Abstract
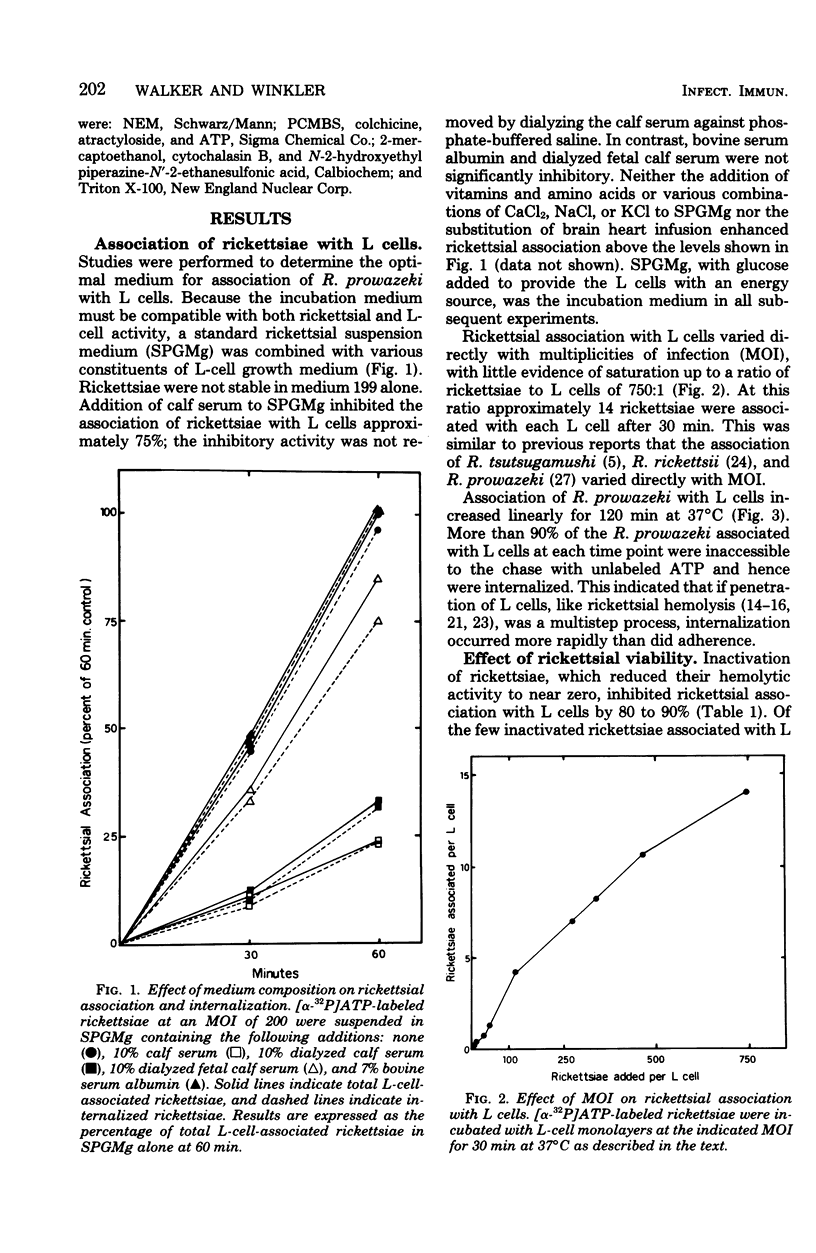
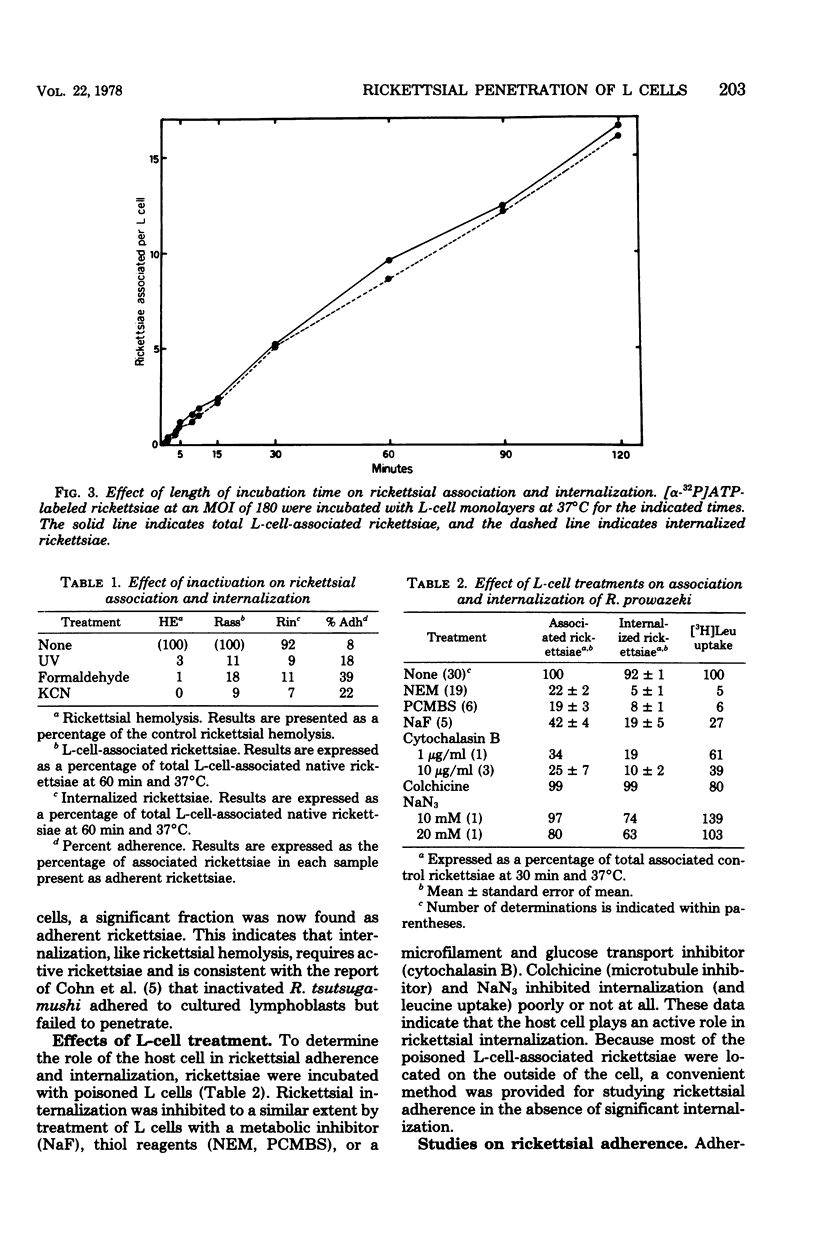
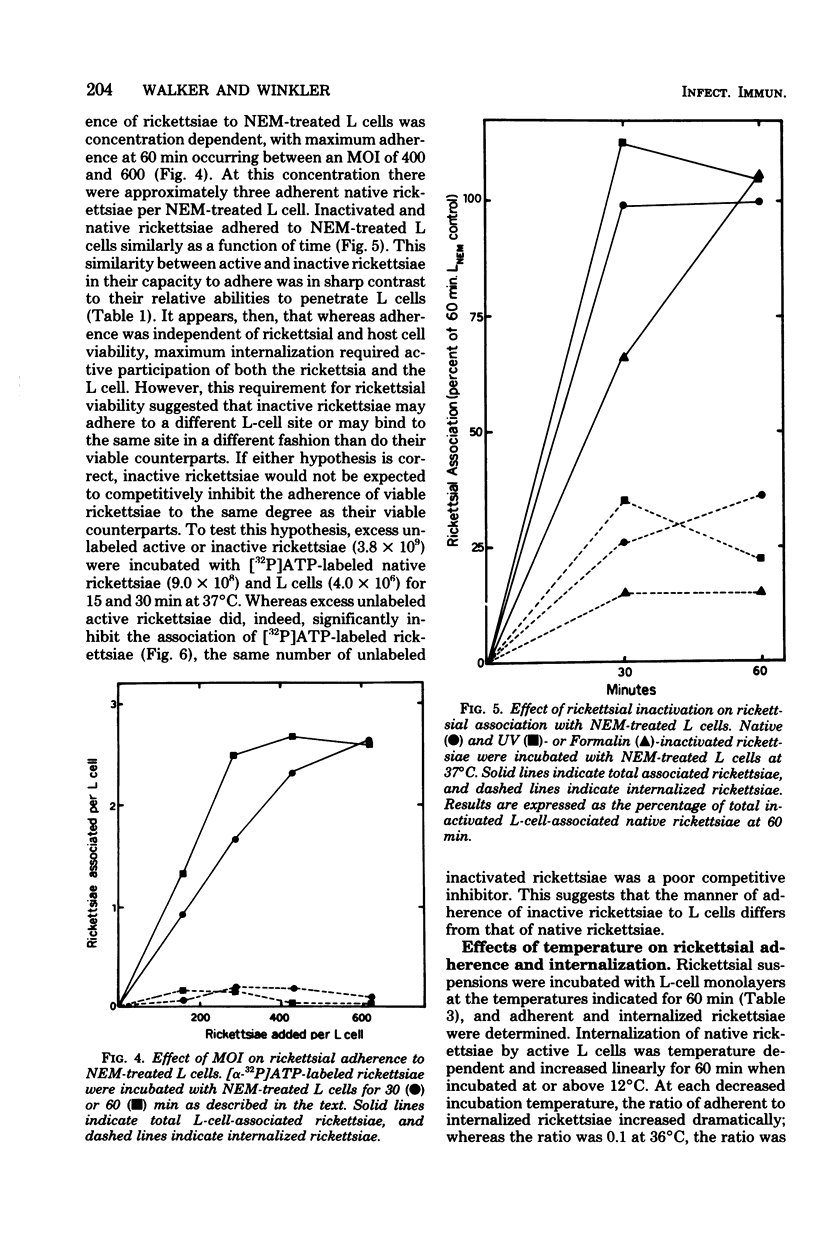
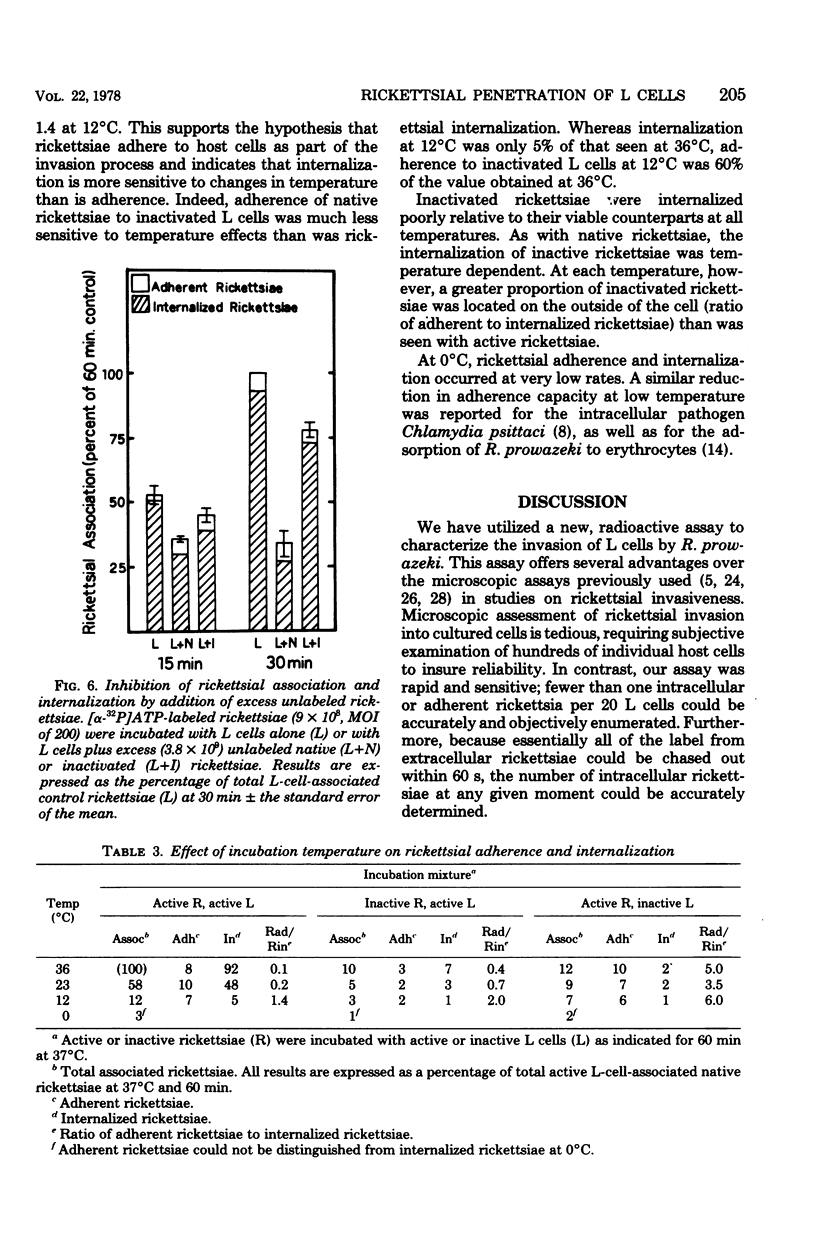
The association of Rickettsia prowazeki with L cells was examined by using a novel radioactive assay in which [α-32P]ATP-labeled rickettsiae were incubated with L-cell monolayers. Rickettsial association with the monolayer involved adherence and internalization steps that could be experimentally distinguished. Since R. prowazeki but not L cells possess an ATP-ADP obligate exchange transport system, addition of excess unlabeled ATP resulted in exchange of the labeled ATP from external, adherent rickettsiae but not from internalized rickettsiae. Rickettsial association was temperature dependent and was a linear function of both time and concentration. More than 90% of the biologically active rickettsiae associated with L cells was internalized. Rickettsial internalization required active participation of both rickettsiae and L cells; inactivation of