Abstract
Objective
Supraventricular tachycardia (SVT) is the most common arrhythmia in infants, and antiarrhythmic medications are frequently used for prophylaxis. The optimal prophylactic antiarrhythmic medication is unknown, and prior randomized trials have been underpowered. We used data from a large clinical registry to compare efficacy and safety of digoxin and propranolol for infant SVT prophylaxis. We hypothesized that SVT recurrence is less common on digoxin compared with propranolol.
Design
Retrospective cohort study.
Setting
Pediatrix Medical Group neonatal intensive care units.
Patients
Infants discharged from 1998–2012 with SVT treated with digoxin or propranolol. We excluded infants discharged prior to completing 2 days of therapy, those with Wolff-Parkinson-White syndrome, structural heart defects (except atrial/ventricular septal defects and patent ductus arteriosus), and those started on multi-drug therapy.
Measurements
We used Cox proportional hazards to evaluate SVT recurrence, defined as need for adenosine or electrical cardioversion while exposed to digoxin vs. propranolol, controlling for infant characteristics, inotropic support, supplemental oxygen, and presence of a central line.
Results
We identified 342 infants exposed to digoxin and 142 infants exposed to propranolol. The incidence rate of treatment failure was 6.7/1000 infant-days of exposure to digoxin and 15.4/1000 infant-days of exposure to propranolol. On multivariable analysis, treatment failure was higher on propranolol compared with digoxin (hazard ratio=1.97 [95% confidence interval: 1.05, 3.71]). Hypotension was more frequent during exposure to digoxin versus propranolol (39.4 versus 11.1/1000 infant-days, p<0.001). There was no difference in frequency of other clinical adverse events.
Conclusions
Digoxin was associated with fewer episodes of SVT recurrence but more frequent hypotension in hospitalized infants relative to propranolol.
Keywords: supraventricular tachycardia, infants, digoxin, propranolol
Supraventricular tachycardia (SVT) is the most common arrhythmia of infancy, occurring in 1/250 infants (1–3). Due to difficulty with diagnosis and the morbidity associated with unrecognized episodes of SVT, pharmacologic prophylaxis is often used in infants to prevent SVT recurrence after initial diagnosis (2,4,5). Digoxin and propranolol are the 2 most commonly used prophylactic medications; however, there is no consensus regarding the optimal choice (5,6).
A recent multicenter, randomized, controlled trial attempted to compare the efficacy and safety of digoxin and propranolol for SVT prophylaxis in infants (6). The study was conducted at 19 member sites of the Pediatric and Congenital Electrophysiology Society across North America but was terminated due to difficulty with enrollment. After almost 4 years, the trial accrued an analyzable sample size of only 61 infants from a targeted sample size of 220. While the observed recurrence rate of SVT was lower in infants treated with digoxin (19%) compared with propranolol (31%), the study was underpowered to identify a statistically significant difference between both groups (p=0.25). This trial highlights the inherent complexities of enrolling infants in clinical trials, particularly for relatively rare diseases and conditions. As a consequence, the question of optimal antiarrhythmic medication for SVT prophylaxis in infants remains unanswered (7).
Well-designed cohort studies from large clinical registries appropriately estimate treatment effects and, therefore, represent the next best alternative in the hierarchy of research designs (8). To this end, we conducted a large retrospective cohort study comparing the safety and efficacy of digoxin versus propranolol for the treatment of SVT in hospitalized infants (9).
METHODS
Study Design and Sample
We performed a retrospective cohort study of infants discharged from 333 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from 1998–2012. This national group of NICUs includes smaller community hospitals to tertiary care medical centers. The data are generated by clinicians caring for these infants for the purpose of documentation and billing in a shared electronic medical record. Data are then extracted, de-identified, and consolidated into the Pediatrix Clinical Data Warehouse. The study was approved by the Duke University Institutional Review Board.
Inclusion and exclusion criteria were designed to mirror the Study of Antiarrhythmic Medications in Infancy (SAMIS) trial, a randomized, controlled trial that failed to reach its end point due to poor patient accrual (6). Potentially eligible for inclusion were all infants with a diagnosis of SVT and without a diagnosis of congenital heart disease other than atrial septal defects, ventricular septal defects, or patent ductus arteriosus, treated with either propranolol or digoxin as first-line antiarrhythmic medication in the first 120 days of life, and hospitalized in the same NICU for 2 or more days following therapy initiation (i.e., adequate time to attain steady-state drug concentrations) (n=730). We excluded infants with a diagnosis of Wolff-Parkinson-White syndrome (n=66), atrial flutter (n=100), and those receiving multi-drug therapy on the day of presentation (n=107).
