Abstract
Background
Prostate-specific antigen (PSA) screening for prostate cancer has high risks of overdiagnosis, particularly in older men, and reports from screening trials indicate that it saves few lives after 11–13 years of follow-up. New clinical guidelines recommend against PSA screening for all men or for men over 70 years, but expected population effects of these guidelines have not been studied.
Methods
Two models of prostate cancer natural history and diagnosis were previously developed using reconstructed PSA screening patterns and prostate cancer incidence in the US. Assuming a survival benefit of PSA screening consistent with the screening trials, we used the models to predict incidence and mortality rates for the period 2013–2025 under continued PSA screening and under discontinued PSA screening for all men or for men over 70 years.
Results
The models predict that continuation of recent screening rates will overdiagnose 710,000–1,120,000 (range between models) men but will avoid 36,000–57,000 cancer deaths over the period 2013–2025. Discontinued screening for all men eliminates 100% of overdiagnoses but fails to prevent 100% of avoidable cancer deaths. Continued screening for men under 70 eliminates 64–66% of overdiagnoses but fails to prevent 36–39% of avoidable cancer deaths.
Conclusions
Discontinuing PSA screening for all men may generate many avoidable cancer deaths. Continuing PSA screening for men under 70 years could prevent more than half of these avoidable cancer deaths while dramatically reducing overdiagnoses relative to continued PSA screening for all ages.
Keywords: mass screening, models, statistical, prostate-specific antigen, prostatic neoplasms, surveillance
Introduction
Prostate cancer is the most common solid organ cancer in US men, with an estimated 233,000 new cases and 29,480 deaths in 2014.1 The high incidence of prostate cancer reflects a combination of high latent prevalence of disease2 and effects of prostate-specific antigen (PSA) screening. Widespread adoption of PSA screening beginning in 1987 led to a doubling of incidence rates and significantly reduced the occurrence of metastatic cancer at presentation.3 However, the role of PSA screening in the 56% drop in prostate cancer mortality rates since 19914 remains controversial. The US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial found no reduction in mortality after 13 years of follow-up.5 In contrast, the European Randomized Study of Screening for Prostate Cancer (ERSPC) found a significant 20% relative reduction in mortality after 11 years of follow-up, but this amounted to an absolute reduction of only one death per 1,000 men screened.6 While the long-term mortality benefit of PSA screening is uncertain, it may exceed that reported for the trials to date.7,8
Counterbalancing mixed reports of benefit, harms of PSA screening are significant. In the US, 23–42% of PSA-detected cancers would never have been detected in the absence of screening;9 by definition, treating these overdiagnosed cancers cannot improve patient outcomes and often leads to erectile, urinary, and bowel dysfunction.10-12 Although cancers detected in young men with high PSA and high Gleason score are unlikely to be overdiagnosed,13 a majority of cancers are found in older men with low-risk characteristics.
Concerns about high rates of overdiagnosis and overtreatment and small absolute numbers of cancer deaths prevented by screening in trial reports led the US Preventive Services Task Force (USPSTF) to recommend against routine PSA screening for all men.14 Other organizations, such as the American Cancer Society,15 American Urological Association,16 and the American College of Physicians,17 advise shared decision-making for men under age 70 years with at least a 10-year life expectancy. An upper age limit for screening was motivated partly by the age group found to benefit from screening in the ERSPC and partly because of higher risks of overdiagnosis and uncertain treatment benefit for older men.18-21
In this article, we quantify expected population effects of these new PSA screening guidelines using two models of prostate cancer natural history. The models are statistical representations of disease progression, detection, treatment, and survival that were previously developed to study the plausible roles of PSA screening22 and changes in initial treatments23 in prostate cancer mortality trends. Because the models separate prostate cancer natural history and non-screen diagnosis from detection by PSA screening, they provide a coherent framework for predicting plausible effects of discontinued screening. We consider perfect adherence to these new guidelines and continuation of contemporary disease management patterns as a reasonable (albeit idealized) substrate for evaluating expected impacts on prostate cancer incidence and mortality patterns.
