Abstract
This pilot study tested the efficacy of an Audio-visual Stimulation (AVS) program for the promotion of sleep in individuals with chronic pain. Insomnia and chronic pain are common comorbid conditions and their relationship has been viewed as bidirectional. Recent studies suggest a relatively dominant role of sleep in this dyad. The premise of this pilot study was that AVS enhances low frequency while reducing high frequency brain activity resulting in decreased hyperarousal and improved sleep with potential consequent reduction in pain. We conducted a pilot intervention study of AVS using a pre-post design. Participants self-administered a 30-minutes AVS program nightly at bedtime for one month. Sleep and pain were assessed at baseline and at the conclusion of the 4-week intervention phase. 9 adults (mean age 33 ± 15.8 years; female, 89%) completed the study. After using the AVS device for 4 weeks, significant improvement was seen in reported insomnia (ISI, p=.003), pain severity (BPI, p=.005), and pain interference with functioning (BPI, p=.001). Large effect sizes (Partial Eta2: .20–.94)(Cohen’s d: 0.44–1.45) were observed. The results of this pilot study suggest that the AVS program may be efficacious in decreasing both insomnia and pain symptoms. In order to better assess the efficacy of AVS for sleep promotion and possible pain reduction, more definitive randomized controlled trials will be needed. These should include appropriate sample sizes, objective measures of sleep and pain, and longitudinal follow-up.
Keywords: Audio-visual stimulation, light-sound stimulation, insomnia, sleep, chronic pain
Introduction
Chronic pain affects 1 in 3 Americans (Johannes, Le, Zhou, Johnston, & Dworkin, 2010), and 67–88% of adults with chronic pain experience some form of sleep disturbance (Morin, LeBlanc, Daley, Gregoire, & Merette, 2006). This striking level of comorbidity has suggested to many that the relationship between pain and sleep may be bi-directional (Alsaadi et al., 2014; Finan, Goodin, & Smith, 2013; Jansson-Frojmark & Boersma, 2012; McCracken & Iverson, 2002; Okifuji & Hare, 2011). That is, pain may serve to precipitate and perpetuate sleep continuity disturbance; and that sleep initiation and maintenance problems and/or sleep loss may serve to exacerbate or reduce the individual’s tolerance of chronic pain. Further, chronic insomnia may increase the risk for the development of other medical and psychiatric conditions (Matteson-Rusby, Pigeon, Gehrman, & Perlis, 2010) which, in turn, as comorbid conditions, increase the risk for the development, or exacerbation, of chronic pain. These issues, when taken together, strongly suggest that sleep disturbance, in the context of chronic pain, should be targeted for treatment.
Sleep and Chronic Pain
As noted above, the relationship between insomnia and chronic pain has been viewed as bi-directional. Recent studies provide laboratory based evidence that individuals with primary insomnia and insomnia comorbid with chronic pain process pain abnormally (Haack et al., 2012). For example, those with primary insomnia experienced spontaneous pain more frequently and intensely, exhibited a higher sensitivity to evoked heat and pressure pain, and had a dysfunctional pain inhibition system. These findings are consistent with the emerging hypothesis that insomnia is associated with increased pain perception and/or amplification. Importantly, findings from a recent systematic review show that sleep impairment not only predicts new onset and the continuation of chronic pain; sleep is a stronger predictor of chronic pain than chronic pain is to sleep impairment (Finan et al., 2013). This hypothesis is further supported by a longitudinal 13-year study in which sleep disturbances at baseline strongly predicted chronic or onset of radiating low back pain over the course of longitudinal follow up (Lusa, Miranda, Luukkonen, & Punakallio, 2014). Finally, results from a recent study show that short-term improvements in sleep predicted long-term improvements in chronic pain, insomnia symptoms, and fatigue (McCurry et al., 2014; Vitiello et al., 2014). Taken together these findings suggest that improving sleep might improve pain outcomes (Finan et al., 2013; M.T. Smith & Haythornthwaite, 2004; Turk & Cohen, 2010).