Study Measurements
We categorized infants based on the initial antiarrhythmic medication received: digoxin or propranolol. We evaluated several covariates as surrogate measures of infant severity of illness: inotropic support, mechanical ventilation, supplemental oxygen administration, and presence of a central venous line (CVL) on a day of antiarrhythmic medication exposure. We defined inotropic support as exposure to any inotrope (dopamine, dobutamine, epinephrine, milrinone, norepinephrine, or phenylephrine) on a day of antiarrhythmic medication exposure. We defined mechanical ventilation as any invasive mechanical ventilation and supplemental oxygen as the administration of FiO2 >21% on a day of antiarrhythmic medication exposure. We defined CVL as the presence of any CVL on a day of antiarrhythmic medication exposure. We defined small-for-gestational age (SGA) status as previously described (10).
The primary outcome was the first treatment failure defined as the need for adenosine or electrical cardioversion after 2 or more days of uninterrupted therapy with digoxin or propranolol. Two secondary outcomes were evaluated in a priori-defined sensitivity analyses. The first secondary outcome was the first treatment failure defined as need for adenosine, electrical cardioversion, or the addition of a second-line antiarrhythmic medication after 2 or more days of uninterrupted therapy with digoxin or propranolol. Second-line antiarrhythmic medications included amiodarone, flecainide, procainamide, sotalol, verapamil, or the combination of digoxin and a beta-blocker (e.g., propranolol, atenolol, esmolol). The second secondary outcome was late treatment failure defined as the first need for adenosine or electrical cardioversion after 5 days of exposure to antiarrhythmic medication.
For safety assessments, we reviewed the Food and Drug Administration drug labels for commonly described laboratory and clinical adverse events (AEs) associated with both drugs, as well as for laboratory-associated precautions reported for use of both drugs. We collected available laboratory information while infants were exposed to antiarrhythmic medications. We attributed a laboratory AE to antiarrhythmic medications if it occurred between the start of exposure through the end of exposure to the antiarrhythmic medication. We classified laboratory abnormalities as an AE or a serious adverse event (SAE) based on pre-specified cut-off values (11) (Supplemental Table 1). We identified clinical AEs associated with digoxin or propranolol including bradycardia, hypotension (defined as the need for inotropic medication), heart block, ventricular tachycardia, and bronchospasm. We considered a diagnosis as a clinical AE if it was made while the infant was exposed to antiarrhythmic medication. Each new diagnosis and laboratory abnormality was counted as a separate AE.
Statistical Methods
The unit of observation for this study was an infant-day of exposure to digoxin or propranolol. We used standard summary statistics to describe demographic and baseline characteristics; we presented continuous variables as median (interquartile range) or mean (standard deviation) and categorical variables as count (proportion). We compared continuous and categorical variables across the 2 antiarrhythmic medication groups using Wilcoxon rank sum, Student’s t-test, chi- square, and Fisher’s exact tests, and evaluated trends over time using Cochrane Armitage tests for trend. We described diagnostic and laboratory AEs and SAEs at the infant-day level (number of days with AE/1000 infant-days on antiarrhythmic medication). We describe changes in antiarrhythmic medication over time by reporting the proportion of infants discharged within a given calendar year exposed to each antiarrhythmic medication. We evaluated the association between antiarrhythmic medication and treatment failure using Cox proportional hazards modeling. Infants were considered at risk after 2 days of exposure to antiarrhythmic medication and censored at the time of discharge or 120 days of life. We included daily antiarrhythmic medication exposure, inotropic support, need for any supplemental oxygen, and the presence of a CVL as time-varying and discharge year and gestational age as non–time-varying covariates in the final model. We tested the proportional hazards assumption using observed (Kaplan-Meier) versus predicted (Cox model) plots and goodness-of-fit tests based on Schoenfeld residuals. All curves of potential covariates were visually inspected, and the p-values from the goodness-of-fit tests were >0.05. We conducted all analyses using Stata 12.0 (College Station, TX) and considered a p <0.05 statistically significant.
