Abstract
Hepatocellular carcinoma (HCC) is a highly complicated disease characterized by comorbid cirrhosis and disease heterogeneity. Given multiple failures in the past, we need to learn from previous experiences and generate novel ideas to increase the chance of success. More effort and patience should be exercised in the selection of a homogeneous patient population and identification of predictive markers during drug development for HCC.
The pace of drug development for hepatocellular carcinoma (HCC) has been astonishingly slow. At present, sorafenib is the only approved systemic agent for treatment of advanced HCC. Previous successes with sorafenib initially triggered a wave of clinical trials of multitargeted agents aiming at angiogenic signaling molecules for HCC. However, such high hope has recently been let down by a series of negative results from randomized clinical trials in both first- and second-line settings (Table 1). Multifactorial reasons exist to account for the difficulty in drug development for HCC. A well-known factor is the presence of comorbid cirrhosis in most HCC patients, leading to impaired drug metabolism and reduced drug tolerability. Recent data on next-generation sequencing of the tumor indicate that HCC is composed of a large number of genetic and epigenetic alterations, and no clinically relevant druggable driver molecules have been identified for HCC [12]. All of these factors could have contributed to failure of the current approach to drug testing of antiangiogenic targeted agents for treatment of HCC.
Table 1.
Key randomized studies of systemic agents for hepatocellular carcinoma
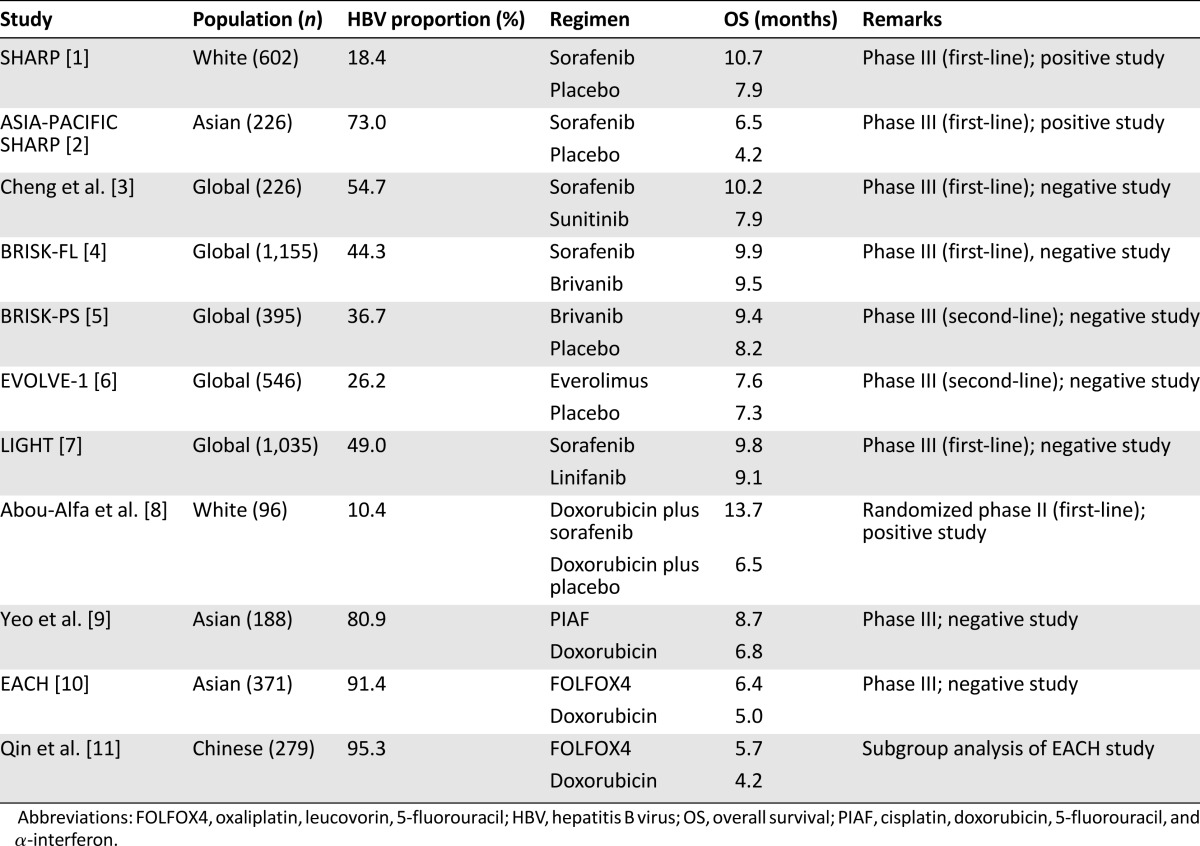
In the current issue of The Oncologist, Qin et al. report on the use of cytotoxic chemotherapy as treatment for HCC in a Chinese population [11]. The study is based on a multicenter randomized clinical trial, known as the EACH study, conducted from March 2007 to May 2009 [10]. The EACH study was carried out in Southeast Asian countries, from which patients with a confirmed diagnosis of advanced HCC were randomized in a 1:1 ratio into one of two chemotherapy regimens, namely, FOLFOX4 (oxaliplatin, leucovorin, 5-fluorouracil) or doxorubicin. The results of the whole EACH study have already been published in full in 2013 [10]. In summary, the study failed to meet its primary objective of improvement in overall survival (6.4 months in the FOLFOX4 arm vs. 5.0 months in the doxorubicin arm; p = .07) despite an improvement in progression-free survival favoring the FOLFOX4 regimen [10]. In the current study, Qin et al. carried out an exploratory analysis in the subgroup of patients of Chinese ethnicity, which accounted for 75% of the EACH study population [11]. In this subgroup, it was found that the FOLFOX4 regimen was associated with better overall survival, which was statistically significant (5.7 vs. 4.3 months; hazard ratio: 0.74; p = .03). Other efficacy endpoints, including progression-free survival and response rate, also favored the FOLFOX4 regimen.