Current treatment options for sleep include pharmacological treatment and Cognitive Behavioral Therapy for Insomnia (CBT-I) (M. Perlis, Gehrman, & Riemann, 2008; Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008). Medical treatment with hypnotics (e.g., benzodiazepines and benzodiazepine receptor agonists) has been shown to have good efficacy, safety, and durability for up to 12 months. Clinical benefits, however, do not persist after treatment discontinuation and some patients may experience loss of effectiveness and/or psychological dependence with extended use of hypnotics (Nowell et al., 1997; Roth, Walsh, Krystal, Wessel, & Roehrs, 2005). CBT-I is considered a gold standard non-pharmacological treatment, yielding treatment effects comparable to or exceeding those observed for medications (Jungquist et al., 2010b; Mitchell, Gehrman, Perlis, & Umscheid, 2012; M. T. Smith et al., 2002; Wang, Wang, & Tsai, 2005). Further, there is evidence that CBT-I for insomnia comorbid with chronic pain is as, or more, efficacious than when the treatment is applied to patients with “Primary Insomnia” (Jungquist et al., 2010a; Jungquist et al., 2012; Vitiello, Rybarczyk, Von Korff, & Stepanski, 2009).
One area in insomnia research that has remained under studied is use of self-care approaches that people can use at the home setting to promote sleep. Specifically, brainwave entrainment through light and sound stimulation is an intervention that may have potential to promote relaxation and sleep, but its potential efficacy for improving sleep has not been well explored.
Audio-visual Stimulation (AVS)
The study of the Audio-visual Stimulation (AVS) can be traced back to 1930s. The term AVS is often used interchangeably with “light and sound stimulation” and “audio photic stimulation.” In 1934, with the availability of encephalogram, the impact of photic stimulation on brain activity was documented in a study in which increased brain activity was found to correspond to the frequency of a given photic stimulation (Adrian & Matthews, 1934). During the following decade, several investigators reported brain activity changes in response to photic stimulation, noting that the rhythm of brain activity tended to assume the rhythm of the photic stimulus, which was termed “entrainment” (Bartley, 1937; Jasper, 1936).
Brain activity AVS entrainment to slow frequencies to promote deep relaxation has been reported clinically and in several older studies. In 1980, one of the biofeedback/neurofeedback pioneers, Dr. Thomas Budzynski reported using light and sound stimulation (stimulation frequency unspecified) to assist his clients in successfully achieving and maintaining theta state (3–6 Hz, pre Stage 1 sleep state) during psychotherapy sessions (Budzynski, 1992; Hutchison, 1990). A study by Harris demonstrated that light and sound stimulation (frequency undefined) promoted better sleep for his AIDS/HIV patients (Hutchison, 1990). In addition, a study by Hauri found that AVS closed-loop training, discussed further below, in the sensory motor rhythm (SMR, 12–15 Hz) promoted sleep in people who were physically relaxed but cognitively preoccupied (P. Hauri, 1981; P. J. Hauri, Percy, Hellekson, Hartmann, & Russ, 1982). Of note, Harris’ and Hauri’s studies are two of the few in the AVS literature that document an effect of AVS on sleep.
