Abstract
Despite the strides that immunotherapy has made in mediating tumor regression, the clinical effects are often transient, and therefore more durable responses still are needed. The temporary nature of the therapy-induced immune response can be attributed to tumor immune evasion mechanisms, mainly the effect of suppressive immune cells and, in particular, T regulatory cells (Treg). Although the depletion of Treg has been shown to be effective in enhancing immune responses, selective depletion of these suppressive cells without affecting other immune cells has not been very successful, and new agents are sought.
We found that PI3K-Akt pathway inhibitors selectively inhibit Treg with minimal effect on conventional T cells (Tconv). Our results clearly show selective in vitro inhibition of activation (as represented by a decrease in downstream signaling) and proliferation of Treg in comparison to Tconv when treated with different Akt and PI3K inhibitors. This effect has been observed in both human and murine CD4 T cells. In vivo treatment with these inhibitors resulted in a significant and selective reduction in Treg both in naïve and tumor-bearing mice. Furthermore, these PI3K-Akt inhibitors led to a significant therapeutic antitumor effect, which was shown to be Treg-dependent.
Here, we report the use of PI3K-Akt pathway inhibitors as potent agents for the selective depletion of suppressive Treg. We show that these inhibitors are able to enhance the antitumor immune response and are therefore promising clinical reagents for Treg-depletion.
Keywords: Regulatory T cells, PI3K-Akt pathway, PI3K-Akt inhibitors, cancer vaccine
Introduction
Cancer immunotherapy has proven successful in mediating disease regression in cancer patients. Several strategies have been used effectively to generate therapeutic tumor antigen-reactive T-cell responses. These include active immunization (1–5), adoptive cell transfer (ACT) of lymphocytes genetically engineered for antitumor function (6–9), and ACT of tumor-infiltrating lymphocytes (TIL)(10–16). Despite these successes, many responses are transient, and improvements are needed to increase durable responses in patients.
A suppressive tumor immune environment is thought to limit the efficacy of ACT and active immunization approaches. Chief among the suppressive cells are regulatory CD4 T cells (Treg) that can be distinguished by the expression of FoxP3 and CD25HI molecules (17). An opposing action of Treg and cytotoxic CD8 T cells in tumor regression has been identified in mouse models (18). A correlation of tumor-infiltrating Treg with poor clinical prognosis has been demonstrated in humans (19–22). In fact, the depletion of Treg was found to enhance antitumor immunity and promote tumor regression in mouse models (23–26).
Despite the evidence of the suppressive role of Treg in the tumor microenvironment (TME), there is still a paucity of Treg-depleting clinical reagents. Concerns over efficacy and specificity have burdened currently available clinical reagents such as low-dose cyclophosphamide and anti-CD25 monoclonal antibody (mAb) daclizumab (27). Novel clinical reagents that specifically target Treg persistence and survival are candidates for tumor immune modulation.
Several reports have shown that Treg and conventional T cells (Tconv) display unique signaling signatures downstream of the T-cell receptor (TCR) (28–31). The phosphatidylinositol-3-kinase/Akt (PI3K-Akt) pathway plays a critical role in the cellular response to TCR engagement and co-stimulation (32, 33). Active PI3K-Akt signaling results in increased cytokine gene expression, a characteristic of productive T-cell activation (34). In addition to transmitting signals critical for T-cell activation, many T-cell functions are governed by PI3K-Akt signaling, which included proliferation, survival, migration, and metabolism (35, 36).
Due to the important role of the PI3K-Akt pathway in T-cell function (35, 36) and the reported differences between Treg and Tconv downstream of the TCR (28–31), we evaluated the effect of PI3K-Akt inhibition on Treg and Tconv. The impact of Akt and PI3K inhibitors on Treg and Tconv activation and proliferation is assessed in vitro. We have also evaluated the effect and therapeutic efficacy of in vivo treatment with these inhibitors on the antitumor immune response.