Methods
Prostate cancer natural history
The two models of prostate cancer natural history and diagnosis24,25 used in this article were independently developed as part of the Cancer Intervention and Surveillance Modeling Network (CISNET), a consortium of investigators using surveillance models to investigate drivers of national cancer trends. Numerous statistical models have produced varying estimates of prostate cancer outcomes associated with PSA screening, but many do not readily generalize beyond the particular setting to which they were applied. The CISNET prostate cancer models were designed to use population-based data sources to disentangle disease natural history and non-screen diagnosis from effects of PSA screening. In this way, the estimated models represent a virtual laboratory for assessing the expected impacts of alternative screening PSA scenarios, such as discontinued screening. By using two models, we are able to examine sensitivity of results to natural history assumptions.
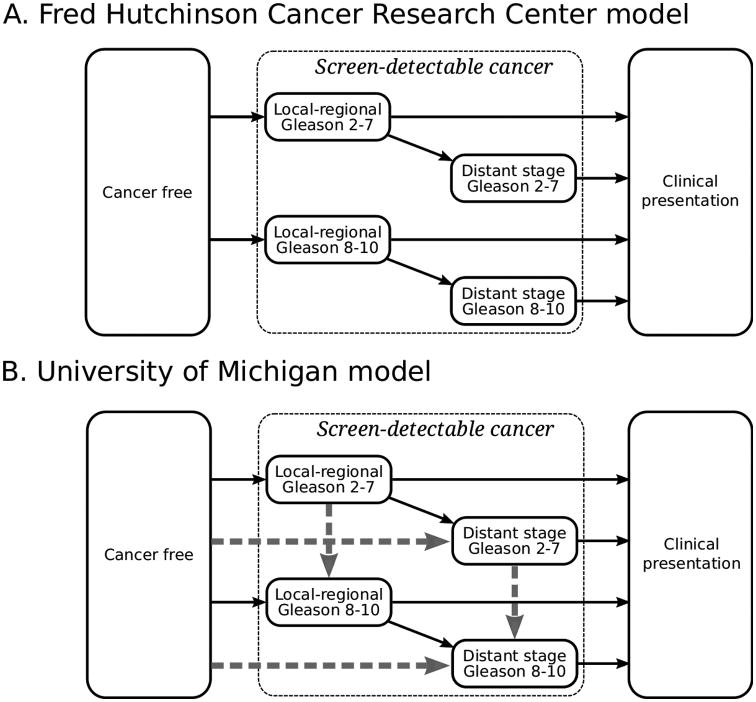
Figure 1 illustrates prostate cancer natural history—health states and transitions between states, representing onset of a screen-detectable cancer, progression through stages and/or grades, and clinical presentation—in the two models. In the Fred Hutchinson Cancer Research Center (FHCRC) model, cancers are localized at onset and may be either low- (Gleason score 2–7) or high-grade (Gleason score 8–10). Risks of metastasis and diagnosis depend on age, time since onset, and tumor stage and grade and are correlated with individual PSA levels. In the University of Michigan (UMICH) model, cancers can be localized or metastatic and low- or high-grade at onset, and risks of stage and grade progression and diagnosis depend on age, year, time since onset, and tumor stage and grade. Detailed model descriptions are given in the Supplemental Materials. Screening according to reconstructed PSA screening patterns in the US26 is superimposed on each model to produce screen-detected and non-screen-detected cases diagnosed each year.
Figure 1.
Health state transitions in two models of prostate cancer natural history. Screen-detectable cancers in both models progress from local-regional to distant stage. In the UMICH model, cancer can also progress to distant stage before becoming screen-detectable (horizontal dashed gray arrows). Cancer grade (Gleason score 2–7 or 8–10) is fixed in the FHCRC model but lower grade can progress to higher grade in the UMICH model (vertical dashed gray arrows).
The models were informed with the same prostate cancer incidence data from the SEER program. The FHCRC model also used PSA test results from the control arm of the Prostate Cancer Prevention Trial27 to estimate PSA growth rates and data on biopsy practice patterns25,28 to model disease detection when PSA exceeds 4 ng/mL, while the UMICH model estimated effective test sensitivity using SEER incidence and US screening patterns. Risks of disease onset, progression, and non-screen diagnosis were estimated so that the models reproduced prostate cancer incidence rates in the SEER program by age (50–84 years), calendar year (1975–2000), stage (local-regional or distant), and grade (Gleason score 2–7 or 8–10). Estimation details are given in the Supplemental Materials.