Audio-visual stimulation (AVS) is a promising self-care intervention option for insomnia that uses preprogrammed light and sound patterns to potentiate sleep-related EEG activity (delta-theta 1–8 Hz) (Budzynski, Budzynski, Sherlin, & Tang, 2011). The AVS devices are inexpensive and readily available without a prescription. Although the AVS mechanism is not fully understood, it is thought that the auditory and visual elements of AVS stimulation modulate endogenous brain activity by activating retinal cells in the eyes and pressure-sensitive cilia within the cochlea of the ears. The evoked electrical potential is then transmitted via neural pathways (audio signals via the medial geniculate; visual signals via the lateral geniculate) to the thalamus where audio and visual sensory information is processed. From the thalamus, the entrained electrical activity is propagated through the cortical thalamic loop to the rest of limbic system and the cerebral cortex (Budzynski et al., 2011; Collura & Siever, 2008) (Figure 1). Importantly, reported adverse effects are minimal; the only known contraindication for adults is a history of a seizure disorder (Rodin, Daly, & Bickford, 1955). While the neural mechanism for AVS remains in debate, a study with six healthy adults demonstrated that repetitive training over a period of 2 months (25 sessions of AVS ramping from 18 down to 2 Hz over 20 minutes, with subjects laying in darkened room with eyes closed) significantly reduced beta and gamma activities and increased the theta/alpha ratio (a brainwave state that is conducive for sleep onset). The findings suggested that with repeated training, AVS cultivates an adaptive self-regulation process and provides exogenous signals to entrain cortical activity to slower frequencies (Teplan, Krakovska, & Stolc, 2006, 2011).
Figure 1.
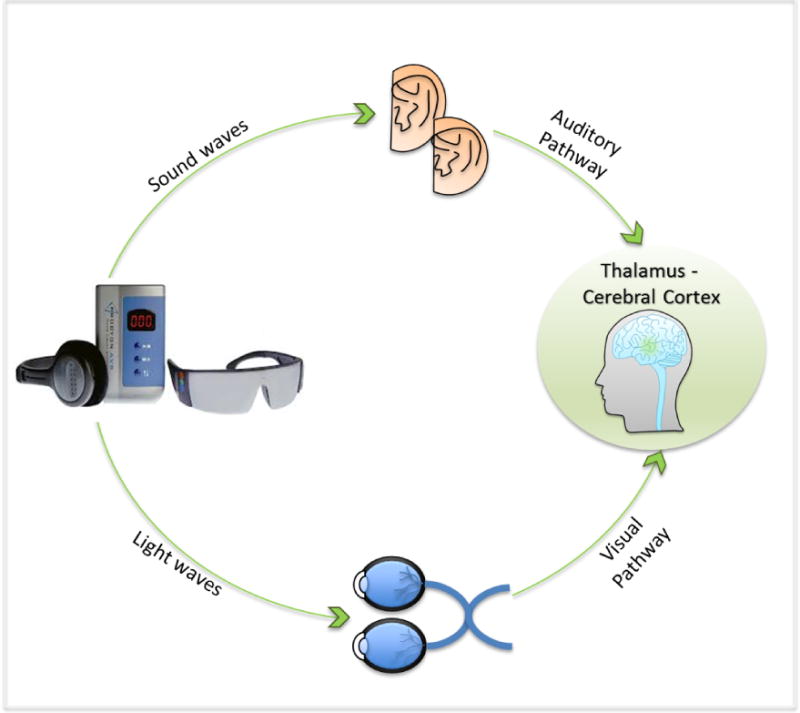
AVS Auditory and Visual Pathways
In AVS, the visual stimulation (flickering light) is delivered through goggles with light-emitting diodes (LEDs) and the audio stimulation is delivered through headphones.
One current line of thought about the ethology and pathophysiology of insomnia, is that chronic insomnia is a hybrid state (hyperarousal) wherein the subject is less disengaged from the environment owing to conditioned cortical arousal and/or local neuronal wakefulness (Buysse, Germain, Hall, Monk, & Nofzinger, 2011; M. L. Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997). This hypothesized state suggests that interventions that facilitate EEG slowing (via feedback, entrainment, conditioning, etc.) may promote improved sleep induction and maintenance. The purpose of this study was to examine the feasibility of a 30 minutes self-administered AVS program for sleep promotion in adults with insomnia and chronic pain in the community setting.