Materials and Methods
Mice and cell lines
Female C57BL/6(H-2b) and BALB/c mice (6–10 weeks old) (NCI, Frederick, MD) were housed under pathogen-free conditions. All procedures were carried out with approved institutional animal protocols. B16, CT26 and EL4 cell lines were obtained from American Type Culture Collection (ATCC) (Manassas, VA) which routinely authenticate and test these cell lines (for mycoplasma, by the Hoechst stain, PCR and the standard culture test). These cells were used within six months of purchase. TC-1 (established by immortalization with the HPV16 E6 and E7 genes and its growth enhanced by Treg (37, 38)) was a gift from Prof. TC Wu. These cells along with B16 were authenticated and tested for mouse parvovirus (MPV) and mouse hepatitis virus (MHV) using PCR at Georgia Regents University. All tests were negative.
Reagents
The PI3K inhibitor Wortmannin (WM) and the Akt inhibitor triciribine (TCN) were obtained from Calbiochem (San Diego, CA). IC87114, a PI3K inhibitor, and MK-2206, an Akt inhibitor, were purchased from SelleckChem. The 9-mer synthetic peptide from HPV16 E749–57, RAHYNIVTF, was obtained from Celltek Bioscience. E749–57 (100µg/mouse) was used as a vaccine along with GM-CSF (5µg/mouse, PeproTech, Rocky Hill, NJ), anti-CD40 (20µg/mouse, BioLegend) and Incomplete Freund’s Adjuvant (IFA)(50 µL/mouse, Sigma, St. Louis, MO). This was reported as the most effective therapeutic combination for this vaccine (39).
Human T-cell cultures
Leukapheresis products were obtained from healthy human donors (Department of Transfusion Medicine, NIH). Peripheral blood mononuclear cells (PBMC) were prepared over Ficoll-Paque Plus gradient centrifugation (GE Healthcare, Little Chalfont, UK) and CD4+CD25HI and CD4+CD25- cells were sorted using the FACSAria II flow cytometer. The cells were then labeled with CFSE (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Fifty thousand cells were cultured with anti-CD3/CD28-conjugated Dynabeads (Life Technologies, Carlsbad, CA) at a 4:1 cell-to-bead ratio in RPMI-1640 supplemented with 5% autologous serum and 100U/mL IL-2 (PeproTech, Rocky Hill, NJ) for three days, in the presence or absence of escalating concentrations of inhibitors. CFSE dilution was then assessed by flow cytometry.
Murine CD4 T-cell cultures
Magnetic bead purification kits (Miltenyi, Auburn, CA) were used to enrich CD4+CD25− and CD4+CD25+ T cells from murine splenocytes following the manufacturer’s instructions. Cells were labeled with CFSE (Life Technologies, Carlsbad, CA) and cultured in 24-well plates at a density of 5×105 cells per well in RPMI 1640 (Life Technologies, Carlsbad, CA) with 10% FCS in the presence of 10µg/mL plate-bound anti-CD3 (BD, San Jose, CA), 1µg/mL soluble anti-CD28 (BD), and 100 IU/mL IL-2 (R&D, Minneapolis, MN). Plates were centrifuged and then incubated at 37°C, 5% CO2 for 72 hours. WM (200nM), MK-2206 (2µM), IC87114 (10µM), or DMSO (carrier) were added to the culture media from the beginning. CFSE dilution was measured by flow cytometry.
The phosphorylation level of S6 was assessed. Murine cells were prepared as described above and stimulated for 15 minutes. Thirty micrograms of cell lysates in RIPA buffer were then run on SDS-PAGE gels, transferred to PVDF membranes probed with primary antibodies (1:1000) (anti-pS6, -S6 (Cell Signaling)) overnight at 4°C and incubated with secondary antibodies (1:2000) for one hour at room temperature. Chemiluminescence was performed with Pierce reagents (Rockford, IL) and densitometric analysis was performed using ImageJ (NIH, Bethesda, MD).
