Abstract
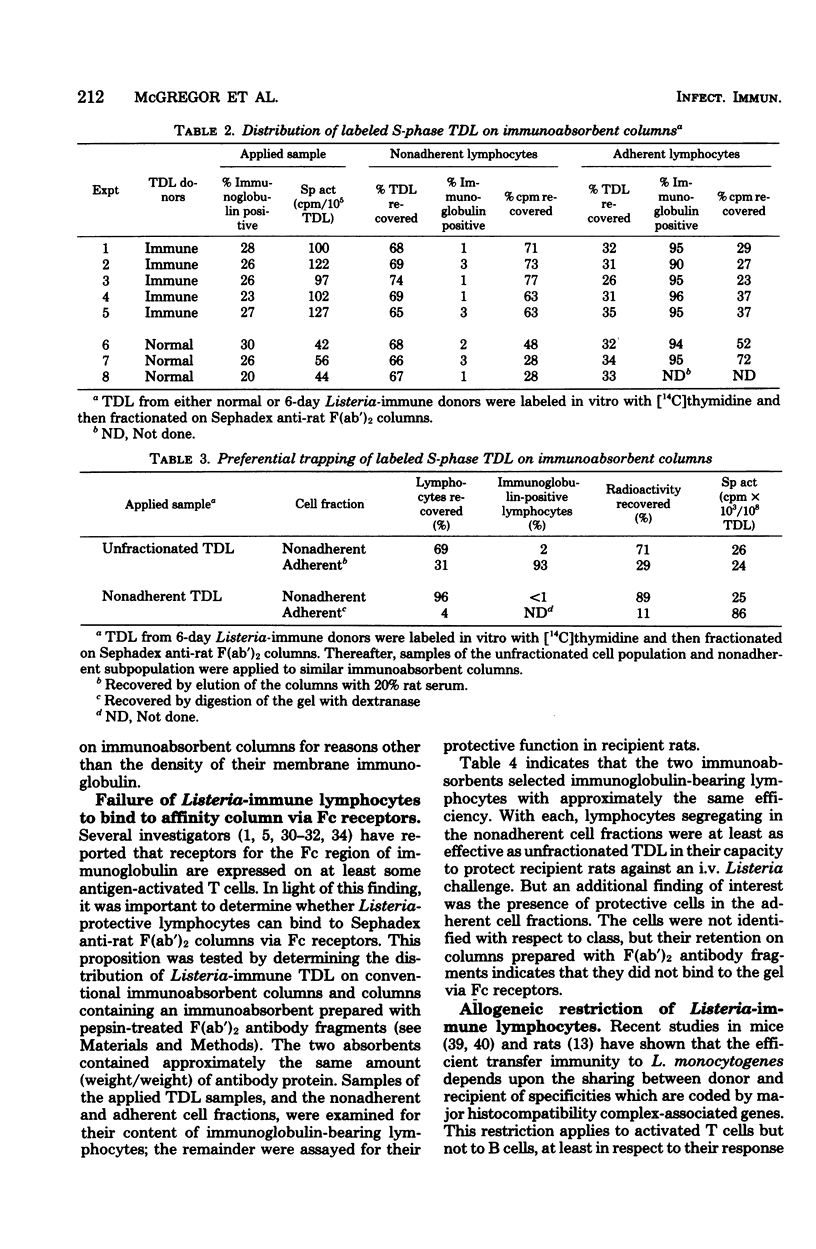
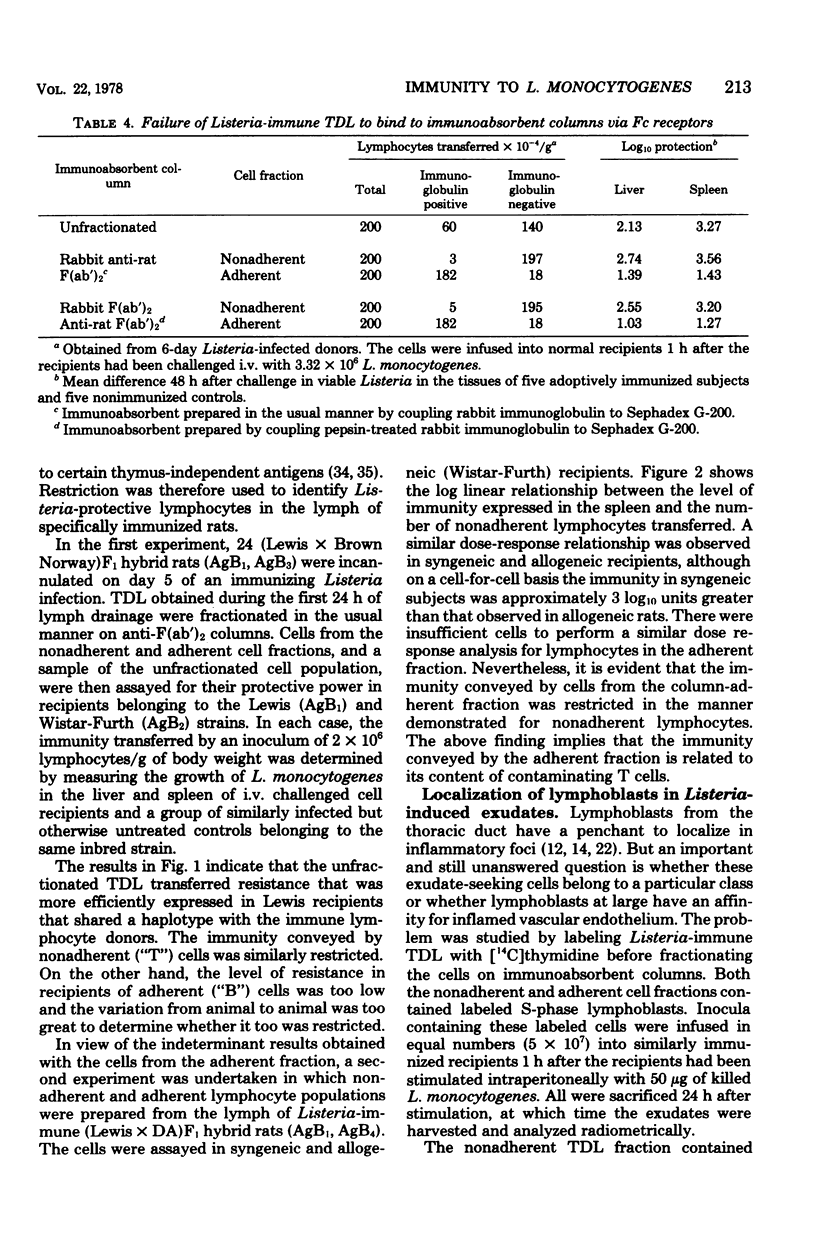
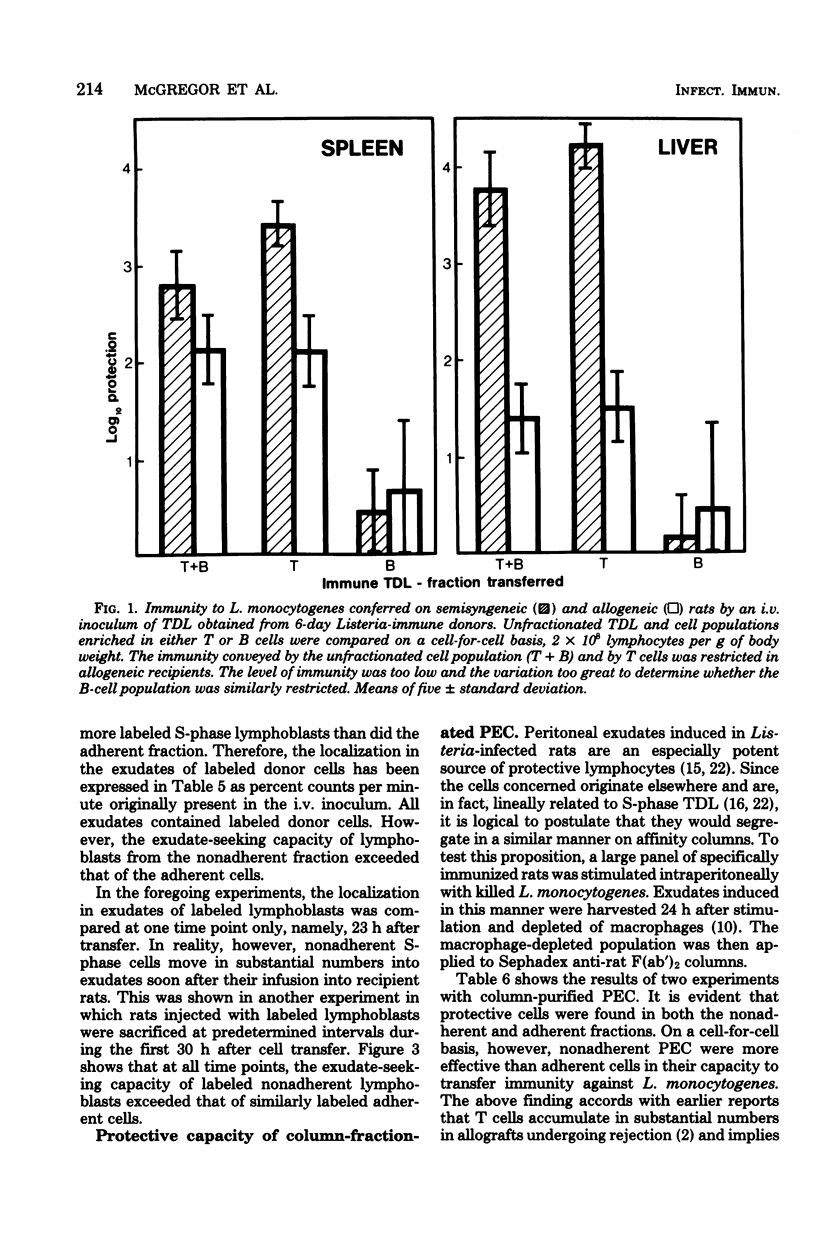
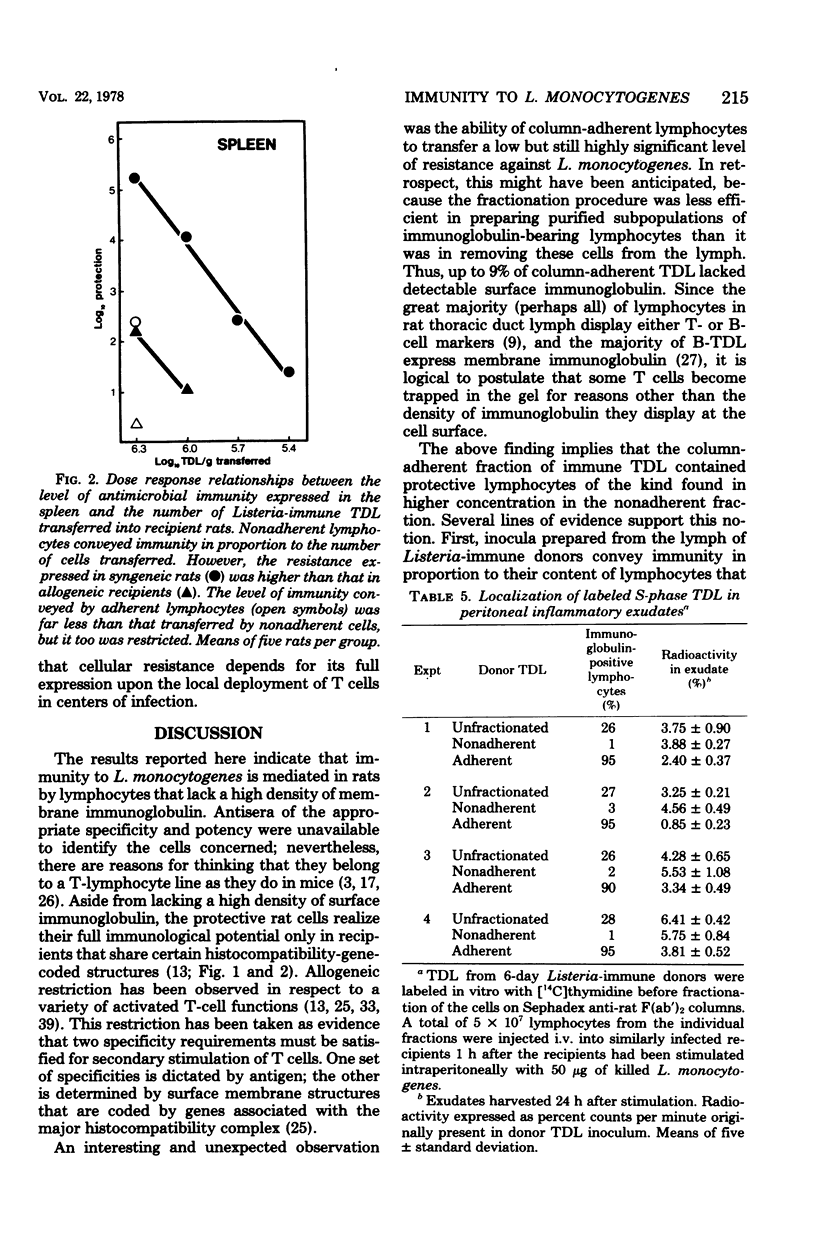
Affinity columns prepared with rabbit antibody to the F(ab′)2 fragment of rat immunoglobulin were used to separate rat thoracic duct lymphocytes into sub-populations that differ with respect to the density of their surface membrane immunoglobulin. Using this technique, it was shown that lymphocytes in the DNA synthetic (S) phase of the mitotic cycle are added in increased number to the lymph of rats infected with Listeria monocytogenes. The great majority of these S-phase cells lacked a high density of surface immunoglobulin as indicated by their failure to bind to the immunoabsorbent. Cells which can protect recipient rats against a challenge infection with L. monocytogenes also segregated with nonadherent thoracic duct lymphocytes obtained from Listeria-immune donors. These protective cells realized their full immunological potential only in recipients that shared histocompatibility-gene-coded structures with the immune lymphocyte donors. The above findings accord with the view that immunity to L. monocytogenes is mediated in rats by activated T cells which are formed as part of the animal's cell-mediated response to infection. Although Listeria-protective lymphocytes concentrate in the nonadherent, T-cell-enriched fraction, it was consistently observed that the adherent, B-cell-enriched fractions of immune donor thoracic duct lymphocytes also could transfer a low level of antimicrobial resistance. This immunity was restricted in allogeneic recipients, a finding which implies that the protection afforded by the adherent population is related to its content of T cells. Nonadherent