleep consultation by a board certified specialist prior to PSG. Thyroid cancer patients were excluded. The study was approved by the MD Anderson Institutional Review Board.
Patients and clinical characteristics included basic demographics (age, sex, body mass index [BMI]), comorbidities, malignancy (region of cancer, pathology, disease status, treatment), sleep history (previous diagnosis of OSA, sleep symptoms), physical examination, PSG parameters (total sleep time [TST], sleep position, sleep efficiency [time asleep in bed/time in bed], sleep stage, apneas, hypopneas, oxygen saturation nadir, positive pressure therapy), and treatment of sleep disorder. Sleep surveys included the Epworth Sleepiness Scale (ESS) and the Pittsburgh Sleep Quality Index (PSQI). Active cancer was defined by actively treated cancer or less than 5 years from cancer diagnosis.
PSG
An all-night-attended, comprehensive PSG was performed using a computerized polygraph to monitor electroencephalogram (O2-A1, O2-A2, C3-A2, C4-A1), left and right electro-oculogram, electrocardiogram, chin and anterior tibialis electromyogram, abdominal and chest wall excursion using impedance plethysmography, airflow by nasal pressure and nasal and oral thermistors, and oxygen saturation (SaO2) by pulse oximetry. Apnea was defined as cessation of breathing for at least 10 seconds, and hypopnea was defined as decreased effort to breathe at least 50% less than baseline and with at least a 4% decrease in SaO2. Respiratory effort-related arousal (RERA) was characterized by increasing respiratory effort followed by an arousal from sleep that does not meet the criteria for apnea or hypopnea.
Sleep-related breathing disorders encompasses a group of disorders characterized by abnormal respiratory patterns or abnormal hypoventilation during sleep. The presence or absence of OSA was determined via PSG based on the American Academy of Sleep Medicine guidelines [19]. OSA is defined as an apnea and hypopnea index (AHI, episodes of apneas and hypopneas per hour of sleep) greater than 5. The severity of OSA was rated as mild (5 to <15), moderate (15 to <30), or severe (≥30). Patients with RERAs associated with daytime sleepiness and an elevated respiratory disturbance index (RDI, total number of apneas, hypopneas, and RERAs per hour of sleep) were classified as Upper Airway Resistance Syndrome, which is considered a subtype of OSA.
Patients with significant sleep-related breathing disorder underwent PSG with positive airway pressure titration using continuous positive airway pressure, bilevel positive airway pressure, and/or adaptive servo-ventilation (ASV). Positive airway pressure (PAP) was titrated to eliminate snoring and sleep-disordered breathing. A split study was conducted when AHI ≥40 in the first 2 hours of sleep, so the second portion of the study was used to titrate PAP therapy.
Statistical Analysis