Methods
This was a pilot intervention study using a pre- and post- design. Thirteen participants were recruited from the community sites in Philadelphia and Seattle, through flyers posted at community centers and advertisement placed in the local newspaper. Inclusion criteria were adults 21 years and older, having nonmalignant pain most days for more than 6 months, difficulty sleeping at least 3 nights per week for 3 months, a score of 8 or higher on the Insomnia Severity Index (ISI), and a score between 3 and 10 on the ‘worst pain’ item of the Brief Pain Inventory (BPI). Exclusion criteria were seizure disorder, night shift worker, known photosensitivity, cognitive impairment (assessed through in-person interview by trained research staff), severe psychiatric disorder, and history of a sleep disorder other than insomnia, such as restless leg syndrome, sleep apnea or narcolepsy. Sleep apnea was self-reported or presumed based on a score higher than 0.5 on the Multivariate Apnea Prediction (MAP) index (Maislin et al., 1995). Restless Leg Syndrome was assessed with the 4 item simplified version of International Restless Legs Syndrome scale (IRLS)(Walters et al., 2003; Wunderlich et al., 2005). This pilot study was approved by the University of Pennsylvania and Seattle University Institutional Review Boards. Inform consent was obtained prior to data collection.
Measures
Insomnia Severity Index (ISI)
The ISI is a validated 7-item (0–4) scale that measures insomnia severity. Norms are: 0–7 = no clinically significant insomnia; 8–14 = sub threshold insomnia; 15–21 = clinical insomnia (moderate severity); 21–28 = clinical insomnia (severe). The ISI has internal consistency (alpha =0.90), sensitivity (86%) and specificity (87%); the scale is well-established and sensitive to changes with intervention (Bastien, Vallieres, & Morin, 2001; Morin, Belleville, Belanger, & Ivers, 2011).
Sleep diary
The diary is a two-page log with standard questions about the quantity and quality of the previous night of sleep, including Time to Bed, Sleep Latency, Number of Awakenings, Wake After Sleep Onset time (WASO), Total Sleep Time, and Time out of Bed. The diary also includes questions about the causes of sleep difficulties if any, caffeine and alcohol consumption, daytime napping, exercise, health issues, hypnotic use and the AVS adherence. The one-week sleep diary was completed once at baseline and again upon completion of the intervention.
Brief Pain Inventory (BPI)
The BPI (short form) is a 9-item assessment of pain severity, impact of pain on daily function, location of pain, pain medications and amount of pain relief in the past 24 hours. Administration takes 5 minutes. The scoring of the pain data yields 2 categories: 1) Pain Severity which is a combination of the four pain items (pain now, average pain, worst pain and least pain in the last 24 hours) [0=no pain, 10=pain as bad as you can imagine], and 2) Pain Interference with 7 daily activities/functioning including general activity, walking, work, mood, enjoyment of life, relations with others, and sleep [0=pain does not interferes, 10=pain completely interferes]. Reliability is adequate (Cronbach alpha = 0.77 – 0.91). The BPI has been tested in various pain conditions such as cancer pain, depressive disorders, fibromyalgia, osteoarthritis, etc. In addition, BPI is available in more than 36 languages and has been validated by confirming the consistency of its 2-factor structure (Cleeland & Ryan, 1994; Keller et al., 2004).
Patient Health Questionnaire (PHQ-9)
The PHQ-9 is a well-established scale measuring mood state. The items ask how often in the past 2 weeks the individual has been bothered by symptoms of depression. Scores on the PHQ-9 range from 0 to 27 (1–4 minimal depression; 5–9 mild depression; 10–14 moderate depression; 15–19 moderately severe depression; and 20–27 severe depression (Kroenke, Spitzer, & Williams, 2001).