S-phase lymphoblasts moved in substantial numbers from the blood into peritoneal inflammatory exudates induced by L. monocytogenes. The above finding encourages the belief that recently activated T cells realize their protective function locally in centers of infection where they have secondary effects on macrophages.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Grey H. M. Receptors for aggregated IgG on mouse lymphocytes: their presence on thymocytes, thymus-derived, and bone marrow-derived lymphocytes. J Exp Med. 1974 May 1;139(5):1175–1188. doi: 10.1084/jem.139.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., Reinisch C. L., Levey R. H., McCluskey R. T., Schlossman S. F. T-cell migration into allografts. J Exp Med. 1975 May 1;141(5):1210–1215. doi: 10.1084/jem.141.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Shevach E. Requirement for T cells in the production of migration inhibitory factor. J Exp Med. 1975 Nov 1;142(5):1306–1311. doi: 10.1084/jem.142.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M., Hogg N. Role of macrophages in the generation of T helper cells. IV. Nature of genetically related factor derived from macrophages incubated with soluble antigens. Eur J Immunol. 1976 May;6(5):365–372. doi: 10.1002/eji.1830060512. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Anatomical distribution of T and B lymphocytes in the rat. Development of lymphocyte-specific antisera. J Exp Med. 1973 Dec 1;138(6):1443–1465. doi: 10.1084/jem.138.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Blomgren H. Further evidence for autonomy of T cells mediating specific in vitro cytotoxicity: efficiency of very small amounts of highly purified T cells. Cell Immunol. 1973 Oct;9(1):127–141. doi: 10.1016/0008-8749(73)90174-3. [DOI] [PubMed] [Google Scholar]