Treatment benefit and prostate cancer survival
To project prostate cancer survival after diagnosis, the models used frequencies of conservative management, radical prostatectomy, and radiation therapy from SEER and frequencies of androgen deprivation therapy (ADT) from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE)29 database.
In the absence of screening, patients assigned to conservative management or ADT monotherapy were assumed to have baseline prostate cancer survival similar to that for untreated cases in SEER in 1983–1986, just before PSA screening began. We assume that contemporary patients who were not detected by screening and who receive active surveillance have similar survival. There are no randomized trials comparing the main primary treatment options; based on a recent observational study,30 we assumed that radical prostatectomy and radiation with ADT are similarly efficacious and that these treatments are more efficacious than radiation alone. Patients assigned to radical prostatectomy (or radiation with ADT) had improved survival based on the Scandinavian trial of radical prostatectomy versus watchful waiting31 (hazard ratio [HR]=0.62) and consistent with the US-based Prostate Cancer Intervention Versus Observation Trial.32 Patients assigned to radiation monotherapy had survival that improved during the early 1990s (HR=0.9 before 1990, linear improvement to HR=0.7 in 1995, and constant thereafter)23 to reflect the increase in radiation dose intensity over time.
Screening benefit
A patient who is diagnosed by screening and would have died of the disease in the absence of screening was assumed to have prostate cancer survival and initial treatment corresponding to the earlier age, stage, and/or grade at screen detection. This “stage-shift” effect of screening was previously shown to be consistent with the mortality reduction observed in the ERSPC after 11 years of follow-up using the FHCRC model.33
In this study, both models used flexible representations of the stage-shift effect. Instead of giving a full stage-shift to all cancers detected early by screening, a parameter controls the scale of the benefit, with cancers that are detected later in their natural history receiving less benefit. Flexible stage-shift effects and parameter estimation details are given in the Supplemental Materials.
Predicted effects of PSA screening on prostate cancer mortality are based on applying estimated stage-shift effects to prostate cancer survival over a lifetime horizon for the US population. In practice, the models independently generate prostate cancer survival (with any early detection and treatment benefit) and other-cause survival from US life tables.34 Actual survival is the shorter of these competing survival times, with cause of death assigned accordingly.
Discontinued vs age-restricted screening
To quantify expected effects of the new PSA screening guidelines, we predicted prostate cancer incidence, including overdiagnoses and distant stage cancers, and mortality under three scenarios: a continuation of recent screening patterns (continued), a continuation of recent screening patterns restricted to men under 70 (age-restricted), and discontinued screening for all ages (discontinued). Recent PSA screening patterns are based on a reconstruction using SEER-Medicare and National Health Interview Survey (NHIS) data in 200026 and updated using NHIS data in 2005 and 2010. Incidence of overdiagnosis reflects patients diagnosed by PSA screening who would not have been diagnosed in the absence of screening (i.e., who would have died of other causes before clinical presentation). Prostate cancer mortality after 2010 assumes that the distribution of initial therapies remains constant as observed in 2010. Predictions are for men ages 50–84 years between January 1, 2013, and December 31, 2025.
To inflate predictions for the SEER population to the US population, incidence and mortality rates were multiplied by US Census projections by 5-year age group and calendar year.35
Model validations
As a partial validation of the natural history models, we compared predicted and observed incidence counts from SEER in the year 2010—i.e., a decade later than data used to estimate the models. General agreement would suggest that (1) the models reflect reasonable approximations to natural history and (2) there have not been large changes in prostate cancer epidemiology or practices related to prostate cancer diagnosis since the year 2000.
As a partial validation of the survival benefit of early detection and treatment, we compared predicted and observed absolute mortality reductions in simulations of the ERSPC trial through 11 years of follow-up based on stage-shift effects calibrated to match relative mortality reductions. General agreement would suggest that the models reflect reasonable approximations to prostate cancer survival, benefits of early detection and treatment, and competing risks of other-cause death.