RESULTS
Infant Characteristics
Our final cohort consisted of 457 infants from 220 NICUs; 315 (69%) received only digoxin, 115 (25%) received only propranolol, and 27 (17%) received both for a total of 342 infants ever exposed to digoxin and 142 infants ever exposed to propranolol. The 342 infants ever exposed to digoxin contributed 3732 infant-days at risk; the 142 infants ever exposed to propranolol contributed 1172 infant-days at risk. Of the 457 infants included in our analysis, 337 (74%) were first exposed to digoxin, and 120 (26%) were first exposed to propranolol (Table 1). For infants for whom dosing was available, total daily dose of digoxin ranged between 4 mcg/kg/day to 12 mcg/kg/day for 95% of all infants, while total daily dose of propranolol was ≥3 mg/kg/day.
Table 1.
Demographics by antiarrhythmic medication of first exposure, n (%)
| Digoxin N=337 | Propranolol N=120 | P | |
|---|---|---|---|
| Birth weight (g) | 0.31 | ||
| <1000 | 30 (9%) | 10 (8%) | |
| 1000–1499 | 41 (12%) | 17 (14%) | |
| 1500–2499 | 94 (28%) | 23 (19%) | |
| 2500–3499 | 110 (33%) | 41 (34%) | |
| ≥3500 | 60 (18%) | 29 (24%) | |
| Gestational age (weeks) | 0.31 | ||
| ≤28 | 49 (15%) | 12 (10%) | |
| 29–36 | 179 (53%) | 62 (52%) | |
| >36 | 109 (32%) | 46 (38%) | |
| Male, | 213 (63%) | 76 (63%) | 0.98 |
| Race/ethnicity | 0.36 | ||
| White | 196 (57%) | 60 (61%) | |
| Black | 64 (19%) | 11 (11%) | |
| Hispanic | 71 (21%) | 24 (24%) | |
| Other | 13 (4%) | 4 (4%) | |
| Inborn | 250 (72%) | 71 (68%) | 0.36 |
| Small for gestational age | 25 (7%) | 3 (2%) | 0.05 |
| Age at initiation of therapy (days) | 0.25 | ||
| ≤7 | 165 (49%) | 49 (41%) | |
| 8–14 | 90 (27%) | 43 (36%) | |
| 15–28 | 46 (14%) | 14 (12%) | |
| >28 | 36 (11%) | 14 (12%) |
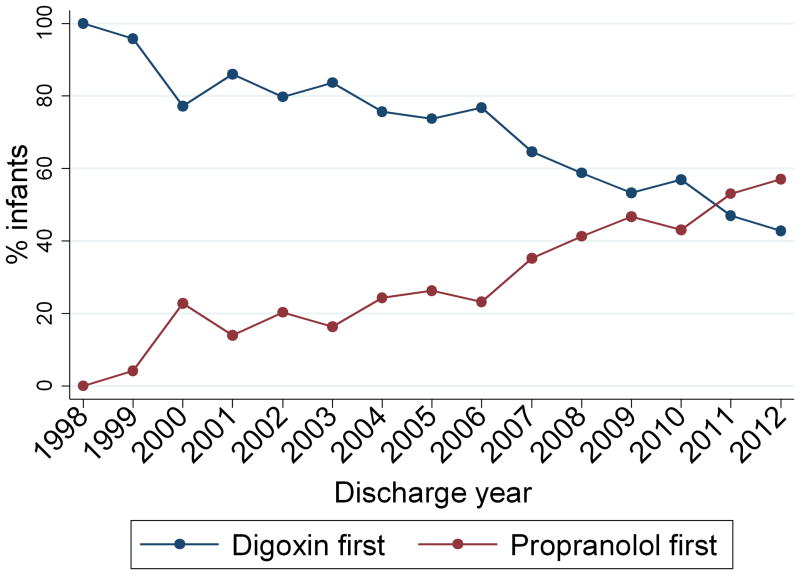
Postnatal age at treatment initiation was similar in infants first exposed to digoxin compared with propranolol (median 7 days [interquartile range 3, 13] vs. 9 days [4, 13], p=0.07). Duration of inpatient therapy was also similar between the 2 groups (5 days [2, 16] vs. 5 days [2, 12], p=0.94). Use of digoxin declined over time from 100% of infants in 1998 to 48% of infants in 2012 (p<0.001) (Figure 1).