Multivariable Apnea Prediction Index (MAP)
The MAP is a 13 items survey that screens for prediction of apnea. The survey assesses common symptoms of apnea such as loud snoring, gasping during sleep, breathing difficulty, and excessive daytime sleepiness. Participants were asked to rate the frequency of these identified symptoms on a numeric scale (0 = never; 4 = always, 5–7 times/week; and do not know). The score is then entered into a formula along with covariates (age, gender, and body mass index) for further computation. A MPA score higher than 0.5 suggests likelihood of sleep apnea (Maislin et al., 1995). In this study, the MPA was assessed at the initial interview. People who scored higher than 0.5 on MPA were excluded from participating in this study.
International Restless Legs Syndrome scale (IRLS)
The IRLS (short form) is a 4 item questionnaire that indexes typical symptoms of restless leg syndrome during the day and sleep (i.e., discomfort sensation in legs, urgency to move or rub legs to relieve discomfort, symptoms worsen when resting). The response option for each item is yes or no (Walters et al., 2003). The IRLS (short form) was used as a screening tool in this study. If a participant answered yes to all 4 questions, then they were not eligible to participate in this study.
Demographic data, brief health history (i.e., smoking, alcohol, drug use) and medication data (name, dosage, frequency, duration, indication, and medication changes) were also collected and used to describe the sample.
Procedure
At the initial meeting, participants completed the ISI, BPI, and PHQ-9 and were instructed to record their sleep patterns (sleep diary) for 1 week during the baseline period; which is a typical length of baseline observation in sleep research. After a 1-week baseline, they were trained to use the AVS program at bedtime and to record their sleep pattern in a sleep diary for 1 month. The AVS program [Procyon by MindPlace] consists of 30-minutes of light flickering (goggles) and sound pulsing (headphones) that gradually descends from alpha (8 Hz) to delta (1 Hz) frequencies. Weekly phone calls were used to address participants’ questions and assess frequency of usage. The ISI, BPI, and PHQ-9 were measured again upon completion of the 1 month intervention.
Data Analysis
The raw data were screened for accuracy, missing values, outliers, and distributional properties prior to analysis (SPSS V21). The sample was characterized using descriptive statistics of demographic and baseline variables. Repeated paired sample T-tests were used to examine pre- and post- intervention differences. The effect size was examined using both ANOVA partial Eta2 and Cohen’s d.
Results
Thirteen adults were enrolled and nine (mean age 33±16, 89% women) (7 from Seattle, 2 from Philadelphia) completed the study. Of the four participants who withdrew from the study, one reported sensitivity to the light stimulation even at the dimmest intensity, two did not like to wear any foreign object (goggles and ear buds) during sleep, and one declined to give a reason. None of the withdrawn participants reported serious adverse effects. There were no significant differences on the demographic characteristics and baseline measures between those who completed and those who withdrew.
Data were collected between November 2012 and March 2013. At baseline, the mean insomnia severity score (ISI) at was 19.2 ± 3.9, in the clinical insomnia moderate range. The total sleep time by self-reported sleep diary was 403.4 ± 70.2 minutes. Mean ratings for worst pain in the last 24 hours (7.0 ± 1.1), pain interference with sleep (8.0 ± 1.6), pain interference with the ability to enjoy life (5.4 ± 1.8), and the impact of pain on mood (5.2± 2.3) were all elevated. The majority of the participants reported having back pain (67%); the rest reported pain that was disease related (i.e., congenital abdominal condition (11%), fibromyalgia (11%), and arthritis (11%). The mood measure (PHQ-9) at baseline was 11.9 ± 5.6, in the moderate depression range.
After the one-month AVS intervention (with self-reported adherence to the AVS of 94%) significant improvement was observed for insomnia (ISI, p=.003). The sleep diary measures (sleep latency, WASO, total sleep time, and sleep efficiency), although not statistically significant, showed trends in the direction of improvement (Table 1). Before Bonferroni adjustment for multiple comparisons, significant improvement in pain severity (BPI, p=.005), pain interference with daily functioning (BPI, p=.001), worst pain (BPI, p=.004), ability to sleep through pain (BPI, p=.015), pain interference with the mood (BPI, p=.012), the degree to which pain interfered with the enjoyment of life (BPI, p=.043), and depression (PHQ-9, p=.035) were observed. Thesefindings suggested large effect sizes (Partial Eta2, range 0.20–0.94)(Cohen’s d, range 0.44–1.45). When Bonferroni correction was performed, Insomnia Severity Index & BPI Pain Interference Functional remained statistically significant (Table 1).