Sensitivity analysis
Because some have argued that the lack of screening benefit reported in the PLCO trial reflects at best a more modest impact of early detection in the US,36,37 we also predicted effects of screening on prostate cancer mortality assuming reduced efficacy. The models recalibrated stage-shift effects of screening to yield a 15% mortality reduction relative to no screening after 11 years of follow-up in simulated ERSPC trials, approximately half the 29% reduction after correction for non-compliance.38 The models then projected mortality rates under continued, age-restricted, and discontinued PSA screening assuming this reduced benefit.
Results
Model validations
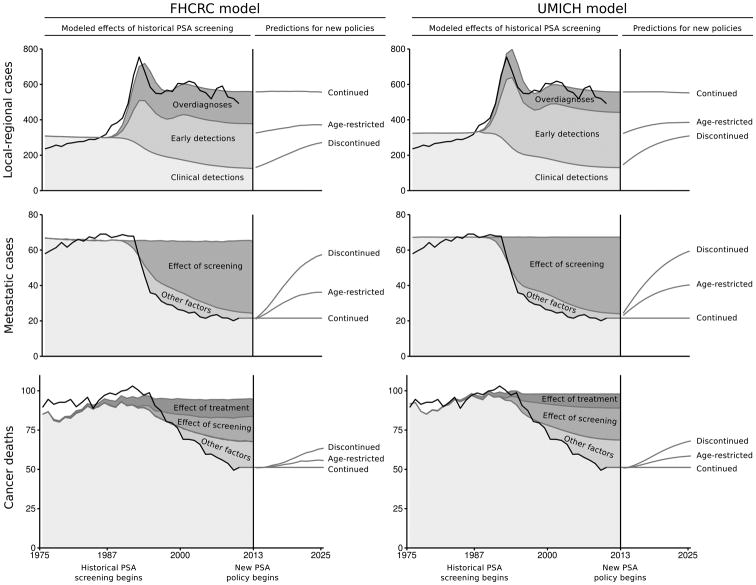
Figure 2 illustrates prostate cancer incidence rates reported in SEER and projected by the models for the calibration (1975–2000) and validation (2001–2010) years. Comparisons by age and stage are shown in the Supplemental Materials. The models closely approximate observed trends in local-regional and metastatic incidence before and after the introduction of PSA screening through 2010. Figure 2 also shows corresponding mortality rates; the models project constant mortality in the absence of screening or changes in initial treatments and similar reductions due to these interventions.
Figure 2.
Historical prostate cancer incidence and mortality rates, modeled effects of historical PSA screening, and model predictions under three PSA screening policies: (1) continuation of recent PSA screening patterns (Continued), (2) continuation of recent PSA screening patterns restricted to men under 70 years (Age-restricted), and (3) discontinued PSA screening for all men (Discontinued). Rates are agestandardized per 100,000 men ages 50–84 years.
Table 1 presents a snapshot of localized cases, metastatic cases, and prostate cancer deaths reported in SEER and projected by the models in 2010. Both models overproject localized cases, though discrepancies are relatively modest (FHCRC: 2.0%; UMICH: 2.3%) over the period 2005–2010. The models estimate that 3 out of 4 cases were detected by PSA screening in 2010, of which 25%–38% (range between models) were overdiagnosed. The models agree that screening explains nearly all the drop in metastatic cases, and the calibrated stage-shift effects of screening explain 48%–52% of the observed drop in prostate cancer deaths relative to no screening.
Table 1.