Figure 1.
Medication use by discharge year.
Infants were more likely to have a CVL and require inotropic support on infant-days of exposure to digoxin compared with propranolol (884/3732 [24%] vs. 199/1172 [17%], p<0.001; 174/3732 [5%] vs. 13/1172 [1%], p<0.001). The results were similar when proportion of infant days of exposure to inotropes and CVL, by antiarrhythmic, were compared separately during the time periods of 1998–2006 and 2007–2012. Similarly, Cochrane-Armitage tests for trend did not identify a significant trend in the use of inotropes (p=0.97) or CVL (p=0.08) over the study period.
Infants were also more likely to require supplemental oxygen and mechanical ventilation on infant-days of exposure to digoxin compared with propranolol (1611/3732 [43%] vs. 298/1172 [25%], p<0.001; 908/3732 [24%] vs. 108/1172 [9%], p<0.001). Again, there were no significant temporal trends in the use of supplemental oxygen (p=0.06) or mechanical ventilation (p=0.93) when evaluated using Cochrane Armitage tests for trend over the study period.
Treatment Failure
A total of 43 infants suffered treatment failures. Adenosine was administered in 42 (98%) of those episodes, and electrical cardioversion was performed once (2%). There were 25 failures during 3732 infant-days at risk among 342 infants on digoxin (incidence rate = 6.7/1000 infant-days) and 18 failures during 1172 infant-days at risk among 142 infants on propranolol (incidence rate = 15.4/1000 infant-days).
Treatment failure was significantly higher on propranolol compared with digoxin (unadjusted hazard ratio = 2.00 [95% confidence interval; 1.09, 3.67]) (Figure 2). The hazard ratio remained significantly higher with propranolol after adjusting for severity of illness, infant gestational age, and discharge year (hazard ratio=1.97 [1.05, 3.71]). Results were similar when excluding the 27 infants exposed to both digoxin and propranolol from the model (hazard ratio=2.01 [1.01, 3.97]). For infants with at least 1 treatment failure, there was no difference in the median time to first treatment failure from initiation of therapy between those first treated with digoxin vs. propranolol (3 days [3, 4] vs. 3 days [3, 6], p=0.29).
Figure 2.
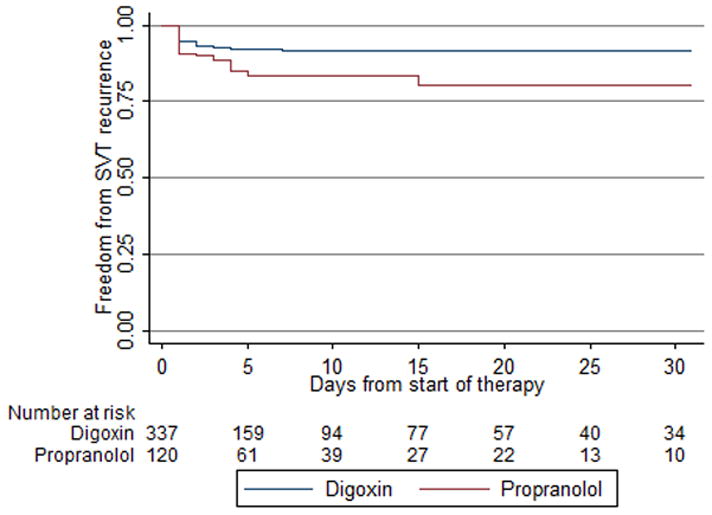
Kaplan-Meier estimated freedom from adenosine or electrical cardioversion in first 30 days of therapy.
We performed 2 sensitivity analyses evaluating our secondary outcome of adenosine, electrical cardioversion or the addition of a second-line antiarrhythmic agent, and late treatment failure. In both cases, results were similar to our primary analysis, with an increased hazard of treatment failure while on propranolol compared with digoxin (Table 2).
Table 2.