Groups were compared with the Fisher’s exact test for categorical variables and with the Wilcoxon rank-sum test for continuous variables. Multivariate logistic regression models were fit to examine the relationship between the presence of OSA and patient and clinical characteristics. Factors that had a univariate p value less than 0.20 were candidates in the multivariate logistic model. The final model retained only factors that had statistical significance. Subgroup analysis was carried out in patients with squamous cell carcinoma. The results were presented in odds ratios (ORs) and 95% confidence intervals (CIs). p values less than 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, NC, http://www.sas.com).
Results
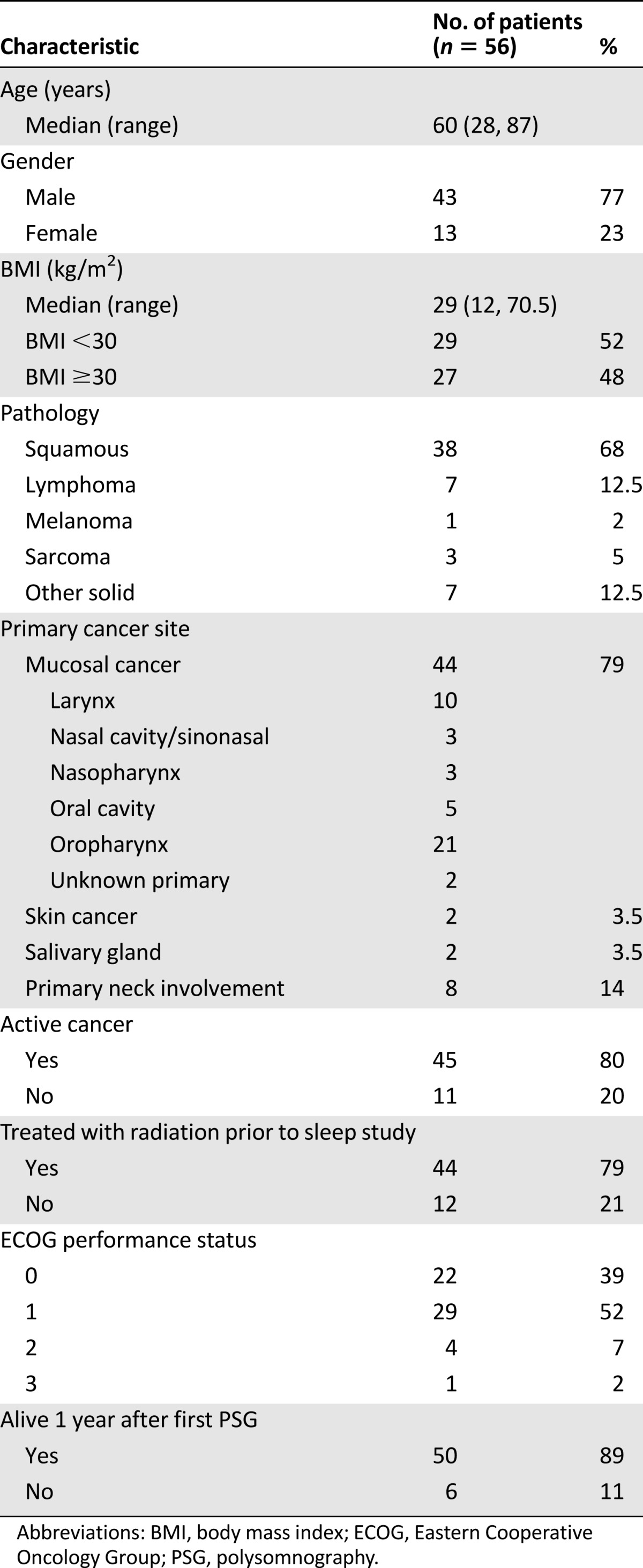
Of the 1,025 patients who met clinical criteria to undergo PSG, a total of 62 had cancers in the head and neck region; among them 6 patients with nonmucosal disease (skin) who received only surgical treatment were excluded. In the remaining 56 patients, sleep-related breathing disorder was noted in 52 patients, and OSA was found in 47 of these patients. Patient and clinical characteristics are shown in Table 1. Most (77%) were men. BMI varied almost equally from <30 (52%) or ≥30 kg/m2 (48%). The majority had active cancer (80%), and some (12.5%) had recurrent disease. Only 2% of patients were current smokers at the time of evaluation. The majority (68%) had squamous pathology, and their tumor stage at diagnosis varied (T0 8%, T1 22%, T2 39%, T3 25%, and T4 6%). Sleep evaluation for squamous cell cancer patients occurred during active treatment (26%) or within 2 years of treatment completion (53%). Patients with nonsquamous cell pathology (n = 18) and squamous cell pathology (n = 38) were compared, but there was no significant difference in age, gender, BMI, therapy (surgery or radiation before PSG), and presence or severity of OSA. Of those with squamous pathology, most were in the oropharynx (47%), larynx (17%), and oral cavity (10%). Four patients underwent PSG with tracheostomy in place. Two were evaluated for OSA with tracheostomy occlusion to assess for possible decannulation. The other two had OSA with significant sleep-related hypoventilation on baseline PSG, and both were referred for repeat PSG after tracheostomy was placed.
Table 1.
Patient demographics and characteristics
Of 95 sleep studies performed in 56 patients, 50 were baseline PSG, 38 were positive airway pressure titration studies, and 7 were split studies. The patients were referred for sleep evaluation by internal medicine (59%), oncology (20%), surgery (16%), and other services (5%). Details regarding sleep evaluation are noted in Table 2. Most patients were referred with OSA symptoms, but the most common complaints included daytime fatigue (93%), snoring (82%), and daytime sleepiness (89%). Hypertension (46%) and hypothyroidism (34%) were the most common comorbid conditions. Facial deformity was documented in six patients, five of whom had OSA.
Table 2.