In vivo experiments to assess splenocyte composition
Tumor-free naïve mice were injected intraperitoneally (i.p.) with 40µg WM, 50µg TCN or 10ug MK2206 dissolved in 35% DMSO in PBS (100µL volume). Mice were injected every other day for a week. Splenocytes were then harvested and the CD4+ and Foxp3+ composition was assessed by flow cytometry.
IFNγ ELISPOT
Tumor-free naïve mice were injected i.p. on alternate days with 40µg WM, 50µg TCN or DMSO vehicle for a week prior to vaccination with E7 vaccine (E7 49–57, GM-CSF, anti-CD40 and IFA) which was given subcutaneously (s.c.) on days 7 and 14. One week after the second E7 vaccination, splenocytes were harvested, and the anti-E7 immune response was assayed by peptide restimulation and interferon-γ (IFN γ) ELISPOT (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions.
Tumor treatment
C57BL/6 female mice were implanted with 50,000 TC-1 cells/mouse s.c. in the right flank on day 0. The mice were then treated with 40µg WM or DMSO for one week after palpable tumors were detected. Mice were then vaccinated with E7 peptide vaccine (E7 49–57, GM-CSF, anti-CD40 and IFA) as described above and tumor growth was monitored. The same model was used with MK-2206, in which mice were challenged with TC-1 cells on day 0, and on days 7 and 14 mice in the appropriate groups were injected with the E7 vaccine and/or MK-2206 (30µg). The tumors were measured and the mice euthanized on day 21. The tumor immune infiltrate was then assessed by flow cytometry. A prophylactic therapeutic model was also used without vaccines. Three tumor models were employed: B16 (200,000 cells per mouse, s.c.) and EL4 (100,000 cells per mouse s.c.), both in C57BL/6 mice, and CT26 (500,000 cells per mouse, s.c.) in BALB/c mice. Mice were treated with 40µg WM, 50µg TCN or DMSO (i.p) on days -7,-4 and -2 prior to subcutaneous tumor inoculation on day 0, and tumor growth was monitored thereafter. Ex-vivo activated Treg (cells were stimulated as described above for 72 hours) were infused i.v. (10,000 cells per mouse) into WM-treated EL4 and CT26 tumor-bearing animals on day 4 following tumor inoculation, and tumor growth was also monitored. Analysis of FoxP3+ T-cell infiltration into CT26 tumor was assessed by flow cytometry on days 20 and 24 following tumor inoculation.
Flow cytometry analysis
Staining for surface markers (CD4 and CD8) was performed using mAbs (BD). Cells were incubated for 20 minutes on ice in PBS, 2% BSA and 0.1% sodium azide. Intracellular staining kits were used to stain for Foxp3 using anti Foxp3-PE mAb (eBioscience). Data acquisition was performed on FACSCalibur or FACScan cytometers (BD). Results were analyzed with Cellquest (BD), WinMDI (Purdue University), or Flowjo (Tree Star).
Statistical analysis
All statistical parameters (average values, SD, significant differences between groups) were calculated using GraphPad Prism Software. Statistical significance between groups was determined by paired t test or ANOVA with post hoc Tukey’s multiple comparison test (p < 0.05 was considered statistically significant).
Results
Human CD4 Treg are more dependent on PI3K-Akt signaling for TCR-induced proliferation compared to conventional CD4 T cells
Due to the vital role that the PI3K-Akt pathway plays in many T-cell functions, including proliferation and survival, we evaluated the effect of inhibiting this pathway on human CD4+ T-cell proliferation. CD4+ Treg were identified as CD4+CD25HI while conventional CD4+ Tconv were identified as CD4+CD25−. Treg and Tconv were fractionated from human PBMCs by fluorescence-activated cell sorting. Proliferation was examined by dilution of CFSE after stimulation with anti-CD3/anti-CD28 in a three-day culture in media containing 100 IU/ml IL-2 with titrated amounts of inhibitors selective for members of the PI3K-Akt pathway.