Prostate cancer cases and deaths extrapolated from SEER and effects of historical PSA screening predicted by two models in the year 2010. Counts are for US men ages 50–84 years.
| SEER | FHCRC | UMICH | |
|---|---|---|---|
|
|
|||
| Localized cases | |||
| Screen detections | |||
| Overdiagnoses | — | 65,500 | 41,300 |
| Early detections | — | 104,900 | 126,100 |
| Clinical detections | — | 51,000 | 53,700 |
| Total | 202,500 | 221,400 | 221,100 |
| Metastatic cases | |||
| Prediction under no screening | — | 24,300 | 25,100 |
| Effect of screening | — | −14,600 | −15,400 |
| Effect of other factors (not modeled) | — | −1,400 | −1,400 |
| Total | 8,300 | 8,300 | 8,300 |
| Prostate cancer deaths | |||
| Prediction under no screening or treatment | — | 33,600 | 34,800 |
| Effect of treatment | — | –4,000 | −3,100 |
| Effect of screening | — | –5,400 | −7,100 |
| Effect of other factors (not modeled) | — | –6,100 | −6,500 |
| Total | 18,100 | 18,100 | 18,100 |
SEER = Surveillance, Epidemiology, and End Results registries; PSA = prostate-specific antigen; FHCRC = Fred Hutchinson Cancer Research Center model; UMICH = University of Michigan model
In simulated ERSPC trials after 11 years of follow-up, both models approximate the relative mortality reduction of 29% after correction for non-attendance estimated by trial investigators38 (FHCRC: 29%; UMICH: 28%) and modestly overproject the observed absolute mortality reduction of 1.1 per 1000 men screened38 (FHCRC: 1.7; UMICH: 1.5) most likely because they did not account for non-attendance or contamination in the actual trial.39
Overall, the correspondence between model projections and empirical data supports using the models to investigate plausible effects of new PSA screening policies. However, to account for factors not in the models that contributed to the declines in distant stage incidence and mortality, we quantified the unexplained portions of the declines in 2010 and subtracted these differences from model projections in subsequent years. In other words, we assumed that other factors that contributed to the observed declines in 2010 would remain constant into the future.
Primary analysis
Figure 2 also illustrates prostate cancer incidence and mortality rates predicted by the models under continued, age-restricted, and discontinued PSA screening for the period 2013–2025. The models predict immediate declines in localized incidence rates under age-restricted and discontinued screening, with steady increases accumulating over this period. In both models, incidence rates nearly return to pre-PSA levels by the year 2025 under discontinued screening. Mortality rates also increase under age-restricted and discontinued screening, with significantly faster increases under discontinued screening. However, mortality rates do not return to pre-PSA levels due to continuation of contemporary patterns of initial treatments and other factors contributing to the observed decline in mortality by 2010.
Table 2 reports localized cases, metastatic cases, and prostate cancer deaths under each PSA screening scenario for the period 2013–2025. Under continued screening, the models project 710,000–1,120,000 overdiagnoses and approximately 130,000 metastatic cases at presentation. Age-restricted screening prevents 470,000–720,000 overdiagnoses (64%–66% decrease) but adds 58,000–73,000 metastatic cases at presentation (46%–57% increase). In contrast, discontinued screening eliminates all overdiagnoses but more than doubles metastatic cases at presentation. The models project over 280,000 prostate cancer deaths through 2025 under continued screening. Age-restricted screening adds 13,000–22,000 prostate cancer deaths (5%–8% increase) while discontinued screening adds 36,000–57,000 prostate cancer deaths (13%–20% increase).
Table 2.
Prostate cancer cases and deaths predicted by two models under three PSA screening policies: (A) continuation of recent PSA screening patterns (Continued), (B) continuation of recent PSA screening patterns restricted to men under 70 years (Age-restricted), and (C) discontinued PSA screening for all men (Discontinued) for the period 2013–2025. Counts are for US men ages 50–84 years.