- Hauschka T. S., Hitt S. A., Zumpft M., Shows T. B., Boyse E. A. Immunoselective loss of parental h antigens by somatic reduction in an H-2 a/H-2 b hybrid mouse leukemia. Transplant Proc. 1975 Jun;7(2):165–171. [PubMed] [Google Scholar]
- Jaffer A. M., Jones G., Kasdon E. J., Schlossman S. F. Local transfer of delayed hypersensitivity by T lymphocytes. J Immunol. 1973 Oct;111(4):1268–1269. [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Activated lymphocytes trigger lymphoblast extravasation. Cell Immunol. 1978 Jun;38(1):76–83. doi: 10.1016/0008-8749(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Allogeneic restriction of acquired antimicrobial resistance in the rat. J Immunol. 1978 Aug;121(2):449–455. [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Allogeneic restriction of the delayed inflammatory reaction in the rat. J Immunol. 1978 Aug;121(2):456–463. [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Lefford M. J. The mediator of cellular immunity. XI. Origin and development of MIF producing lymphocytes. Cell Immunol. 1976 Jun 15;24(2):318–327. doi: 10.1016/0008-8749(76)90215-x. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W. The requirement for C3 receptors on the precursors of 19S and 7S antibody-forming cells. J Exp Med. 1976 May 1;143(5):1111–1121. doi: 10.1084/jem.143.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Kostiala A. A. Role of Lymphocytes in Cellular resistance in infection. Contemp Top Immunobiol. 1976;5:237–266. [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VI. Effect of the antimitotic drug vinblastine on the mediator of cellular resistance to infection. J Exp Med. 1973 Mar 1;137(3):660–674. doi: 10.1084/jem.137.