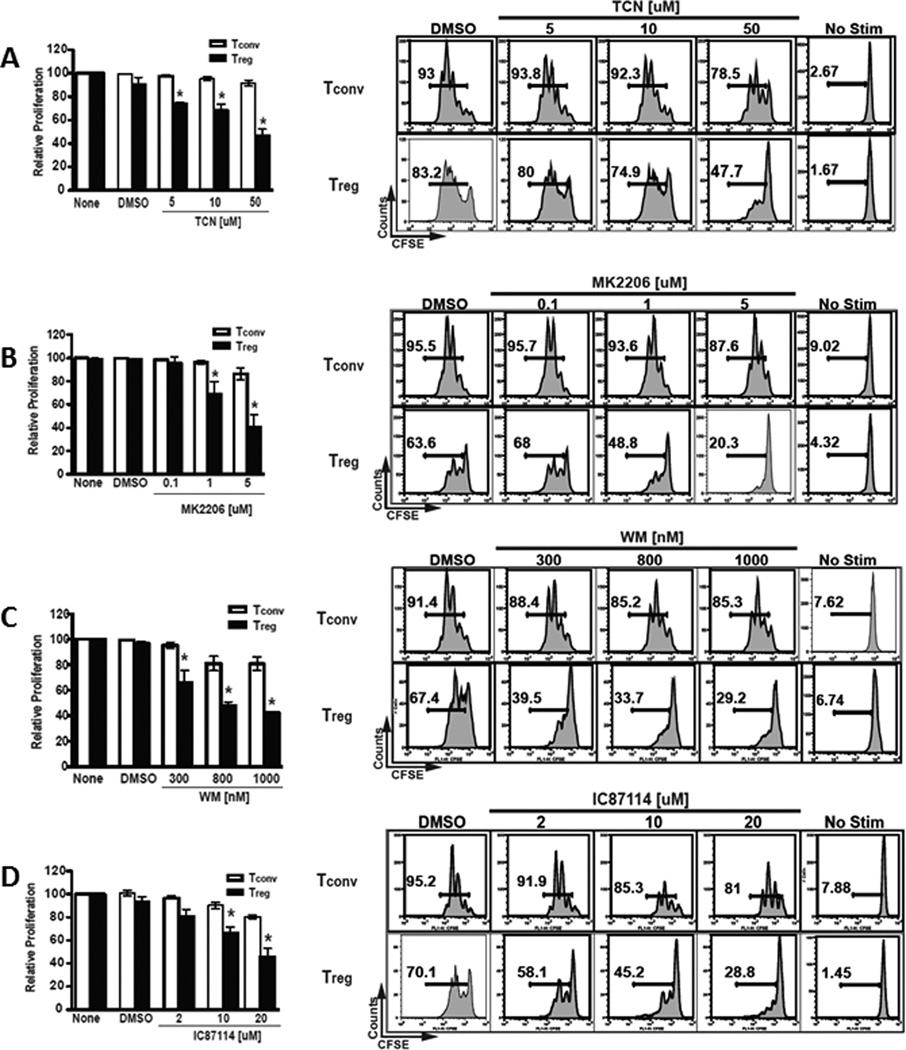
We evaluated the effect of Akt inhibition on proliferation using the pan-Akt inhibitors TCN and MK-2206. A higher sensitivity of Treg to proliferative inhibition by both inhibitors compared to Tconv is shown in Figure 1A, 1B. The average of three experiments revealed a significant inhibition of Treg proliferation compared to Tconv by TCN (Figure 1A) and MK-2206 (Figure 1B) at all doses tested.
Figure 1.
The inhibition of PI3K and Akt in human T cells selectively inhibits the proliferation of human Treg compared to Tconv in a dose-dependent manner.