Sleep evaluation
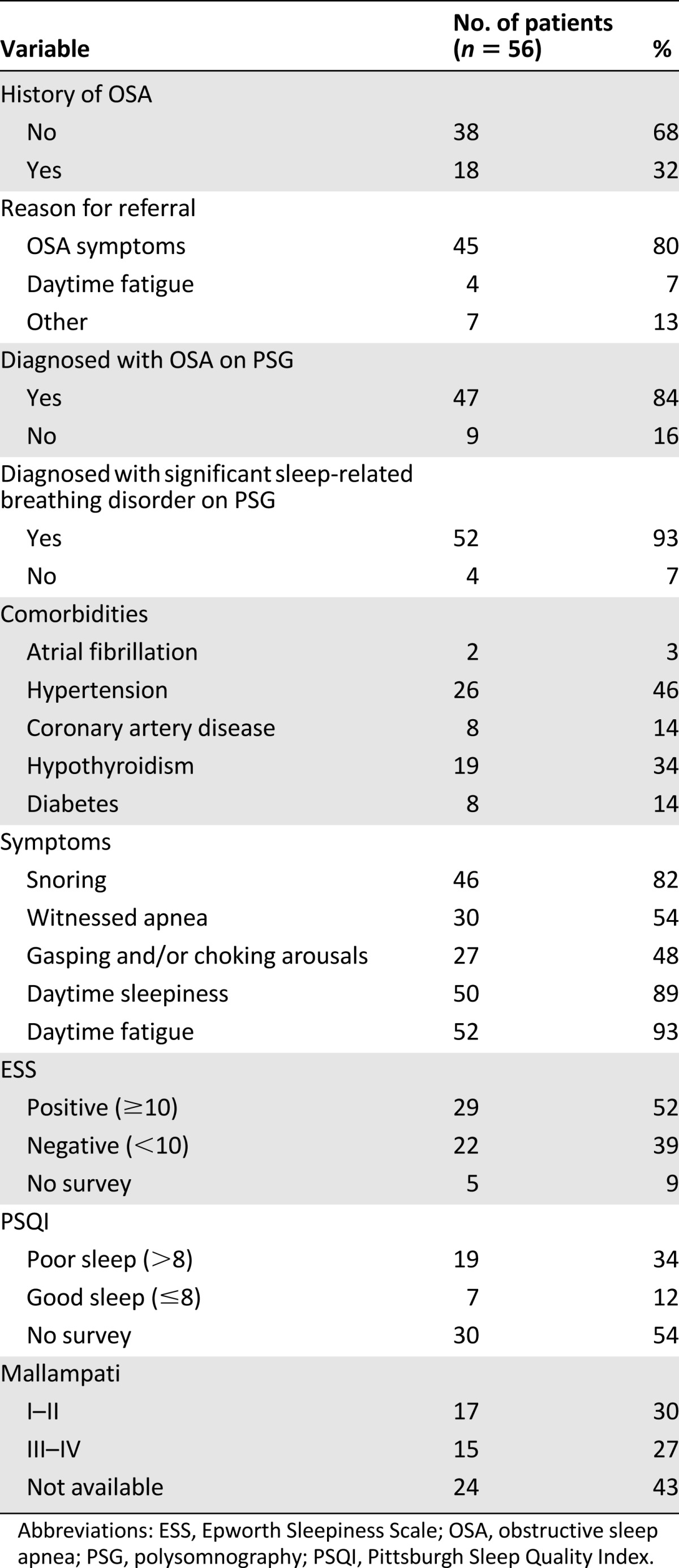
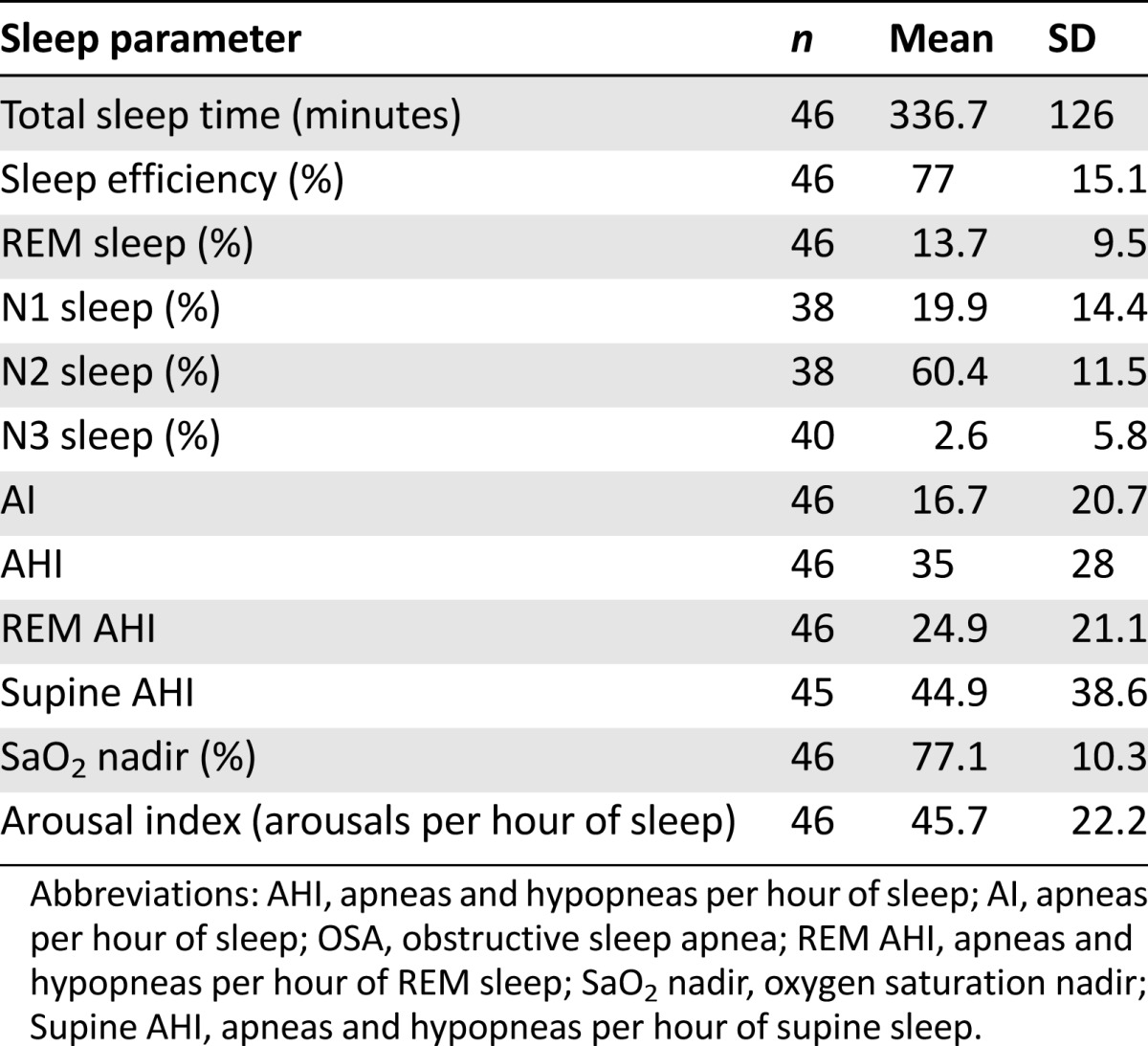
Table 3 describes the diagnostic PSG data in OSA patients (n = 46). The severity of OSA was mild (22%), moderate (32%), or severe (46%). Five patients had split-night studies with TST less than 2 hours because of severe obstructive events. Sleep efficiency was low (<85%) in 28 patients. Latency to persistent sleep was short (<10 minutes) in 18 patients, normal (10–20 minutes) in 16 patients, and long (>20 minutes) in 12 patients. A short sleep latency is consistent with hypersomnia. Latency to REM sleep was available in 45 patients, and it was short (<60 minutes) in 11, normal (60–120 minutes) in 13, and long (>120 minutes) in 17. A short REM sleep latency is seen in narcolepsy but may also be due to prior REM-sleep deprivation or disruption as seen in patients with sleep apnea, depression, irregular sleep/wake schedule, or upon withdrawal of medications such as stimulants and antidepressants. Four patients did not enter REM sleep, and sleep stage data were unavailable in two patients. Of the 39 patients with sleep stage data, the majority of patients (62%) did not have N3 (delta) sleep.
Table 3.
Polysomnographic data in patients with obstructive sleep apnea
There were nine patients without OSA. Five patients had significant sleep-related breathing disorder with a RDI ≥20. Three of these patients underwent PAP titration. In the four remaining patients, all complained of snoring, daytime fatigue, and daytime sleepiness; three complained of gasping