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VII. Localization of sensitized lymphocytes in inflammatory exudates. J Exp Med. 1974 Jun 1;139(6):1415–1430. doi: 10.1084/jem.139.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Vadas M. A. Antigen activation of T lymphocytes: influence of major histocompatibility complex. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):579–588. doi: 10.1101/sqb.1977.041.01.067. [DOI] [PubMed] [Google Scholar]
- North R. J., Spitalny G. Inflammatory lymphocyte in cell-mediated antibacterial immunity: factors governing the accumulation of mediator T cells in peritoneal exudates. Infect Immun. 1974 Sep;10(3):489–498. doi: 10.1128/iai.10.3.489-498.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., Hayward J. A. The lymphocyte surface. II. Separation of Fc receptor, C'3 receptor and surface immunoglobulin-bearing lymphocytes. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):65–81. doi: 10.1098/rspb.1974.0061. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Hudson L. Specific purification of lymphocyte populations on a digestible immunoabsorbent. J Immunol. 1973 Jan;110(1):313–319. [PubMed] [Google Scholar]
- Soteriades-Vlachos C., Gyöngyössy M. I., Playfair J. H. Rosette formation by mouse lymphocytes. III. Receptors for immunoglobulin on normal and activated T cells. Clin Exp Immunol. 1974 Oct;18(2):187–192. [PMC free article] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes. I. Detection of a subpopulation of murine T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Sep 1;142(3):611–621. doi: 10.1084/jem.142.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes: II. Mitogen responsiveness of T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Nov 1;142(5):1041–1051. doi: 10.1084/jem.142.