- A significant reduction in Treg proliferation was observed in response to Akt inhibition by TCN compared to that of Tconv at all doses tested: 5uM (p=0.04), 10uM (p=0.03), and 50uM (p=0.01).
- A significant reduction of Treg proliferation in response to MK-2206 treatment was observed compared to that of Tconv at the 1uM (p=0.05) and 5uM (p=0.005) doses.
- Proliferation of Treg was significantly reduced by WM compared to that of Tconv at all doses tested: 300nM (p=0.03), 800nM (p=0.01) and 1000nM (p=0.002).
- Treg proliferation was significantly reduced by IC87114 compared to that of Tconv at the 10uM (p=0.04) and 20uM (p<0.005) doses.
To confirm that this selective effect is not unique to Akt inhibition, we evaluated the effect of upstream PI3K inhibition by WM and IC87114 on the proliferation of Treg and Tconv. Results consistent with a higher sensitivity of Treg were observed. The average of three experiments demonstrated that proliferation of Treg was significantly inhibited by WM compared to Tconv at all doses tested (Figure 1C) and by IC87114 at the 10uM and 20uM doses (Figure 1D).
To rule out selective drug toxicity as an explanation for the above findings, we next evaluated the effect of PI3K-Akt pathway inhibition on CD4 T-cell subset viability. The same Treg and Tconv examined above were assayed for viability by 7-AAD incorporation. Culture viability was inversely related to the amount of inhibitor present across the dose-range used; however, the viability was reduced equally in Treg and Tconv. This was observed with all inhibitors (WM, IC87114, TCN and MK-2206) (data not shown).
Taken together, our data demonstrate that human Treg are more dependent on the PI3K-Akt signaling pathway for proliferation in response to anti-CD3/anti-CD28/IL-2 stimulation compared to Tconv.
PI3K-Akt pathway is necessary for CD4 Treg maintenance in mice
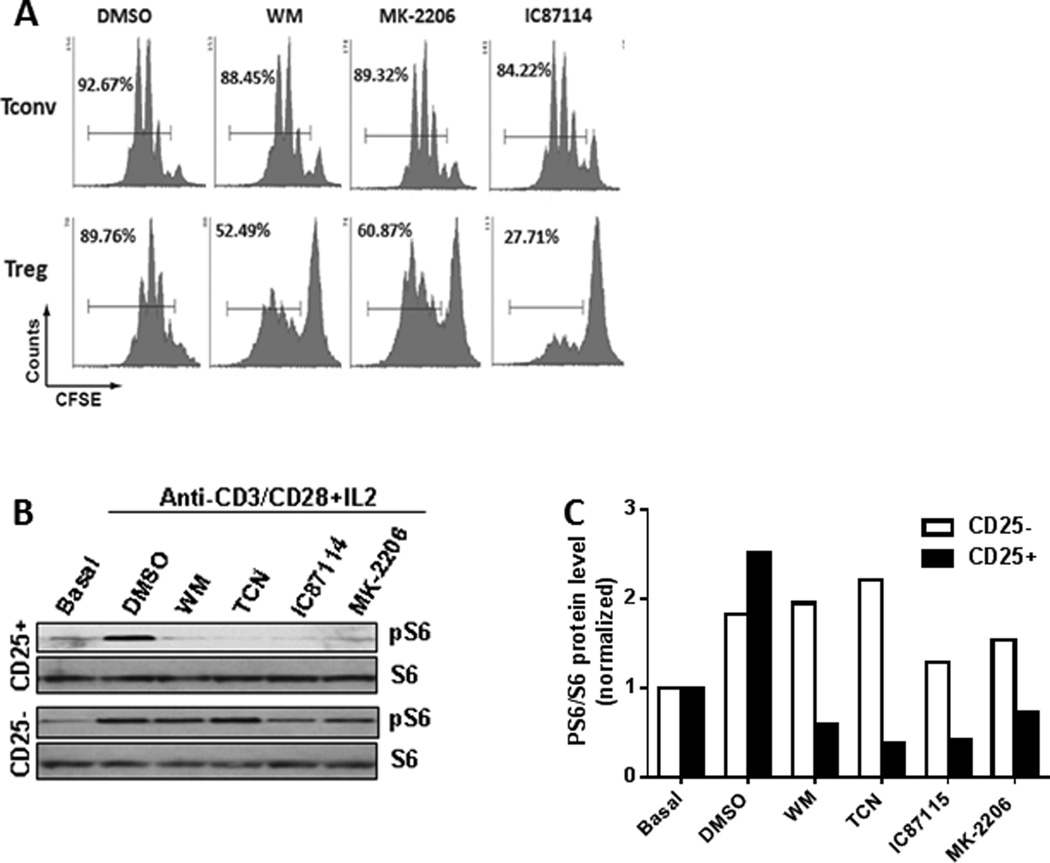