and/or choking arousals; and one complained of witnessed apneas. Two patients were noted to have significant periodic limb movements during the night, and one was placed on pharmacologic therapy. The third was noted to have restless legs syndrome and insomnia, and she was treated with pharmacologic therapy and referred for cognitive behavioral therapy. The fourth patient had anxiety disorder with insomnia and poor sleep hygiene, and she was counseled and referred to psychiatry.
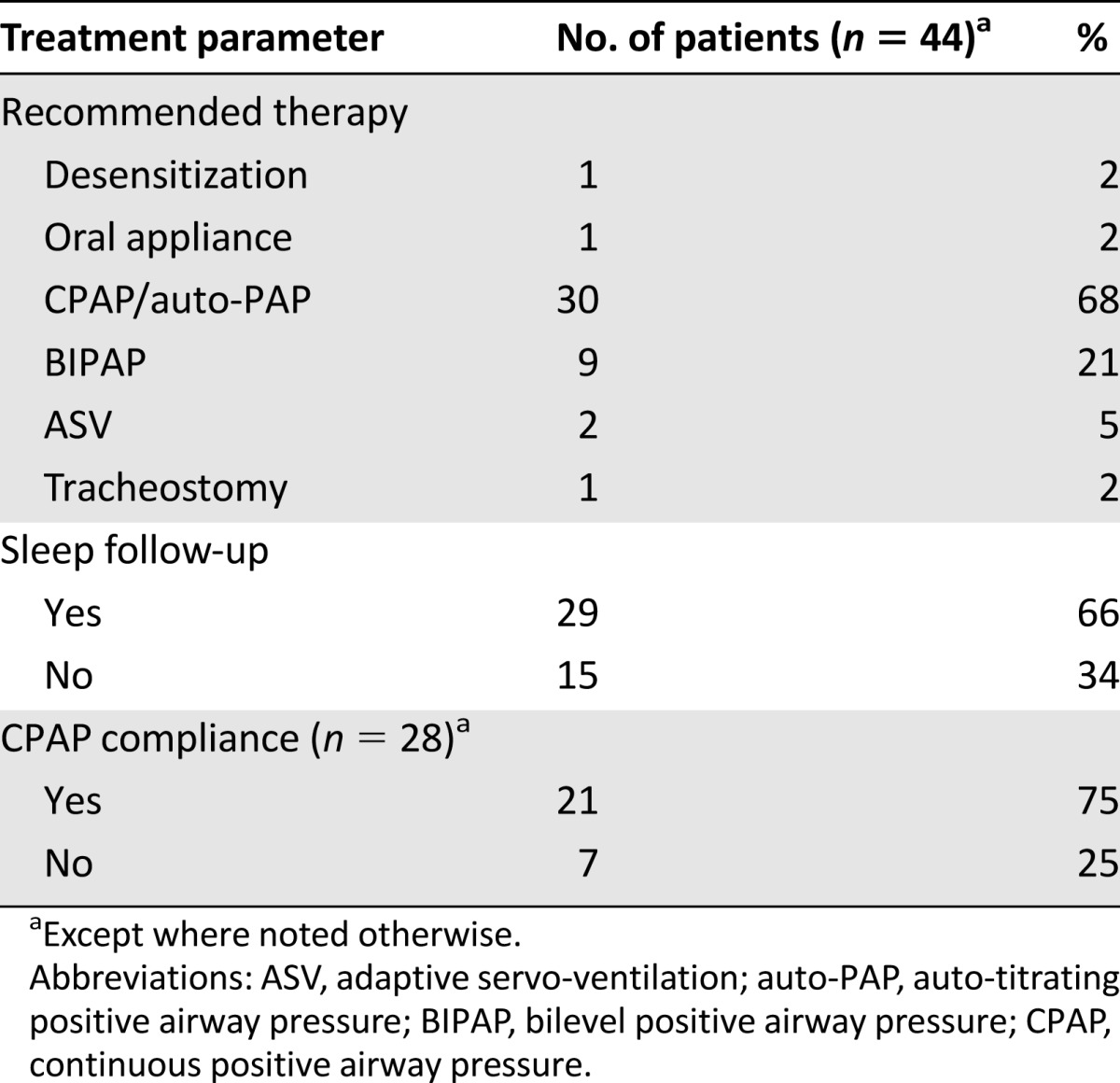
Treatment of sleep disordered breathing in patients who underwent PAP titration is further illustrated in Table 4. Most patients with significant sleep-related breathing disorder (77%) were prescribed positive airway pressure (PAP) therapy. Many patients (65%) followed up in sleep clinic, and 75% were compliant with PAP therapy. Two patients had persistent hypoxemia despite PAP therapy, and ASV was used, but both patients eventually required tracheostomy. In total, four patients underwent tracheostomy after sleep studies. Two patients underwent elective tracheostomy for complications related to radiation (laryngeal radionecrosis and osteroradionecrosis of mandible). Two patients had OSA with sleep-related hypoventilation further complicated by respiratory insufficiency, thus necessitating semiemergent tracheostomy.
Table 4.
Treatment of sleep-related breathing disorder among patients who had positive-pressure titration