How should these results be interpreted in the context of the existing literature? The use of cytotoxic chemotherapy in HCC is not a novel concept. Its history dates back to the 1980s, and for the past 30 years, potential activities of a wide spectrum of cytotoxic chemotherapeutic agents have been reported by various studies, with doxorubicin as the most commonly used regimen. According to large-scale randomized clinical trials using chemotherapy as first-line systemic treatment, the overall survival of patients undergoing doxorubicin treatment was in the range of 6–7 months, with radiological and serological response rates of around 5% and 20%, respectively [8, 9, 13]. Unfortunately, there has never been a single prospective randomized study to compare chemotherapy with placebo for treatment of HCC. In addition, the toxicity issue of chemotherapy has been a concern for patients with more severe cirrhotic damage. Consequently, cytotoxic chemotherapy has not been widely considered to be a standard treatment for HCC [14]. In the EACH study, the investigators decided to use doxorubicin, instead of sorafenib, as the control drug. It was noted that the overall survival of 4 months observed in the doxorubicin arm of the current study was worse than the previously reported figure. This discrepancy could be explained by a portion (25%) of patients having received prior systemic treatment and the lower dose of doxorubicin used in the study. With this study design, the authors have provided data to suggest that the FOLFOX4 regimen could be a potentially more efficacious alternative to the conventional chemotherapy of doxorubicin in a Chinese population. However, due to a lack of head-to-head comparison between FOLFOX4 and sorafenib, it remains too early to conclude that FOLFOX4 is a standard treatment for advanced HCC in either the first- or second-line setting. The future direction of research should be geared toward validation of efficacy and toxicity of FOLFOX4 in comparison with sorafenib in the first-line setting or in comparison with placebo in the second-line setting.
The current study by Qin et al. has raised another important issue about drug development for HCC: the tumor is known to occur in the background of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection. Although sorafenib is approved for all patients with inoperable HCC regardless of etiology, emerging evidence suggests that the benefits of sorafenib vary in patients with different etiologies. According to subgroup analyses of the Asia-Pacific SHARP study (sorafenib vs. placebo) [2], a phase III clinical trial comparing sunitinib versus sorafenib [9], and the BRISK-FL study (brivanib vs. sorafenib) [4], there was a persistent trend for patients with chronic HBV infection to derive less benefit from sorafenib when compared with patients without HBV infection. In fact, a vast amount of literature indicates that the clinicopathological features and prognoses differ significantly between HBV- and HCV-related HCC [15, 16]. Generally, HBV-related HCC tends to be associated with younger age at onset, larger tumor, lower rate of cirrhosis, higher level of α-fetoprotein, and more aggressive disease course than HCV-related HCC. Deep sequencing work on HBV-related tumor has also indicated that distinct molecular events are involved in hepatocarcinogenesis and progression of the tumor [6]. Currently, most international clinical trials allow recruitment of HCC patients with different etiologies, including both HBV and HCV infection. Consequently, the trial outcome is of efficacy observed in a heterogeneous population of HCC, which renders interpretation of data difficult [17]. Taking this evidence into consideration, HBV-related HCC should be considered as a separate disease entity from HCV-related HCC, and clinical trials focusing only on HBV-related populations should be seriously considered.