Treatment failure
| Incidence rate per 1000 infant-days
|
Unadjusted hazard ratio (95% CI)a | Adjusted hazard ratio (95% CI)b | ||
|---|---|---|---|---|
| Digoxin n=3732 | Propranolol n=1172 | |||
| Adenosine or cardioversion after 2 days | 6.7 | 15.4 | 2.00 (1.09, 3.67) | 1.97 (1.05, 3.71) |
| Adenosine or cardioversion after 5 days | 0.7 | 5.7 | 6.65 (1.29, 34.3) | 6.72 (1.19, 37.8) |
| Adenosine, cardioversion, or second-line drug after 2 days | 6.7 | 16.3 | 2.13 (1.18, 3.87) | 2.04 (1.10, 3.80) |
CI=confidence interval.
Digoxin as reference.
Digoxin as reference, adjusted for gestational age, discharge year, daily inotrope use, supplemental oxygen, and presence of a central venous line.
Safety
Overall clinical AEs were more common on days of exposure to digoxin compared with days of exposure to propranolol (45.3/1000 infant-days vs. 13.7/1000 infant-days, p<0.001). This difference was largely due to reports of hypotension requiring inotropic support, which was the most common AE overall (32.6/1000 infant-days with an incidence rate of 39.4/1000 infant-days for digoxin vs. 11.1/1000 infant-days for propranolol, p<0.001). There was no significant difference between the proportion of infant-days with bradycardia or bronchospasm while on digoxin compared with propranolol (1.1/1000 infant-days vs. 1.0/1000 infant-days, p=0.66; 5.1/1000 infant-days vs. 1.7/1000 infant-days, p=0.20). No cases of complete heart block or ventricular tachycardia were diagnosed while exposed to either digoxin or propranolol. There was no significant difference in mortality at hospital discharge between infants first treated with digoxin compared with those first treated with propranolol (7/337 [2%] vs. 1/120 [1%], p=0.23).
There was no significant difference in the overall frequency of laboratory AEs and SAEs while exposed to digoxin vs. propranolol (142/3732 infant-days [4%] vs. 54/1172 infant-days [5%], p=0.22; 45/3732 [1%] vs. 22/1172 [2%], p=0.08). Hypocalcaemia was less common on infant-days of exposure to digoxin compared with propranolol (AE: 6.2/1000 infant-days vs. 23/1000 infant-days, p<0.001; SAE: 5.6/1000 infant-days vs. 14.5/1000 infant-days, p=0.002) (Table 3). An elevated blood urea nitrogen (BUN) was reported more frequently on infant-days of exposure to digoxin compared with propranolol (5.4/1000 infant-days vs. 1/1000 infant-days, p=0.04). There was no significant difference in the proportion of infants with at least 1 laboratory AE or SAE first exposed to digoxin compared with propranolol (109/337 [32%] vs. 33/120 [28%], p=0.32; 40/337 [12%] vs. 10/120 [8%], p=0.29).
Table 3.
Laboratory adverse events per 1000 infant-days
| AE | SAE | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Digoxin | Propranolol | P | Digoxin | Propranolol | P | |
| Serum electrolytes | ||||||
| Hypoglycemia | 1.1 | 1.7 | 0.59 | <1 | 1.0 | 0.07 |
| Hyperkalemia | 20.1 | 18.8 | 0.78 | 2.1 | 1.7 | 0.77 |
| Hypokalemia | 5.1 | <1 | 0.01 | <1 | <1 | 0.58 |
| Hypercalcemia | <1 | 1.0 | 0.07 | <1 | <1 | 0.99 |
| Hypocalcaemia | 6.2 | 23.0 | <0.001 | 5.6 | 14.5 | 0.002 |
| Hypomagnesaemia | 1.0 | 1.7 | 0.22 | <1 | 1.0 | 0.07 |
| Renal dysfunction | ||||||
| Elevated BUN | 5.4 | 1.0 | 0.04 | 2.1 | 1.0 | 0.37 |
| Elevated creatinine | 2.7 | 2.6 | 0.95 | 1.0 | <1 | 0.33 |
DISCUSSION
To our knowledge, we report the largest retrospective comparative effectiveness study of digoxin vs. propranolol for the prophylaxis of SVT in infants. Although time trends indicate an increasing provider preference for propranolol, we found that treatment failure was more common on propranolol compared with digoxin. This was true even after adjusting for infant severity of illness.
