Abstract
Background.
In this study, we compared the efficacy and safety of the oral fluoropyrimidine S-1 as monotherapy or in combination with leucovorin as the second-line treatment for patients with metastatic pancreatic cancer whose disease had progressed on gemcitabine treatment.
Methods.
The study was a randomized, open-label, controlled study. Patients randomly received S-1 or S-1 in combination with leucovorin (SL arm) in 21-day cycles. The primary endpoint was the 6-month survival rate.
Results.
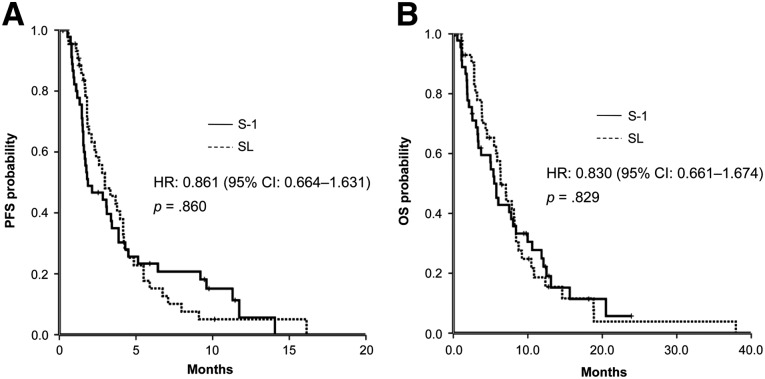
A total of 92 patients were randomized to S-1 (n = 47) and SL (n = 45). No statistically significant differences were observed between the two arms with regard to 6-month survival rates (40% vs. 49%), median overall survival (5.5 vs. 6.3 months), median progression-free survival (1.9 vs. 3.0 months), and overall response rate (4.7% vs. 8.3%). The rate of major grade 3–4 adverse events of digestive toxicity was significantly higher in the SL arm than in the S-1 arm.
Conclusion.
Compared with S-1, SL did not improve the survival of patients with metastatic pancreatic cancer who had failed to benefit from prior gemcitabine treatment, but SL had a higher adverse event rate.
Abstract
摘要
背景. 本研究旨在比较口服氟尿嘧啶 S-1 单药或联合亚叶酸钙用于二线治疗吉西他滨治疗后疾病进展的转移性胰腺癌的有效性和安全性。
方法. 本研究为随机、开放标签、对照试验。患者随机接受 S-1 或 S-1 联合亚叶酸钙(SL组)治疗,每 21 天为 1 周期。主要终点为 6 个月生存率。
结果. 共 92 例患者随机分配至 S-1 组(n=47)和 SL 组(n=45)。两组 6 个月生存率(40% vs. 49%)、中位总生存(5.5个月 vs. 6.3个月)、中位无进展生存(1.9个月 vs. 3.0个月)、总缓解率(4.7% vs. 8.3%)无统计学差异。SL 组主要 3∼4 级不良事件发生率显著高于 S-1 组。
结论. 对于吉西他滨治疗后未能获益的转移性胰腺癌患者,与 S-1 相比,SL 并未提高生存率,反而提高了不良事件发生率。The Oncologist 2014;19: 1133-1134
Author Summary
Discussion
There is no standard second-line treatment regimen after failure of first-line therapy for patients with locally advanced or metastatic pancreatic cancer (PC), mainly because of the poor condition of the patients.
S-1 has been approved to treat patients with metastatic PC in Japan and Taiwan. In the GEST study, median overall survival (OS) in the gemcitabine monotherapy group (8.8 months) was significantly longer than that in previously reported phase III studies, possibly because in Japan, a higher proportion of patients (50.5%) received S-1-based therapy in second-line treatment. For patients with gemcitabine-resistant advanced PC, several phase II studies have shown that S-1 in second-line treatment could achieve promising efficacy and good tolerability. In this study, we evaluated a combination of S-1 and leucovorin (SL) because preclinical studies have confirmed that leucovorin could enhance the efficacy of S-1. Its main mechanism is thought to be the formation of more covalent triple complexes (ternary complex; i.e., TS-FdUMP-CH2FH4) with the addition of leucovorin.
In this study, SL did not further enhance the overall response rate or the clinical benefit rate and did not prolong median OS and progression-free survival (PFS) compared with S-1 (Fig. 1). These results are different from previously reported results in metastatic colorectal cancer. It is possible that by enhancing the incidence of grade 3–4 adverse events compared with S-1 (57.8% vs. 31.1%), SL resulted in higher rates of dose reductions (40.0% vs. 14.9%)—an observation that is similar to previous reports. In this study, the overall response rate in the two arms was lower than in previous phase II studies of S-1 monotherapy as second-line treatment for advanced PC. Through analysis, we concluded that the lower response rate may be explained by the poor general condition of the patients (53.7% with Karnofsky performance status 70–80) and the higher proportions of withdrawal (10.9%) and treatment at reduced dose levels (30.5%) due to adverse events. Compared with the S-1 arm, patients in the SL arm had significantly higher digestive toxicity but no significant increase in grade 3 or 4 bone marrow toxicity; however, in the SL arm, after reducing the dose level of S-1, 83.3% (15 of 18) of patients could tolerate it. OS and PFS in the two arms were similar to the results of previously reported studies that enrolled some locally advanced PC, whereas in this study, most patients had metastatic PC and poor prognosis. Patients with metastasis in two or more organs accounted for 71.4% of the S-1 arm and 65% of the SL arm, showing that S-1 or SL after dose adjustment had some effect for patients with metastatic PC.
Figure 1.
Kaplan-Meier estimates of progression-free survival (A) and overall survival (B) in the two arms.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; SL, S-1 plus leucovorin.
Supplementary Material
Acknowledgment
Feijiao Ge and Nong Xu contributed equally to this work.
Footnotes
Access the full results at: Xu-14-223.theoncologist.com
ClinicalTrials.gov Identifier: NCT01074996
Sponsor(s): Taiho Pharmaceutical Company Limited
Principal Investigator: Jianming Xu
IRB Approved: Yes
For Further Reading:Jason E. Faris, Lawrence S. Blaszkowsky, Shaunagh McDermott et al. FOLFIRINOX in Locally Advanced Pancreatic Cancer: The Massachusetts General Hospital Cancer Center Experience. The Oncologist 2013;18:543–548.
Implications for Practice:The prognosis for patients with locally advanced pancreatic cancer, who constitute about almost a third of patients presenting with a new diagnosis of pancreatic cancer, is quite poor, with a median survival of approximately 1 year. The ideal treatment paradigm for these patients is unclear, but based on the experience with FOLFIRINOX in the metastatic setting, multiple institutions have begun to treat with FOLFIRINOX for patients with locally advanced disease. In this paper, the authors describe their institutional experience with FOLFIRINOX followed by chemoradiation in patients with locally advanced pancreatic cancer. Evidence is provided for substantial activity, with conversion to surgical resectability in more than 20% of patients. The authors suggest that further study is warranted on this promising treatment approach for patients with locally advanced pancreatic cancer.
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.