| Continued (A) | Age-restricted (B) | Discontinued (C) | Percent relative effects of age-restricted vs discontinued screening 100 ×(A−B)/(B−C) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| FHCRC | UMICH | FHCRC | UMICH | FHCRC | UMICH | FHCRC | UMICH | |
|
|
||||||||
| Localized cases | ||||||||
| Screen detections | ||||||||
| Overdiagnoses | 1,122,900 | 705,200 | 399,700 | 237,100 | 0 | 0 | 64.4 | 66.4 |
| Early detections | 1,763,600 | 2,071,400 | 1,130,000 | 1,163,900 | 0 | 0 | 35.9 | 43.8 |
| Clinical detections | 795,600 | 890,900 | 1,008,800 | 1,216,900 | 1,372,400 | 1,679,500 | 37.0 | 41.3 |
| Total | 3,682,100 | 3,667,400 | 2,538,400 | 2,617,800 | 1,372,400 | 1,679,500 | 49.5 | 52.8 |
| Metastatic cases | 127,900 | 129,300 | 186,200 | 202,600 | 271,100 | 291,300 | 40.7 | 45.3 |
| Prostate cancer deaths | ||||||||
| Base case PSA efficacy | 283,500 | 284,600 | 296,400 | 306,900 | 319,400 | 342,000 | 35.9 | 38.9 |
| Reduced PSA efficacy | 284,300 | 285,400 | 290,300 | 299,400 | 301,800 | 320,700 | 33.9 | 39.6 |
PSA = prostate-specific antigen; FHCRC = Fred Hutchinson Cancer Research Center model; UMICH = University of Michigan model
In summary, the models concur that age-restricted screening substantially reduces overdiagnoses while preventing a majority of the additional metastatic cases at presentation and prostate cancer deaths predicted under discontinued screening.
Sensitivity analysis
Under reduced PSA screening efficacy, age-restricted screening adds 6,000–14,000 prostate cancer deaths (2%–5% increase) while discontinued screening adds 18,000–35,000 prostate cancer deaths (6%–12% increase). As in the primary analysis, age-restricted screening prevents a majority of the additional prostate cancer deaths predicted under discontinued screening.
Discussion
In the last two years, there have been major revisions to prostate cancer screening policy recommendations by influential US guidelines panels, most notably the USPSTF.14,40 Motivated largely by the results of the PLCO and ERSPC trials, the new recommendations are generally conservative and advocate cessation of routine PSA screening for all men or for men above 70 years or with a limited life expectancy. The response to these recommendations in terms of clinical practice is evolving, but screening rates could decline dramatically. Our analysis suggests that discontinued screening could have profound consequences for prostate cancer deaths and advanced disease in this country.
Continuation of current screening is expected to overdiagnose as many as 1 million US men but prevent large numbers of metastatic cases at presentation and prostate cancer deaths by 2025. Discontinued screening indiscriminately eliminates both the harms and benefits of screening, eliminating overdiagnosis and overtreatment of low-risk prostate cancer but at great cost. Restricting screening to men under 70 eliminates a majority of overdiagnosed cases and preserves more than half of the metastatic cases avoided and lives saved with contemporary screening patterns, and this finding is insensitive to whether screening efficacy is similar to or lower than that reported in the ERSPC.
The models confirm that PSA screening generates substantial numbers of overdiagnosed cases, but the estimates are below other reported figures,41 which were based on coarse approximations with limited accounting for age or period effects.42 The wide range for the absolute number of overdiagnoses predicted by the two models is not unexpected given that this harm is not directly observable and estimates are sensitive to unknown aspects of prostate cancer natural history. In particular, the UMICH model estimates fewer overdiagnoses and more early detections than the FHCRC model because its allowance for faster cancer progression during the screen-detectable window implies shorter lead times.43 Nonetheless, the reduction in overdiagnoses expected under age-restricted PSA screening relative to discontinued PSA screening is highly consistent between models.
Our results confirm that if screening improves survival by a “stage-shift” effect, then PSA screening appears to have played an important role in the observed decline in prostate cancer mortality. However, screening and changes in primary treatments do not explain the entire observed decline in prostate cancer mortality. Other factors, such as increasing obesity rates44 or decreasing smoking rates,45 or other interventions, such as treatment at biochemical recurrence,46 might have reduced mortality. Our results assume the unexplained contribution of these factors to the decline in mortality in 2010 will remain constant. Provided these factors do not interact with effects of screening, they should not affect our main results.