In a recent survey of pediatric cardiologists and electrophysiologists, 98% of respondents recommended pharmacological therapy to prevent recurrence of SVT episodes in infants diagnosed with a first episode (5). However, there is no consensus regarding the appropriate prophylactic agent in this population. In the same survey, pediatric electrophysiologists predominantly chose propranolol as a prophylactic agent, while non–electrophysiology-trained pediatric cardiologists preferred digoxin (5). This uncertainty regarding the optimal prophylactic agent is reflected in the time trends in our analysis. Over the past decade, there has been a clear shift in provider preference, with propranolol gradually replacing digoxin as the preferred prophylactic agent.
It is unclear why providers increasingly favor propranolol to digoxin as there are very few data to guide treatment. Single-center, retrospective analyses have provided conflicting results regarding efficacy of 1 or both agents in infants and have all been limited by inclusion of a heterogeneous mix of SVT subtypes (12–14). The Study of Antiarrhythmic Medications in Infancy (SAMIS) trial was a well-designed, randomized, controlled, multicenter trial in a relatively homogenous patient population (infants with SVT but no pre-excitation or atrial flutter) (6). Unfortunately, despite best efforts to enroll infants at 19 North American sites over almost 4 years, the SAMIS trial fell short of its target size of 220 infants, instead enrolling and randomizing 72 infants and ultimately including only 61 in the final analysis. The trial was underpowered to detect a statistically significant difference; however, the SVT recurrence rate was higher in infants treated with propranolol (31%) compared with those receiving digoxin (19%). Our study confirms these findings and, even though our digoxin cohort had increased baseline comorbidities and therefore a presumptive increased baseline risk for SVT recurrence, recurrence rates were still substantially lower.
Our analysis is limited by its retrospective study design. However, as the SAMIS trial demonstrates, there are inherent difficulties in conducting randomized controlled trials in infants. These difficulties have been well documented, particularly when investigating cardiovascular drugs (15,16). Relative rarity of disease and difficulties enrolling vulnerable infants in clinical trials are often-cited causes, and these obstacles are not easily overcome (6,16). Therefore, alternative trial designs need to be considered. Well-designed, careful use of clinical registry data can be beneficial, providing an opportunity to study safety and efficacy in the real-world setting. Previous meta-analyses have demonstrated that retrospective analyses of large observational cohorts appropriately estimate treatment effects, particularly when they adapt principles of the design of randomized controlled trials (8). We have attempted to do so in our study by using similar inclusion criteria, adjusting for differences in baseline susceptibility to the outcome, and using similar statistical methods to those employed in the trial. In addition to the easier feasibility of retrospective analyses, these studies also may have the added advantage of greater generalizability due to their “real-world” context. The Pediatrix Clinical Data Warehouse combines clinical data from >300 NICUs across North America. Its design allows for unbiased extraction of information from a diverse neonatal population, including small community NICUs as well as large tertiary referral centers (9). As such, it is highly representative of the scope of practice in the United States and has been previously used to identify or confirm differences in efficacy of several drugs in infants (11,17,18).
Clinical databases also provide a valuable source for safety data. Safety concerns exist when using both digoxin and propranolol in infants. Digoxin has a narrow therapeutic index, with a risk of digitalis toxicity reported as high as 12% in neonates and infants (19). This manifests as general symptoms (fatigue, nausea, vomiting, headaches, and blurred vision) and pro-arrhythmic effects (atrioventricular nodal block, profound sinus bradycardia, and sinoatrial block), both of which may be difficult to identify in infants. While beta-blockers are generally thought to have a more favorable risk profile, side effects include bradycardia, atrioventricular nodal block, hypotension, heart failure, and bronchospasm. Although AEs were commonly reported in the randomized trial of SVT prophylaxis in both treatment arms, none were thought to be related to study medication (6). In our analysis, we did not identify a significant difference in the proportion of infants experiencing bradycardia or bronchospasm while treated with either drug, and no infants demonstrated atrioventricular nodal block or ventricular tachycardia while on therapy. We did, however, note higher rates of hypotension during digoxin therapy, as well as more frequent elevations of BUN. Given that infants receiving digoxin appeared more acutely ill at baseline than those treated with propranolol—based on a higher proportion requiring supplemental oxygen, mechanical ventilation, and inotropic support—it is possible that this higher rate of AEs is not related to direct effects of digoxin therapy. However, it is also possible that the frequency of hypotension in infants treated with digoxin is part of clinicians’ motivation to switch towards propranolol therapy in recent years. Indeed, it may be reasonable to judge that short-lived breakthrough SVT episodes are better tolerated than hypotension caused by secondary SVT prophylaxis.