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Yamashita U., Shevach E. M. The role of Ia antigens in T cell activation. Immunol Rev. 1977;35:95–120. doi: 10.1111/j.1600-065x.1977.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A., Sorvari T. Incomplete restoration of the bursa-dependent immune system after transplantation of allogeneic stem cells into immunodeficient chicks. Proc Natl Acad Sci U S A. 1974 Mar;71(3):957–961. doi: 10.1073/pnas.71.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Rosenstreich D. L. Binding of aggregated gamma-globulin to activated T lymphocytes in the guinea pig. J Exp Med. 1974 Apr 1;139(4):1002–1012. doi: 10.1084/jem.139.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Iverson G. M., Oppenheim J. J. Induction of guinea pig B-cell lymphokine synthesis by mitogenic and nonmitogenic signals to Fc, Ig, and C3 receptors. J Exp Med. 1974 Dec 1;140(6):1631–1645. doi: 10.1084/jem.140.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Rosenstreich D. L. Role of B lymphocytes in cell-mediated immunity. I. Requirement for T cells or T-cell products for antigen-induced B-cell activation. J Exp Med. 1976 Nov 2;144(5):1175–1187. doi: 10.1084/jem.144.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Adler B., Blanden R. V., Davidson W. F., Kees U., Dunlop M. B., Shreffler D. C. H-2 restriction of cell-mediated immunity to an intracellular bacterium: effector T cells are specific for Listeria antigen in association with H-21 region-coded self-markers. J Exp Med. 1977 May 1;145(5):1353–1367. doi: 10.1084/jem.145.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Restriction by H-2 gene complex of transfer of cell-mediated immunity to Listeria monocytogenes. Nature. 1974 Sep 20;251(5472):230–233. doi: 10.1038/251230a0. [DOI] [PubMed] [Google Scholar]