The majority of patients (79%) underwent radiation prior to sleep study. Of those with radiation prior to sleep study, 88% had OSA, and in the group without prior radiation, 67% also had OSA. Subgroup analysis of OSA by primary site (mucosal cancer, skin cancer, salivary gland, primary neck involvement) and radiation prior to sleep study (radiation versus nonradiation) did not show a significant statistical difference.
The univariate analysis between all patients without OSA and all patients with OSA revealed that male gender (p = .024), radiation prior to sleep study (p = .09), active cancer (p = .06), higher Epworth sleepiness score (p = .09), and ECOG performance status 1–3 (p = .08) were significant predictors of the presence of OSA. In the logistic regression model for all patients, patients who had radiation prior to sleep study were more likely to have OSA (OR = 11.47; 95% CI = 1.49 to 88; p = .019). Patients who had active head and neck cancer were more likely to have OSA (OR = 13; 95% CI = 1.65 to 102; p = .015). In the logistic regression model for squamous cell pathology, the results were very similar.
Discussion
Sleep-related breathing disorders were common in patients with tumors in the head and neck region referred with sleep disruption. Daytime fatigue and sleepiness were the most common complaints. This is consistent with previous studies suggesting that sleep disturbance is common in patients with cancer and may worsen with chemotherapy and/or radiation [20]. The presence of malignancies involving the head and neck region should raise the level of clinical suspicion as a risk factor for sleep-related breathing disorder and more specifically OSA.