The status quo of widespread, relatively late screening47 irrespective of life expectancy48 and nearly universal treatment29 clearly is far from optimal. Yet, as the present analysis demonstrates, wholesale abandonment of screening efforts may be a costly solution. While an age-restricted policy is a compelling improvement, it does not account for life expectancy or screening history; a 66-year-old healthy man with no prior PSA exposure faces a very different risk profile than a counterpart with multiple comorbidities and multiple prior PSAs below 1 ng/mL.49,50 Other screening strategies may yield more favorable harm-benefit tradeoffs,33 particularly when combined with greater use of active surveillance.51
We accounted for key sources of uncertainty by using two models of prostate cancer natural history and a sensitivity analysis to screening benefit. Nonetheless, other sources of uncertainty remain. Despite relying on population-based datasets and conditioning effects of interventions on patient and tumor characteristics, the varied populations and settings in certain data sources may not be perfectly compatible with each other or representative of the general US setting. Restriction to coarse grade categories (Gleason scores 2–7 vs 8–10) was necessary to avoid bias due to upward grade migration over time. We also did not incorporate life expectancy in selecting men to be screened in the models, but it is likely that men who choose to be screened for prostate cancer are healthier than the general population. Thus, our estimates of overdiagnoses under screening may be modestly inflated. Finally, we previously showed that, due to widespread contamination and lower-than-expected mortality, the stage-shift effect of screening is neither supported nor contradicted by results from the PLCO trial.52 Nevertheless, the screening benefit in our primary analysis has not been confirmed over a long-term horizon.
In summary, recently revised screening guidelines are poised to yield a large shift in prostate cancer epidemiology, reducing overdiagnosis and overall incidence at the expense of increasing the burden of prostate cancer metastasis and mortality. Our projections indicate that discontinuing screening may significantly erode observed reductions in prostate cancer mortality over a relatively short time frame. Continuing screening but restricting to men below age 70 is one approach that could preserve many of the benefits of contemporary patterns of screening while still reducing harms. Rather than abandoning screening entirely, our results support finding ways to continue screening that mitigate harm while preserving as much of the benefit as possible.
Supplementary Material
Acknowledgments
We thank Dr. Rafael Meza for helpful comments.
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (U01CA157224) as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Disclosures: None
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Etzioni R, Cha R, Feuer EJ, Davidov O. Asymptomatic incidence and duration of prostate cancer. Am J Epidemiol. 1998;148(8):775–785. doi: 10.1093/oxfordjournals.aje.a009698. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni R, Gulati R, Falcon S, Penson D. Impact of PSA screening on the incidence of advanced stage prostate cancer in the US: A surveillance modeling approach. Med Decis Making. 2008;28:323–331. doi: 10.1177/0272989X07312719. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated with State, Total U.S. (1969-2010) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- 5.Andriole GL. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder FH. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulati R, Mariotto AB, Chen S, Gore JL, Etzioni R. Long-term projections of the harm-benefit trade-off in prostate cancer screening are more favorable than previous short-term estimates. J Clin Epidemiol. 2011 Dec;64(12):1412–1417. doi: 10.1016/j.jclinepi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanley JA. Measuring mortality reductions in cancer screening trials. Epidemiol Rev. 2011 Jul;33(1):36–45. doi: 10.1093/epirev/mxq021. [DOI] [PubMed] [Google Scholar]
- 9.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008 Mar 20;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 11.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term followup. J Urol. 2010 Jun;183(6):2206–2212. doi: 10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013 Jan 31;368(5):436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulati R, Inoue LY, Gore JL, Katcher J, Etzioni R. Individualized Estimates of Overdiagnosis in Screen-Detected Prostate Cancer. J Natl Cancer Inst. 2014 Jan 7; doi: 10.1093/jnci/djt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer VA on behalf of the USPSTF. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012 May 21;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 15.Wolf AM. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 16.Carter HB, Albertsen PC, Barry MJ, et al. Early Detection of Prostate Cancer: AUA Guideline. J Urol. 2013 May 6; doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P for the Clinical Guidelines Committee of the American College of P. Screening for Prostate Cancer: A Guidance Statement From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013 Apr 9; doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 18.Ross KS, Guess HA, Carter HB. Estimation of treatment benefits when PSA screening for prostate cancer is discontinued at different ages. Urology. 