To the best of our knowledge, this is the largest retrospective comparative effectiveness study of digoxin vs. propranolol for SVT prophylaxis in infants and the first study to show a statistically significant benefit of digoxin. The strengths of our study include its large sample size, the ability to control for several infant characteristics and surrogates of severity of illness that were not available in prior retrospective studies, and the ability to provide a description of changes in drug use over time. In addition, we have attempted to adapt the principles of the only previously conducted randomized controlled trial of SVT prophylaxis in infants. However, as already noted, our study remains limited by its retrospective nature. While our sample size is large, our final cohort included infants from only 220 (67%) of the 330 NICUs contributing data to the clinical data warehouse over the study period. This discrepancy may be due to the fact that the Pediatrix Medical Group covers a wide range of neonatal ICUs including major academic medical centers and small community hospitals. The latter are less likely to diagnose and treat infants with SVT with either digoxin or propranolol and hospitalize the infants for at least 2 days to meet all inclusion criteria. Further, not all 330 NICUs were part of the Pediatrix Medical Group for the full duration of the study. We were only able to include in our study infants receiving treatment for SVT and thus, similarly to the SAMIS trial, are unable to comment on the differences in SVT recurrence related to drug therapy versus spontaneous resolution. The data for our study were derived from electronic medical documentation and not prospective collection and, as such, have not been subject to randomization to protect from confounders, inclusion and exclusion criteria to homogenize the patient population, and standardized drug dosing regimen. Most importantly, we do not know the drug dosage and intervals administered, which may have affected their efficacy especially at the initiation of therapy when doses often get escalated or loading doses are administered. We have attempted to address potential confounders and the issue of loading doses and dose escalation at the initiation of therapy by including several important covariates in our final model and by conducting a sensitivity analysis limited to SVT recurrence 5 days after drug initiation. We are also able to identify the range of doses infants received in our study and found that, while total daily dose varied widely, both propranolol (≥3 mg/kg/day in all infants) and digoxin (4–12 mcg/kg/day in 95% of infants) were generally dosed within the recommended treatment ranges for premature and/or term infants. We chose SVT recurrence after 2 days of therapy as our primary outcome based on the large proportion of infants discharged prior to having completed 5 days of therapy and the known shorter half-life of digoxin and propranolol in infants (20,21). Further, our data source limited the analysis to SVT recurrence in hospitalized infants, with a high proportion of premature infants and a median in-patient admission of only 5 days after initiation of prophylactic therapy. Nonetheless, in-patient SVT recurrence is a reasonable surrogate for long-term recurrence and also an important end point in and of itself. We defined the outcome of SVT recurrence based on the need for adenosine or electrical cardioversion. This is a meaningful outcome given the impact of these interventions; however, recurrent SVT episodes may have been left untreated or aborted with vagal maneuvers, which are not captured in the database. This may underestimate the incidence of SVT recurrence. Lastly, we did not have access to electrocardiographic data to provide a more detailed description of the type of SVT or its rate, nor did we have information about in-utero diagnoses of SVT, both of which may have influenced drug selection. In particular, certain infants may have been suffering from more treatment refractory types of SVT such as ectopic atrial tachycardia or paroxysmal junctional reciprocating tachycardia. However, these conditions are rare, and our low rate of infants with multiple treatment failures (20/457, 4%) suggests that this population might be small in our cohort.
In conclusion, we found that, in hospitalized infants with SVT without pre-excitation or significant congenital heart disease, SVT recurrence was more common when infants received prophylaxis with propranolol compared with digoxin. These findings remained significant after adjusting the analysis for several risk factors and evaluating recurrence at different times after drug initiation. Digoxin, however, was associated with higher incidence of hypotension requiring inotropic support. In the absence of interpretable data from clinical trials, these findings support the use of digoxin over propranolol for SVT prophylaxis in hospitalized infants, although at the potential risk of higher adverse event rates.
Supplementary Material
Acknowledgments
Source of funding:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117.
Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117). Dr. Smith receives salary support for research from the National Institutes of Health and the National Center for Advancing Translational Sciences (HHSN267200700051C, HHSN275201000003I, and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Footnotes
Conflict of interest
The authors have no financial relationships relevant to this article to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Lundberg A. Paroxysmal tachycardia in infancy. A clinical and experimental study. Acta Paediatr. 1963;52:192–195. doi: 10.1111/j.1651-2227.1963.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 2.Nadas AS, Daeschner CW, Roth A, et al. Paroxysmal tachycardia in infants and children; study of 41 cases. Pediatrics. 1952;9:167–181. [PubMed] [Google Scholar]
- 3.Garson A, Jr, Gillette PC. Electrophysiologic studies of supraventricular tachycardia in children. I. Clinical-electrophysiologic correlations. Am Heart J. 1981;102:233–250. doi: 10.1016/s0002-8703(81)80015-4. [DOI] [PubMed] [Google Scholar]
- 4.Salerno JC, Seslar SP. Supraventricular tachycardia. Arch Pediatr Adolesc Med. 2009;163:268–274. doi: 10.1001/archpediatrics.2008.547. [DOI] [PubMed] [Google Scholar]
- 5.Wong KK, Potts JE, Etheridge SP, et al. Medications used to manage supraventricular tachycardia in the infant a North American survey. Pediatr Cardiol. 2006;27:199–203. doi: 10.1007/s00246-005-1126-x. [DOI] [PubMed] [Google Scholar]
- 6.Sanatani S, Potts JE, Reed JH, et al. The study of antiarrhythmic medications in infancy (SAMIS): A multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol. 2012;5:984–991. doi: 10.1161/CIRCEP.112.972620. [DOI] [PubMed] [Google Scholar]
- 7.Perry JC. Supraventricular tachycardia treatment efficacy in infants: On further review. Circ Arrhythm Electrophysiol. 2012;5:882–883. doi: 10.1161/CIRCEP.112.977454. [DOI] [PubMed] [Google Scholar]
- 8.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzer AR, Ellsbury DL, Handler D, et al. The Pediatrix Babysteps Data Warehouse and the Pediatrix Qualitysteps Improvement Project system—tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 11.Hornik CP, Herring AH, Benjamin DK, Jr, et al. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatr Infect Dis J. 2013;32:748–753. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Sullivan JJ, Gardiner HM, Wren C. Digoxin or flecainide for prophylaxis of supraventricular tachycardia in infants? J Am Coll Cardiol. 1995;26:991–994. doi: 10.1016/0735-1097(95)00291-9. [DOI] [PubMed] [Google Scholar]
- 13.Tortoriello TA, Snyder CS, Smith EO, et al. Frequency of recurrence among infants with supraventricular tachycardia and comparison of recurrence rates among those with and without preexcitation and among those with and without response to digoxin and/or propranolol therapy. Am J Cardiol. 2003;92:1045–1049. doi: 10.1016/j.amjcard.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Weindling SN, Saul JP, Walsh EP. Efficacy and risks of medical therapy for supraventricular tachycardia in neonates and infants. Am Heart J. 1996;131:66–72. doi: 10.1016/s0002-8703(96)90052-6. [DOI] [PubMed] [Google Scholar]
- 15.Laughon MM, Avant D, Tripathi N, et al. Drug labeling and exposure in neonates. JAMA Pediatr. 2014;168:130–136. doi: 10.1001/jamapediatrics.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JS, Colan SD, Sleeper LA, et al. Lessons learned from a pediatric clinical trial: The Pediatric Heart Network Angiotensin-converting Enzyme Inhibition in Mitral Regurgitation study. Am Heart J. 2011;161:233–240. doi: 10.1016/j.ahj.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark RH, Bloom BT, Spitzer AR, et al. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117:67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]
- 18.Trembath A, Hornik CP, Clark R, et al. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–960. doi: 10.1016/j.jpeds.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halkin H, Radomsky M, Blieden L, et al. Steady state serum digoxin concentration in relation to digitalis toxicity in neonates and infants. Pediatrics. 1978;61:184–188. [PubMed] [Google Scholar]
- 20.Filippi L, Cavallaro G, Fiorini P, et al. Propranolol concentrations after oral administration in term and preterm neonates. J Matern Fetal Neonatal Med. 2013;26:833–840. doi: 10.3109/14767058.2012.755169. [DOI] [PubMed] [Google Scholar]
- 21.Lang D, von Bernuth G. Serum concentration and serum half-life of digoxin in premature and mature newborns. Pediatrics. 1977;59:902–906. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.