The documented dependence of murine T-cell proliferation and other vital functions on the PI3K-Akt pathway, in addition to the necessity of this pathway in human Treg proliferation shown above, prompted a similar evaluation in murine Treg proliferation. Mouse CD4+ splenocytes were fractionated into CD25+ (Treg) and CD25− (Tconv) subsets using magnetic bead enrichment kits. After enrichment, Treg and Tconv were >92% and >95% pure, respectively, based on CD4 and FoxP3 expression (data not shown). Proliferation was assayed by dilution of CFSE after stimulation with anti-CD3/anti-CD28 and three-day culture in media containing 100 IU/ml IL-2 with WM, MK-2206, or IC87114 (TCN was found to be toxic to murine T cells in these prolonged in vitro culture conditions). Similar to results obtained with human CD4 T-cell subsets, data from at least two independent experiments demonstrated that mouse Treg were significantly more sensitive to proliferative inhibition by WM (200nM), IC87114 (10µM) and MK-2206 (2µM) than Tconv (Figure 2A).
Figure 2.
PI3K and Akt inhibition differentially inhibits the proliferation and downstream activation of murine Treg compared to that of Tconv.
- Representative example of the selective proliferation inhibition in Treg (lower panel) by WM, MK-2206 and IC87114 compared to that of Tconv (upper panel).
- Treatment with WM, TCN, IC87114, or MK-2206 resulted in a marked decrease of phosphorylated S6 in Treg (CD25+) in comparison to Tconv (CD25−).
- Densitometry of phosphorylated S6 levels to S6 ratio showed significantly lower levels in Treg when compared to Tconv when the stimulated cells were treated with PI3K/ Akt pathway inhibitors. Densitometry was performed on gel shown in Figure 2B.
Inhibition of PI3K-Akt signaling by inhibitors in Treg was also confirmed by Western blot analysis in which phosphorylation of S6 was used to identify active signaling through the PI3K-Akt pathway. Confirming the inhibition of PI3K-Akt signaling, treatment of anti-CD3/anti-CD28/IL-2-stimulated Treg with WM, TCN, IC87114, or MK-2206 resulted in a marked decrease of phosphorylated S6 in comparison to Tconv (Figure 2B, 2C).