Table 1.
AVS for Sleep Promotion in Adults with Chronic Pain (N=9) Mean Scores at Baseline Compared with Mean Scores in Post-Testing 4 Weeks Later
| Pre-test | Post-test | Significance | Partial Eta2 | Cohen’s d | |
|---|---|---|---|---|---|
| Insomnia Severity Index | 19.2 ± 3.9 | 12.8 ± 5.0 | 0.003* | .79 | 1.45 |
| Sleep Diary sleep latency | 68.8 ± 27.3 | 56.6 ± 28.3 | 0.456 | .20 | 0.44 |
| Sleep Diary wakes after sleep onset (WASO) | 35.7 ± 17.2 | 22.6 ± 19.6 | 0.090 | .47 | 0.71 |
| Sleep Diary total sleep time | 403.4 ± 70.2 | 444.0 ± 106.5 | 0.463 | .11 | 0.45 |
| Sleep Diary sleep efficiency | 80.5 ± 6.6 | 88.5 ± 4.2 | 0.129 | .48 | 1.45 |
| BPI Pain Severity (4 severity items) | 5.0 ± 1.2 | 4.0 ± 1.6 | 0.047 | .50 | 0.71 |
| BPI Pain Interference Items (7 functional items) | 5.39 ± 1.9 | 3.8 ± 2.0 | 0.001* | .94 | 0.84 |
| PHQ-9 Depression | 11.9 ± 5.6 | 9.0 ± 2.8 | 0.035 | .80 | 0.66 |
Significance after Bonferroni adjustment – P value at 0.05 / 8 items: 0.006
Discussion
The results of this pilot study of a small sample with comorbid insomnia and pain suggest that the AVS program ramping from 8 to 1 Hz over the course of 30 minutes may be efficacious in decreasing insomnia symptoms. In addition to the positive changes in sleep, improvements were also observed in pain although pain was not directly treated. This result indirectly supports the emerging hypothesis that sleep may have a more prominent role than pain in the comorbid dyad (Finan et al., 2013) and illustrates the feasibility of this intervention approach.
Limitations
While the results of this preliminary study were encouraging, taking into account that sleep and pain are highly subjective phenomena, the interpretation of the findings is limited by the small sample size, lack of a control group, the lack of objective measures of sleep and pain, and the pre post design. In addition, the high self-reported adherence rate for AVS usage could be indicative of highly committed subjects or social desirability effects. To further the understanding of the AVS intervention in sleep promotion and pain reduction, we recommend that future study include a randomized controlled design with a larger sample size, an objective measure of sleep and pain, and longitudinal follow up.
Implications
This pilot study is the first to examine the efficacy of a 30 minutes self-administered portable in-home AVS self-care intervention for sleep induction in adults with chronic pain. Considering that treatment options for insomnia are somewhat limited, AVS could serve as an initial intervention in a stepped care approach for the management of insomnia in people with chronic pain.
Acknowledgments
This project was conducted with the support of 1) John A. Hartford Foundation Claire M. Fagin Fellowship – National Hartford Center of Gerontological Nursing Excellence, 2) National Institute of Nursing Research T-32 Post-doctoral fellowship (NINR 5-T32-NR009356) from the NewCourtland Center for Transitions and Health, School of Nursing University of Pennsylvania, 3) the pilot study fund from the Biobehavioral Research Center, School of Nursing University of Pennsylvania, 4) the Sinegal Faculty Development Fund, College of Nursing, Seattle University, and 5) Center for Research on the Management of Sleep Disturbances (P30 NR011400), University of Washington.