OSA was present in the majority of our cohort of patients that were referred with sleep complaints. Traditional hallmark OSA symptoms include snoring, daytime sleepiness, fatigue, gasping arousals, witnessed apneas, and unrefreshed sleep [19, 21]. Although a large proportion of our patients did report snoring (82%), a greater number complained of daytime fatigue (93%) and daytime hypersomnia (89%) (Table 2). Other compounding causes for sleep disruption specific to head and neck cancers include pain, depression, nicotine and alcohol use, and xerostomia [22]. The latter, occurring after radiation therapy, may adversely impact sleep via excessive consumption of liquids resulting in nocturia, frequent arousals, and difficulty returning to sleep after awakening. Disfiguring therapies (surgery, radiation) and socially distressing losses of function (eating, speaking, and swallowing) may also predispose these patients to depression [23]. Thus, a heightened clinical awareness is important because the presentation of OSA in cancer patients may not be typical. Often systemic manifestations of the malignancy or its treatments may obscure other etiologies of the patient’s complaints. A proactive approach to identifying OSA in these patients may also help decrease their overall symptom burden.
The use of an established sleep survey in addition to the clinician’s simple sleep history may help identify patients at risk. Although not diagnostic of OSA, the ESS is a brief eight-question validated reliable tool to assess daytime hypersomnolence, and it is commonly used to screen patients with possible OSA [5, 24]. The ESS was positive in 52% of our patients. Although no survey has been validated to detect OSA in the cancer population, other surveys such as the PSQI may provide some insight into sleep disruption. In cancer patients, fatigue from the systemic manifestations of the cancer or its treatment, suboptimal sleep hygiene, use of analgesics and narcotics, and concomitant respiratory or neurologic disease may also be contributing to their sleep disruption. Other disorders such as insomnia and sleep-related movement disorders should also be addressed.
In the general population, treatment of OSA with PAP therapy improves psychological and physical impairments in patients presenting primarily with fatigue, neurocognitive impairment, and depression [4]. Treatment options for OSA in patients with tumors in the head and neck region, however, may provide additional challenges to this treatment modality. PAP therapy was recommended for most of our patients. Of those that followed up, adherence to therapy (Table 4) was slightly better when compared with that reported in the general population (54%) [25]. However, PAP therapy may be difficult to administer if facial deformities exist. Other common issues such as dry mouth may be amplified in this population. Finally, there was a subset of patients in our cohort for whom hypoxia was persistent despite advanced forms of PAP, and tracheostomy was the only treatment option.
Although the impact of untreated OSA on the outcome of the cancer itself in human subjects has not been well defined, intermittent hypoxia associated with repetitive episodes of reduction or cessation of breathing during sleep can have additional potential adverse consequences at both tissue and systemic levels [26]. Two large observational studies have also suggested the possible effect of intermittent hypoxia and cancer by showing an association between a worse outcome of patients with malignancies and OSA and an increased risk of cancer and OSA. A population based cohort study from Wisconsin (1,522 subjects, 22-year follow-up) demonstrated that both the overall and cancer-specific mortality increased linearly and significantly with increasing OSA severity [27]. This association remained significant after adjusting for potential confounding variables such as age, sex, smoking, BMI, physical activity, diabetes, waist circumference, and sleep duration. This association persisted after exclusion of those patients treated with PAP therapy [27]. A multicenter, clinical cohort study from Spain (4,910 patients, median follow-up of 5 years) concluded that OSA severity, based on the percentage of nocturnal desaturation below 90% (TSat90), was independently associated with increased risk of incident cancer after correcting for important confounding variables, such as age, sex, smoking, alcohol consumption, and BMI [28]. Our cohort of patients with cancer mirrors the severity of OSA found in the previously described population-based studies in that 78% of patients had moderate to severe OSA, and the SaO2 nadir was 77% ± 9%, reflecting significant hypoxia. In our study, we could not determine the temporal relationship between onset of OSA and cancer diagnosis or the effect of OSA (with and without treatment) on cancer outcome.
Frequent medical comorbidities associated with OSA including hypertension, atrial fibrillation, and diabetes were also seen in our patients. However, hypertension and hypothyroidism were most common. Although OSA can be prevalent in patients with untreated hypothyroidism, we believe thyroid dysfunction in our patients with cancers in the head and neck region is likely a sequella from radiation therapy, and optimization of thyroid supplementation should be addressed. Common risk factors for OSA include male gender, obesity, increased neck circumference, enlarged tonsils and adenoids, decreased upper airway diameter, decreased upper-airway muscle responsiveness during sleep, and craniofacial abnormalities [4, 6, 21]. Our cohort has a male predominance (77%), but contrary to the perceived body type associated with OSA, a significant portion of these patients were not obese (BMI <30 in 52%). Furthermore, patients who received radiation prior to the sleep study and those with active cancer were more likely to have OSA in our cohort. Thus, we speculate that structural changes as a result of the tumor or therapy (surgery and/or radiation) may be more important by reducing upper airway compliance, altering neurosensory feedback and interfering with adequate ventilation and oxygenation during sleep. Of note, most of our patients had active cancer (80%) and a good performance status (ECOG 0–2 in 98%), and the majority were alive 1 year after their baseline PSG (89%). Thus, this supports the importance of appropriate work up and therapy for symptoms in these highly functional patients.