Apart from resorting to cytotoxic chemotherapy, a number of different approaches have been proposed and attempted to develop new systemic treatment for HCC. One of the strategies is to exploit targets other than angiogenic signaling molecules. A wide variety of targets, including nonangiogenic signaling molecules (e.g., mammalian target of rapamycin [mTOR], mitogen-activated protein kinase [MEK]) and non-signaling-related targets (e.g., glypican-3, histone deacetylase, arginase), have already undergone clinical testing in HCC. There have been no winners to date. Recent clinical trials of agents targeting mTOR (everolimus) [18], glypican-3 [19], MEK (selumetinib) [20], and histone deacetylase (belinostat) [21] have failed to yield remarkable results. Another approach is to develop targeted agents in patient populations enriched with a predictive biomarker. This method is based on the hypothesis that the targeted agent confers significant benefit only in a small number of patients harboring the relevant markers or targets. Examples include c-MET inhibitors for HCC with overexpression of c-MET proteins or gene copies [22]. It is unclear whether this approach will be successful until the results of these clinical trials become available. Finally, a number of trials have commenced to test the combination of different systemic agents, with the aim of producing synergistic effects. At present, there have not been successful combinations of targeted agents for HCC, but, as a chemotherapy backbone, there was a promising phase II result for the combination of doxorubicin and sorafenib, with overall survival of longer than 13 months [8]. This encouraging result has led to initiation of an ongoing phase III trial comparing sorafenib and sorafenib plus doxorubicin (ClinicalTrials.gov identifier NCT01015833).
In conclusion, the current work by Qin et al. has undoubtedly provided us with one more drug option for future testing [11]. However, HCC is a highly complicated disease characterized by comorbid cirrhosis and disease heterogeneity. Given multiple failures in the past, we need to learn from previous experiences and generate novel ideas to increase the chance of success. Apart from testing more different drugs, more effort and patience should be exercised in the selection of a homogeneous patient population and identification of predictive markers during drug development for HCC.
Footnotes
Editor's Note: See the related article, “Efficacy and Safety of the FOLFOX4 Regimen Versus Doxorubicin in Chinese Patients With Advanced Hepatocellular Carcinoma: A Subgroup Analysis of the EACH Study,” on pages 1169–1178 of this issue.
Disclosures
Stephen L. Chan: Novartis, Pfizer (RF); Novartis (C/A).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto A, Totoki Y, Abe T, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 7.Cainap C, Qin S, Huang W, et al. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2013;31(suppl 4):249a. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: A randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 9.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 10.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 11.Qin S, Cheng Y, Liang J, et al. Efficacy and Safety of the FOLFOX4 Regimen Versus Doxorubicin in Chinese Patients With Advanced Hepatocellular Carcinoma: A Subgroup Analysis of the EACH Study. The Oncologist. 2014;19:1169–1178. doi: 10.1634/theoncologist.2014-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa H, Shibata T. Comprehensive genome sequencing of the liver cancer genome. Cancer Lett. 2013;340:234–240. doi: 10.1016/j.canlet.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: Serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Hiotis SP, Rahbari NN, Villanueva GA, et al. Hepatitis B vs. hepatitis C infection on viral hepatitis-associated hepatocellular carcinoma. BMC Gastroenterol. 2012;12:64. doi: 10.1186/1471-230X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, Huang GT, Yang PM, et al. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42:2524–2529. doi: 10.1016/j.ejca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: Present and future. J Gastroenterol Hepatol. 2012;27:862–872. doi: 10.1111/j.1440-1746.2012.07096.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 19.Yen CJ, Daniele B, Kudo M, et al. Randomized phase II trial of intravenous RO5137382/GC33 at 1600 mg every other week and placebo in previously treated patients with unresectable advanced hepatocellular carcinoma. J Clin Oncol. 2014;32(suppl 5):4102a. [Google Scholar]
- 20.O’Neil BH, Goff LW, Kauh JS, et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011;29:2350–2356. doi: 10.1200/JCO.2010.33.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo W, Chung HC, Chan SL, et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: A multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J Clin Oncol. 2012;30:3361–3367. doi: 10.1200/JCO.2011.41.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You H, Ding W, Dang H, et al. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–889. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]