either greatly reduced internalization. Rickettsial adherence to poisoned L cells was a saturable function of time and concentration. Adherence showed less temperature dependence than did internalization, but like rickettsial internalization, the extent of adherence was extremely low at 0°C. The rate and extent of adherence by inactivated and native rickettsiae to inactivated L cells were similar. Although inactive rickettsiae adhered to active and inactive L cells to a similar extent, inactive rickettsiae were internalized poorly by active L cells. These data form the basis for the hypothesis that R. prowazeki are internalized by the host cell through a process of “induced phagocytosis” and that inactivated rickettsiae adhere to the host cell differently from native rickettsiae, failing to trigger the endocytosis mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Identification of cholesterol in the receptor site for rickettsiae on sheep erythrocyte membranes. Infect Immun. 1976 Jan;13(1):120–126. doi: 10.1128/iai.13.1.120-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: adsorption of rickettsiae to erythrocytes. Infect Immun. 1973 Jan;7(1):93–99. doi: 10.1128/iai.7.1.93-99.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: effect of metabolic inhibitors upon hemolysis and adsorption. Infect Immun. 1973 Apr;7(4):550–555. doi: 10.1128/iai.7.4.550-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER J. C., BOVARNICK M. R., MILLER J. C., CHANG R. S. M. Observations on the hemolytic properties of typhus rickettsiae. J Bacteriol. 1954 Jun;67(6):724–730. doi: 10.1128/jb.67.6.724-730.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman R., Fiset P. Method for counting Rickettsiae and Chlamydiae in purified suspensions. J Bacteriol. 1968 Jan;95(1):259–261. doi: 10.1128/jb.95.1.259-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork E., Wisseman C. L., Jr Growth of Rickettsia prowazeki in enucleated cells. Infect Immun. 1976 Jun;13(6):1743–1748. doi: 10.1128/iai.13.6.1743-1748.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Ramm L. E. Adsorption of typhus rickettsiae to ghosts of sheep erythrocytes. Infect Immun. 1975 Jun;11(6):1244–1251. doi: 10.1128/iai.11.6.1244-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Edlinger E. A., Waddell A. D., Jones M. R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976 Oct;14(4):1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Silverman D. J. In vitro studies on Rickettsia-host cell interactions: lag phase in intracellular growth cycle as a function of stage of growth of infecting Rickettsia prowazeki, with preliminary observations on inhibition of rickettsial uptake by host cell fragments. Infect Immun. 1976 Jun;13(6):1749–1760. doi: 10.1128/iai.13.6.1749-1760.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. Mechanisms of immunity in typhus infections. IV. Failure of chicken embryo cells in culture to restrict growth of antibody-sensitized Rickettsia prowazeki. Infect Immun. 1974 Mar;9(3):571–575. doi: 10.1128/iai.9.3.571-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