2005 Nov;66(5):1038–1042. doi: 10.1016/j.urology.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Schaeffer EM, Carter HB, Kettermann A, et al. Prostate specific antigen testing among the elderly--when to stop? J Urol. 2009 Apr;181(4):1606–1614. doi: 10.1016/j.juro.2008.11.117. discussion 1613-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009 Nov;182(5):2242–2248. doi: 10.1016/j.juro.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Vickers A, Bennette C, Steineck G, et al. Individualized estimation of the benefit of radical prostatectomy from the scandinavian prostate cancer group randomized trial. Eur Urol. 2012 Aug;62(2):204–209. doi: 10.1016/j.eururo.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008 Mar;19(2):175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etzioni R, Gulati R, Tsodikov A, et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer. 2012;118(23):5955–5963. doi: 10.1002/cncr.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat in Med. 2006 Aug 30;25(16):2846–2866. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 25.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010 Oct;11(4):707–719. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariotto A, Etzioni R, Krapcho M, Feuer EJ. Reconstructing prostate-specific antigen (PSA) testing patterns among black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109(9):1877–1886. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 27.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003 Jul 17;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 28.Pinsky PF, Andriole GL, Kramer BS, Hayes RB, Prorok PC, Gohagan JK. Prostate biopsy following a positive screen in the Prostate, Lung, Colorectal and Ovarian cancer screening trial. J Urol. 2005;173(3):746–750. doi: 10.1097/01.ju.0000152697.25708.71. discussion 750-751. [DOI] [PubMed] [Google Scholar]
- 29.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010 Mar 1;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59(6):893–899. doi: 10.1016/j.eururo.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011 May 5;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 32.Wilt T, Brawer MK, Jones K, Barry M. Radical Prostatectomy versus Observation for Localized Prostate Cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: Model estimates of potential benefits and harms. Ann Intern Med. 2013;158(3):145–153. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics. Vital statistics of the United States, Volume II: Mortality, part A. Washington DC: Government Printing Office; various years. [Google Scholar]
- 35.United States Census Bureau. 2004 Interim National Population Projections. Population Division United State Census Bureau; 2004. [Google Scholar]
- 36.Chou R, LeFevre ML. Prostate cancer screening--the evidence, the recommendations, and the clinical implications. JAMA. 2011 Dec 28;306(24):2721–2722. doi: 10.1001/jama.2011.1891. [DOI] [PubMed] [Google Scholar]
- 37.Miller AB. New data on prostate-cancer mortality after PSA screening. N Engl J Med. 2012 Mar 15;366(11):1047–1048. doi: 10.1056/NEJMe1200185. [DOI] [PubMed] [Google Scholar]
- 38.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012 Mar 15;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roobol MJ, Kerkhof M, Schroder FH, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2009;56(4):584–591. doi: 10.1016/j.eururo.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Tasian GE, Cooperberg MR, Cowan JE, et al. Prostate specific antigen screening for prostate cancer: knowledge of, attitudes towards, and utilization among primary care physicians. Urol Oncol. 2012 Mar-Apr;30(2):155–160. doi: 10.1016/j.urolonc.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst. 2009 Oct 7;101(19):1325–1329. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013 Jun 4;158(11):831–838. doi: 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulati R, Wever EM, Tsodikov A, et al. What if i don't treat my PSA-detected prostate cancer? Answers from three natural history models. Cancer Epidemiol Biomarkers Prev. 2011;20(5):740–750. doi: 10.1158/1055-9965.EPI-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fesinmeyer MD, Gulati R, Zeliadt S, Weiss N, Kristal AR, Etzioni R. Effect of population trends in body mass index on prostate cancer incidence and mortality in the United States. Cancer Epidemiol Biomarkers Prev. 2009 Mar;18(3):808–815. doi: 10.1158/1055-9965.EPI-08-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011 Jun 22;305(24):2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008 Jun 18;299(23):2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011 May 1;29(13):1736–1743. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006 Nov 15;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 49.Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grenabo Bergdahl A, Holmberg E, Moss S, Hugosson J. Incidence of Prostate Cancer After Termination of Screening in a Population-based Randomised Screening Trial. Eur Urol. 2013 May 17; doi: 10.1016/j.eururo.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 51.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012 Apr 17;156(8):591–595. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulati R, Tsodikov A, Wever EM, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23(6):827–835. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.