Special thanks to Akiko Miller, Yip-Han Lee, Taylor Goulding, Cara Mcguinness, and Regina Belche for their exceptional assistance and contribution on the project.
Contributor Information
Hsin-Yi (Jean) Tang, Email: jeantang@uw.edu, Assistant Professor, School of Nursing; University of Washington, Health Science Center, 1959 NE Pacific St. Box 357263, Seattle, WA 98195-7263, TEL: 206-685-0816, FAX: 206-685-9551.
Michael V. Vitiello, Professor, Psychiatry and Behavioral Sciences, School of Medicine, University of Washington, Seattle, Washington, 98195.
Michael Perlis, Associate Professor, Director of the Behavioral Sleep Medicine Program, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19106.
Jun James Mao, Associate Professor, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19106.
Barbara Riegel, Professor and Edith Clemmer Steinbright Chair of Gerontology, School of Nursing, University of Pennsylvania, Philadelphia, Pennsylvania 19106.
References
- Adrian E, Matthews B. The Berger rhythm: Potential changes from the occipital lobes of man. Brain. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Alsaadi SM, McAuley JH, Hush JM, Lo S, Bartlett DJ, Grunstein RR, Maher CG. The Bidirectional Relationship Between Pain Intensity and Sleep Disturbance/Quality in Patients with Low Back Pain. Clin J Pain. 2014 doi: 10.1097/ajp.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Bartley S. Some observations on the organization of the retinal response. American Journal of Physiology. 1937;120:184–189. [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Budzynski T. Clinical considerations of sound/light. Seattle, WA: Synetics; 1992. [Google Scholar]
- Budzynski T, Budzynski H, Sherlin L, Tang HY. Audio-Visual Stimulation: Research and Clinical Practice. In: Berger J, Turow G, editors. Music, Science, and the Rhythmic Brain. New York: Routledge; 2011. pp. 137–153. [Google Scholar]
- Buysse DJ, Germain A, Hall M, Monk TH, Nofzinger EA. A Neurobiological Model of Insomnia. Drug Discov Today Dis Models. 2011;8(4):129–137. doi: 10.1016/j.ddmod.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Collura T, Siever D. Audio-visual entainment in relation to mental health and EEG. In: Budzynski T, Budzynski H, Evans J, Abarbanel A, editors. Introduction to Quantitative EEG and Neurofeedback: Advanced Theory and Applications. 2. Boston: Elsevier; 2008. pp. 193–223. [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. European Journal of Pain. 2012;16(4):522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri P. Treating psychophysiologic insomnia with biofeedback. Archives of General Psychiatry. 1981;38(7):752–758. doi: 10.1001/archpsyc.1981.01780320032002. [DOI] [PubMed] [Google Scholar]
- Hauri PJ, Percy L, Hellekson C, Hartmann E, Russ D. The treatment of psychophysiologic insomnia with biofeedback: a replication study. Biofeedback and self-regulation. 1982;7(2):223–235. doi: 10.1007/BF00998785. [DOI] [PubMed] [Google Scholar]
- Hutchison Michael. Megabrain Report. Vol. 1. Sausalito, CA: Megabrain, Inc; 1990. The Megabrain Report Special Issue on Sound and Light Technologies. [Google Scholar]
- Jansson-Frojmark M, Boersma K. Bidirectionality between pain and insomnia symptoms: a prospective study. Br J Health Psychol. 2012;17(2):420–431. doi: 10.1111/j.2044-8287.2011.02045.x. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Cortical excitatory state and synchronism in the control of bioelectric autonomous rhythms. Cold Spring Harbor Symposia on Quantitative Biology. 1936;4:320–332. doi: 10.1101/sqb.1936.004.01.033. [DOI] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. The journal of pain : official journal of the American Pain Society. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, Perlis ML. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010a;11(3):302–309. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, Perlis ML. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Medicine. 2010b;11(3):302–309. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungquist CR, Tra Y, Smith MT, Pigeon WR, Matteson-Rusby S, Xia Y, Perlis ML. The durability of cognitive behavioral therapy for insomnia in patients with chronic pain. Sleep Disord. 2012;2012:679648. doi: 10.1155/2012/679648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusa S, Miranda H, Luukkonen R, Punakallio A. Sleep disturbances predict long-term changes in low back pain among Finnish firefighters: 13-year follow-up study. Int Arch Occup Environ Health. 2014 doi: 10.1007/s00420-014-0968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Dinges DF. A survey screen for prediction of apnea. Sleep. 1995;18(3):158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- Matteson-Rusby SE, Pigeon WR, Gehrman P, Perlis ML. Why treat insomnia? Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00743. doi: 10.4088/PCC.08r00743bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7(2):75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Shortreed SM, Von Korff M, Balderson BH, Baker LD, Rybarczyk BD, Vitiello MV. Who benefits from CBT for insomnia in primary care? Important patient selection and trial design lessons from longitudinal results of the Lifestyles trial. Sleep. 2014;37(2):299–308. doi: 10.5665/sleep.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA : the journal of the American Medical Association. 1997;278(24):2170–2177. [PubMed] [Google Scholar]
- Okifuji A, Hare BD. Do sleep disorders contribute to pain sensitivity? Curr Rheumatol Rep. 2011;13(6):528–534. doi: 10.1007/s11926-011-0204-8. [DOI] [PubMed] [Google Scholar]
- Perlis M, Gehrman P, Riemann D. Intermittent and long-term use of sedative hypnotics. Curr Pharm Des. 2008;14(32):3456–3465. doi: 10.2174/138161208786549290. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. Journal of Sleep Research. 1997;6(3):179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Rodin EA, Daly DD, Bickford RG. Effects of photic stimulation during sleep; a study of normal subjects and epileptic patients. Neurology. 1955;5(3):149–159. doi: 10.1212/wnl.5.3.149. [DOI] [PubMed] [Google Scholar]
- Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Medicine. 2005;6(6):487–495. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, Buysse DJ. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. The American journal of psychiatry. 2002;159(1):5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain interrelate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Teplan M, Krakovska A, Stolc S. EEG responses to long-term audio-visual stimulation. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2006;59(2):81–90. doi: 10.1016/j.ijpsycho.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Teplan M, Krakovska A, Stolc S. Direct effects of audio-visual stimulation on EEG. Computer Methods & Programs in Biomedicine. 2011;102(1):17–24. doi: 10.1016/j.cmpb.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Turk DC, Cohen MJ. Sleep as a marker in the effective management of chronic osteoarthritis pain with opioid analgesics. Seminars in Arhritis and Rheumatism. 2010;39(6):477–490. doi: 10.1016/j.semarthrit.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, McCurry SM, Shortreed SM, Baker LD, Rybarczyk BD, Keefe FJ, Von Korff M. Short-term Improvement in Insomnia Symptoms Predicts Long-term Improvements in Sleep, Pain, and Fatigue in Older Adults with Co-Morbid Osteoarthritis and Insomnia. Pain. 2014 doi: 10.1016/j.pain.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, Trenkwalder C. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Wang MY, Wang SY, Tsai PS. Cognitive behavioural therapy for primary insomnia: a systematic review. J Adv Nurs. 2005;50(5):553–564. doi: 10.1111/j.1365-2648.2005.03433.x. [DOI] [PubMed] [Google Scholar]
- Wunderlich GR, Evans KR, Sills T, Pollentier S, Reess J, Allen RP, Walters AS. An item response analysis of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2005;6(2):131–139. doi: 10.1016/j.sleep.2004.10.010. [DOI] [PubMed] [Google Scholar]


