Abstract
Background.
Combination chemotherapy consisting of ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) was active as first-line and second-line treatment for extranodal natural killer/T-cell lymphoma (NTCL).
Methods.
Forty-four patients with chemo-naïve stage I/II NTCL were enrolled in a prospective, multicenter, phase II study and received six cycles of IMEP (ifosfamide 1.5 g/m2 on days 1–3; methotrextate 30 mg/m2 on days 3 and 10; etoposide 100 mg/m2 on days 1–3; and prednisolone 60 mg/m2 per day on days 1–5) followed by involved field radiotherapy (IFRT).
Results.
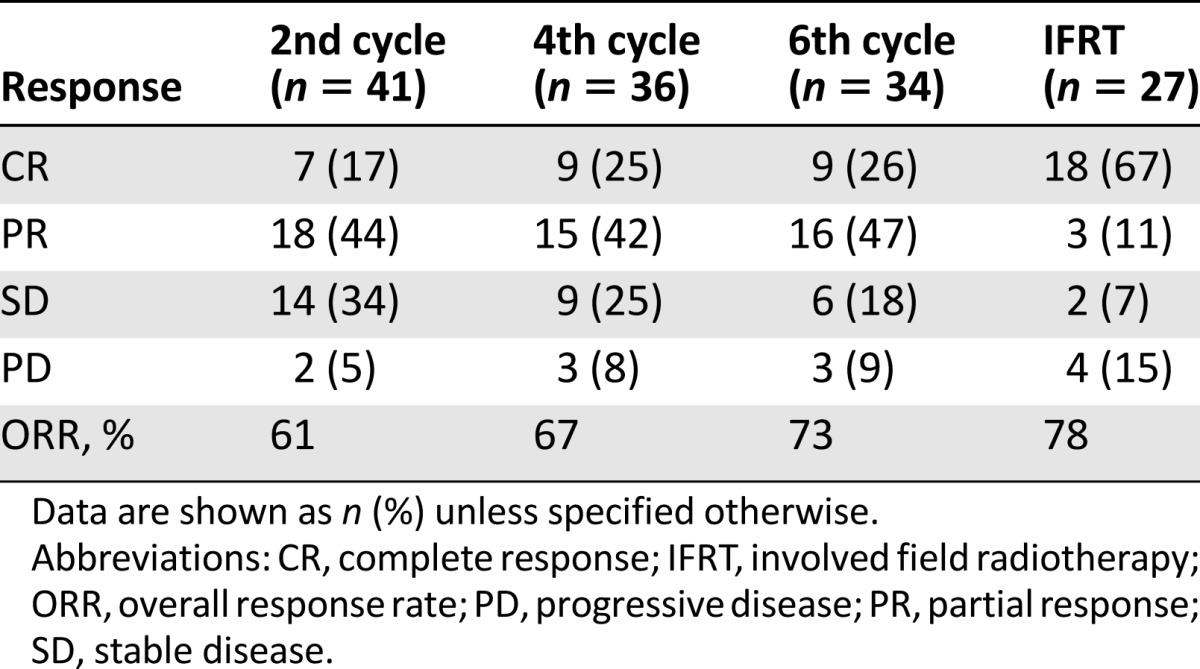
Overall response rates were 73% (complete remission [CR] in 11 of 41 evaluable patients [27%]) after IMEP chemotherapy and 78% (CR 18 of 27 evaluable patients [67%]) after IMEP followed by IFRT. Neutropenia and thrombocytopenia were documented in 33 patients (75%) and 7 patients (16%), respectively. Only 8 patients (18%) experienced febrile neutropenia. Three-year progression-free survival (PFS) and overall survival (OS) were 66% and 56%, respectively. High Ki-67 (≥70%) and Ann Arbor stage II independently reduced PFS (p = .004) and OS (p = .001), respectively.
Conclusion.
Due to the high rate of progression during IMEP chemotherapy, IFRT needs to be introduced earlier. Moreover, active chemotherapy including an l-asparaginase-based regimen should be use to reduce systemic treatment failure in stage I/II NTCL.