Although we used an electronic medical record and the sleep laboratory database to minimize loss of patient data, our study has the limitations inherent to most retrospective studies. We included data only from patients referred to the sleep clinic. Therefore, patients with cancers in the head and neck region for whom a sleep consult was not requested or a PSG was not performed were not included in the study. Furthermore, because these patients were referred with sleep complaints, the percentage of sleep-related breathing disorder and/or OSA may be higher in our cohort as compared with all patients with tumors in the head and neck region. This also highlights the clinical importance of a systematic method for eliciting a sleep history and for recognizing symptoms that may suggest OSA. Also, coexisting sleep disturbances such as insomnia may make it difficult to identify underlying OSA. Patients or caregivers minimizing symptoms can also be a confounding factor in these studies, because it has been shown that cancer patients can marginalize their sleep disruption as “expected” with cancer or “not as important.”
Further prospective study in this patient population could provide meaningful clinical information. Validations of sleep surveys in this population, as well as their correlation to the presence of OSA, are important to identify patients at risk. The identification and treatment of OSA may have a significant physical and psychosocial impact. The goal of future research projects would include assessing the cause and effect of head and neck cancers on OSA with sleep studies before and after treatment, evaluating the psychosocial and economic impact of OSA diagnosis and treatment, and determining methods in which the risk of OSA may be reduced in these patients. Mechanistically further evaluation for neuromuscular dysfunction after radiation and surgery, as well endoscopic evaluation for sites of upper airway obstruction, may further elucidate the pathophysiology of OSA in this population. Currently, there is no consensus statement from otolaryngology or sleep societies to evaluate for OSA in these patients. Systematic evaluation with a sleep history and sleep surveys followed by formal consultation by a boarded sleep specialist and PSG would likely be beneficial. Further study is warranted to optimize outcomes in patients with head and neck cancer and manage their sleep disturbances before, during, and after cancer treatment.
Conclusion
There is clear association between sleep-related breathing disorder and cancers in the head and neck region referred with sleep complaints. We hypothesize that the architectural distortion from the tumor and/or therapy (surgery or radiation) likely predisposes these patients to OSA, probably because of both anatomic and neural factors. Unlike OSA in the general population, these patients are not typically obese and may present with daytime fatigue and hypersomnia. Comorbid medical conditions typically include hypertension and hypothyroidism. Clinical suspicion of OSA may be higher in patients with active disease or in those who have received radiation therapy. Although adherence to PAP therapy may be therapeutic in these patients, certain limitations for the use of these therapies exist, and persistent hypoxia despite advanced PAP therapy may warrant tracheostomy. Further research to evaluate the prevalence of OSA in cancer in the head and neck region after definitive treatment could have significant clinical implications. Finally, based on epidemiologic studies, the identification and treatment of OSA may influence tumor behavior and affect the patient’s clinical course. Given the significant amount of sleep-related breathing disorders in our cohort, a heightened awareness for complaints of sleep disruption and subsequent referral for evaluation of potential OSA is paramount in patients with tumors in the head and neck region.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank the members of the sleep laboratory (Brenda Aaron-Remmert, Leendert Keus, Stephen Mahoney, Vickie Murphy, and Guadelupe Pachecho) for their participation in the diagnostic study and clinical care of these patients. We express our gratitude to Dr. Rodolfo Morice for his assistance in the editorial review of the manuscript. This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672.
Footnotes
For Further Reading:Elisabeth C.W. Neefjes, Maurice J.D.L. van der Vorst, Susanne Blauwhoff-Buskermolen et al. Aiming for a Better Understanding and Management of Cancer-Related Fatigue. The Oncologist 2013;18:1135–1143.
Implications for Practice:Cancer-related fatigue (CRF) is a serious symptom of patients with cancer and deteriorates their daily quality of life. Whereas fatigue is a common problem in the general population, with a prevalence of about 30%, up to 99% of patients with cancer have fatigue of more intense severity. CRF is directly related to the biology of cancer, but it can also be caused by anticancer treatment. We reviewed current evidence about the potential pathophysiological mechanisms causing CRF. Clinical methods to determine the presence and severity of CRF and potential treatment options to reduce CRF will be discussed. After reading this review, the reader will have knowledge of the current understanding of CRF and will be able to give evidence-based advice to patients with CRF.
Author Contributions
Conception/Design: Saadia A. Faiz
Provision of study materials or patients: Saadia A. Faiz
Collection and/or assembly of data: Saadia A. Faiz, Beth M. Beadle, William N. William Jr.