Abstract
摘要
背景. 异环磷酰胺、甲氨蝶呤、依托泊苷以及泼尼松龙(IMEP)联合化疗是结外鼻型自然杀伤/T 细胞淋巴瘤(NTCL)的有效一线和二线治疗。
方法. 本次前瞻性、多中心、 II 期研究入组 44 例既往未接受过化疗的 I/II 期 NTCL 患者,给予 IMEP 6 周期治疗(异环磷酰胺 1.5 mg/m2,第 1∼3 天;甲氨蝶呤 30 mg/m2,第 3、10 天;依托泊苷 100 mg/m2,第 1∼3 天;泼尼松龙 60 mg/m2,第 1∼5 天),继以受累野放疗(IFRT)。
结果. IMEP 化疗后,总缓解率为 73%[11/41 例可评估患者完全缓解(CR,27%)],IMEP 继以 IFRT 后,总缓解率为 78%[18/27 例可评估患者 CR(67%)]。确认的中性粒细胞减少和血小板减少分别为 33 例(75%)和 7 例(16%)。仅 8 例患者(18%)发生发热性中性粒细胞减少。3 年无进展生存(PFS)率和总生存(OS)率分别为 66% 和 56%。高Ki-67(≥ 70%)和 Ann Arbor II 期与 PFS(p=0.004)和 OS(p=0.001)较短独立相关。
结论. 由于 IMEP 化疗期间疾病进展率很高,IFRT 需要早期给予。而且,应该使用包括基于左旋门冬酰胺酶的方案在内的有效化疗,以减少 I/II 期 NTCL 的系统性治疗失败。The Oncologist 2014;19: 1129-1130
Author Summary
Discussion
Our trial was based on the scheme of chemotherapy followed by radiotherapy (sequential). However, other trials used concurrent chemoradiation followed by ifosfamide plus etoposide-based combination chemotherapy [4, 5]. Although the designs of two concurrent chemoradiation trials [4, 5] were very similar, large differences were observed regarding survival data. We chose the JCOG0211 study [4] for comparison with our data because the other study provided limited information on patterns of failure due to short-term follow-up [5]. Our study showed a relatively higher locoregional failure rate (18% vs. 4%) but lower rates of systemic failure than in the JCOG0211 study (14% vs. 33%). These data suggest that upfront chemoradiation is favorable for locoregional control, but it is unfavorable for systemic failure in stage I/II natural killer/T-cell lymphoma (NTCL). Extended chemotherapy of up to six cycles in our study might be a factor for the high incidence of locoregional failure. Better coordination of the sequence between chemotherapy and radiotherapy might reduce both locoregional and systemic failures. A reduced number of cycles of chemotherapy, for example, followed by involved field radiotherapy (IFRT), concurrent chemoradiation, or sandwich radiotherapy during chemotherapy may be more efficacious for untreated stage I/II NTCL. Considering that the planned doses were reduced for 11 patients (25%) in 35 of 229 cycles (15%) in our study, the ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) regimen was safe and relatively well tolerated.
Recently, regimens based on l-asparaginase (l-asp) have shown promising efficacy in patients with refractory/relapsed or newly diagnosed advanced NTCL (overall response rate of 78%–81% and complete response [CR] of 45%–66%) [6–8] and with untreated stage I/II NTCL (CR of 90%, 2-year overall survival [OS] of 88%, and 2-year progress-free survival [PFS] of 90%) [10]. Furthermore, gemcitabine, which is active against NTCL [11], plus oxaliplatin and l-asp followed by IFRT resulted in a CR rate of 74% and 2-year PFS of 86% in stage I/II upper aerodigestive tract NTCL [12]. Furthermore, sandwich l-asp, vincristine, and prednisolone chemotherapy with IFRT showed a promising outcome (2-year OS of 88.5% and 2-year PFS of 80.6%) [13]. Consequently, more active l-asp-based regimens should be introduced in patients with stage I/II NTCL.
The IMEP regimen is effective and safe in patients with stage I/II NTCL before the introduction of l-asp, and IMEP followed by IFRT resulted in improved treatment outcomes in localized NTCL (Table 1). However, a short-course of the l-asp-based regimen followed by IFRT or concurrent or sandwich radiation with an l-asp-based regimen should be introduced in patients with untreated stage I/II NTCL.
Table 1.
Treatment outcomes after completion of each treatment
Supplementary Material
Footnotes
Access the full results at: Heo-14-305.theoncologist.com
Trial Registry: Korean Cancer Study Group: KCSG LY04-03
Sponsor(s): Seoul National University Hospital
Principal Investigator: Dae Seog Heo
IRB Approved: Yes
For Further Reading:Xi Li, Xiaobo Tian, Bo Zhang et al. Polymorphisms in MicroRNA-Related Genes Are Associated With Survival of Patients With T-Cell Lymphoma. The Oncologist 2014;19:243–249.
Implications for Practice:Besides the characteristics of a tumor itself, genetic polymorphisms in relevant genes are considered to be important in influencing the clinical outcomes of patients. Previous studies have suggested that microRNAs are associated with survival of patients with T-cell lymphoma (TCL); therefore, we performed association analyses of 13 carefully selected polymorphisms in microRNA-related genes. The results suggested that four polymorphisms in microRNA-related genes are associated with survival of patients with TCL. These polymorphisms may be used to predict the survival of patients with TCL individually or collectively. Although the results are exploratory, they provide clues to warrant further investigation.
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