Data analysis and interpretation: Saadia A. Faiz, Diwakar Balachandran, Amy Hessel, Xiudong Lei, Beth M. Beadle, William N. William Jr., Lara Bashoura
Manuscript writing: Saadia A. Faiz, Diwakar Balachandran, Lara Bashoura
Final approval of manuscript: Saadia A. Faiz, Diwakar Balachandran, Amy Hessel, Xiudong Lei, Beth M. Beadle, William N. William Jr., Lara Bashoura
Disclosures
The authors indicated no financial relationships.
References
- 1.Davidson JR, MacLean AW, Brundage MD, et al. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 2.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 3.Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. The Oncologist. 2007;12(suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 4.Banno K, Kryger MH. Sleep apnea: Clinical investigations in humans. Sleep Med. 2007;8:400–426. doi: 10.1016/j.sleep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Roehrs T, Carskadon MA, Dement WC, et al. Daytime sleepiness and alertness. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2005. pp. 39–50. [Google Scholar]
- 6.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 7.Friedman M, Landsberg R, Pryor S, et al. The occurrence of sleep-disordered breathing among patients with head and neck cancer. Laryngoscope. 2001;111:1917–1919. doi: 10.1097/00005537-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Dedhia RC, Rosen CA, Soose RJ. What is the role of the larynx in adult obstructive sleep apnea? Laryngoscope. 2014;124:1029–1034. doi: 10.1002/lary.24494. [DOI] [PubMed] [Google Scholar]
- 9.Zorick F, Roth T, Kramer M, et al. Exacerbation of upper-airway sleep apnea by lymphocytic lymphoma. Chest. 1980;77:689–690. doi: 10.1378/chest.77.5.689. [DOI] [PubMed] [Google Scholar]
- 10.Luigetti M, Cianfoni A, Scarano E, et al. Mycosis fungoides as a cause of severe obstructive sleep apnea. Intern Med. 2011;50:1753–1755. doi: 10.2169/internalmedicine.50.5541. [DOI] [PubMed] [Google Scholar]
- 11.Giddings CE, Bray D, Rimmer J, et al. Pleomorphic adenoma and severe obstructive sleep apnoea. J Laryngol Otol. 2005;119:226–229. doi: 10.1258/0022215053561602. [DOI] [PubMed] [Google Scholar]
- 12.Smadi T, Raza MA, Woodson BT, et al. Obstructive sleep apnea caused by carotid body tumor: Case report. J Clin Sleep Med. 2007;3:517–518. [PMC free article] [PubMed] [Google Scholar]
- 13.Farboud A, Pratap R, Helquist H, et al. An unusual cause of obstructive sleep apnoea. J Laryngol Otol. 2009;123:e22. doi: 10.1017/S0022215109990910. [DOI] [PubMed] [Google Scholar]
- 14.Ballesteros F, Jose Sanz J, Maria Guilemany J, et al. Bulky cervical liposarcoma associated with sleep apnea syndrome. Acta Otolaryngol. 2006;126:209–213. doi: 10.1080/00016480500266784. [DOI] [PubMed] [Google Scholar]
- 15.Payne RJ, Hier MP, Kost KM, et al. High prevalence of obstructive sleep apnea among patients with head and neck cancer. J Otolaryngol. 2005;34:304–311. doi: 10.2310/7070.2005.34502. [DOI] [PubMed] [Google Scholar]
- 16.Nesse W, Hoekema A, Stegenga B, et al. Prevalence of obstructive sleep apnoea following head and neck cancer treatment: A cross-sectional study. Oral Oncol. 2006;42:108–114. doi: 10.1016/j.oraloncology.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Steffen A, Graefe H, Gehrking E, et al. Sleep apnoea in patients after treatment of head neck cancer. Acta Otolaryngol. 2009;129:1300–1305. doi: 10.3109/00016480802613113. [DOI] [PubMed] [Google Scholar]
- 18.Qian W, Haight J, Poon I, et al. Sleep apnea in patients with oral cavity and oropharyngeal cancer after surgery and chemoradiation therapy. Otolaryngol Head Neck Surg. 2010;143:248–252. doi: 10.1016/j.otohns.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: A review. Eur J Cancer Care (Engl) 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 22.Shuman AG, Duffy SA, Ronis DL, et al. Predictors of poor sleep quality among head and neck cancer patients. Laryngoscope. 2010;120:1166–1172. doi: 10.1002/lary.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archer J, Hutchison I, Korszun A. Mood and malignancy: Head and neck cancer and depression. J Oral Pathol Med. 2008;37:255–270. doi: 10.1111/j.1600-0714.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Garcia MA, Campos-Rodriguez F, Farre R. Sleep apnoea and cancer: Current insights and future perspectives. Eur Respir J. 2012;40:1315–1317. doi: 10.1183/09031936.00127912. [DOI] [PubMed] [Google Scholar]
- 27.Nieto FJ, Peppard PE, Young T, et al. Sleep-disordered breathing and cancer mortality: Results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]