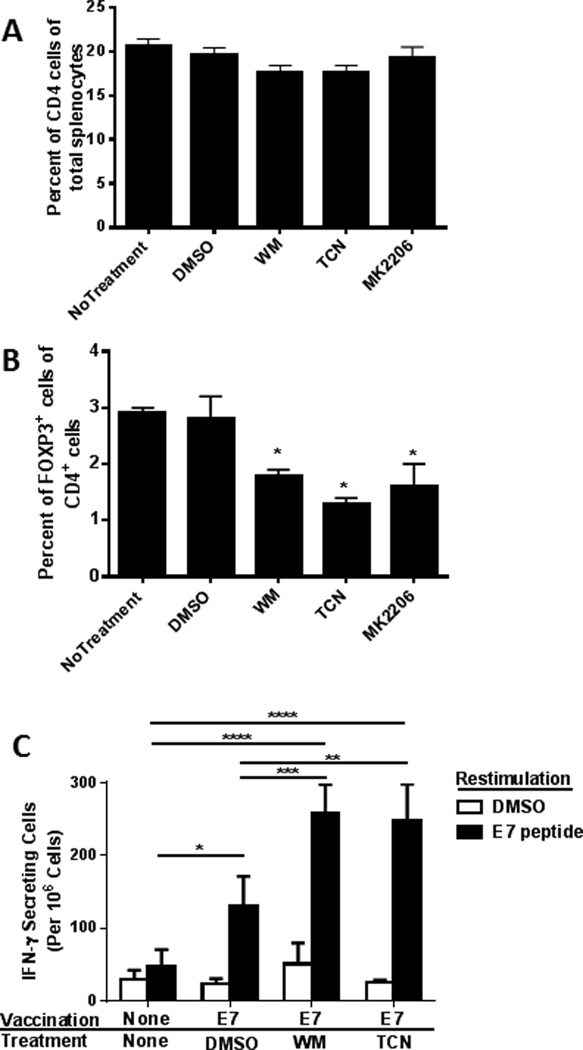
As a result of the significant in vitro difference between the proliferation inhibition of Treg and Tconv by PI3K and Akt inhibitors, the role of PI3K-Akt signaling on the in vivo cellular composition of spleens was examined. In these analyses, mice were treated with WM (40µg), TCN (50µg), MK-2206 (10µg), or DMSO for one week on alternate days prior to flow cytometry analysis of their splenocytes composition. Mice treated with DMSO contained a similar percentage of CD4 T cells compared to WM-, TCN- or MK-2206-treated mice (Figure 3A). Similarly, spleens of DMSO and Inhibitor-treated mice exhibited no differences in the percentages of CD8 T cells (data not shown). Importantly, despite not affecting total CD4 T cells, the percentage of FoxP3+ cells within the CD4+ T cell population was significantly reduced in mice treated with WM, TCN or MK-2206 (Figure 3B).
Figure 3.
The PI3K/ Akt pathway is essential for the in vivo maintenance of murine Treg.
- Mice treated with DMSO vehicle contained similar percentages of CD4 T cells compared to WM, TCN or MK-2206-treated animals (p=0.1).
- The number of FoxP3+ cells in CD4+ T cells was significantly reduced in the mice treated with the inhibitors compared to DMSO (p<0.05).
- Vaccination resulted in a significant increase in E7-reactive T cells (p=0.007). WM and TCN treatment significantly increased the number of E7-reactive cells compared to DMSO treated controls (p<0.01).
The in vitro dependence of Treg proliferation on the PI3K-Akt pathway coupled with the in vivo reduction in the actual numbers of these suppressive cells in response to PI3K and Akt inhibition led us to evaluate the effect of these inhibitors on Treg immune suppressive ability in vivo. Mice were conditioned on alternate days with WM, TCN or DMSO for a week prior to vaccination with HPV16 E7 peptide-based vaccine which was given s.c. on days 7 and 14. One week after the second vaccination, splenocytes were harvested and the E7-specific immune response was assayed by IFNγ ELISPOT after restimulation with E7 peptides. As expected, vaccination-induced E7-reactive T cells and importantly, addition of WM and TCN to vaccine treatment, significantly increased the number of E7-reactive T cells compared to those of the controls (Figure 3C).
Taken together, these data demonstrated that the PI3K-Akt pathway is necessary for Treg proliferation, FoxP3+CD4+ Treg maintenance in mice, and inhibition of this pathway resulted in an augmented T-cell response to peptide vaccination otherwise suppressed by the presence of Treg.
PI3K-Akt inhibition mitigates tumor growth
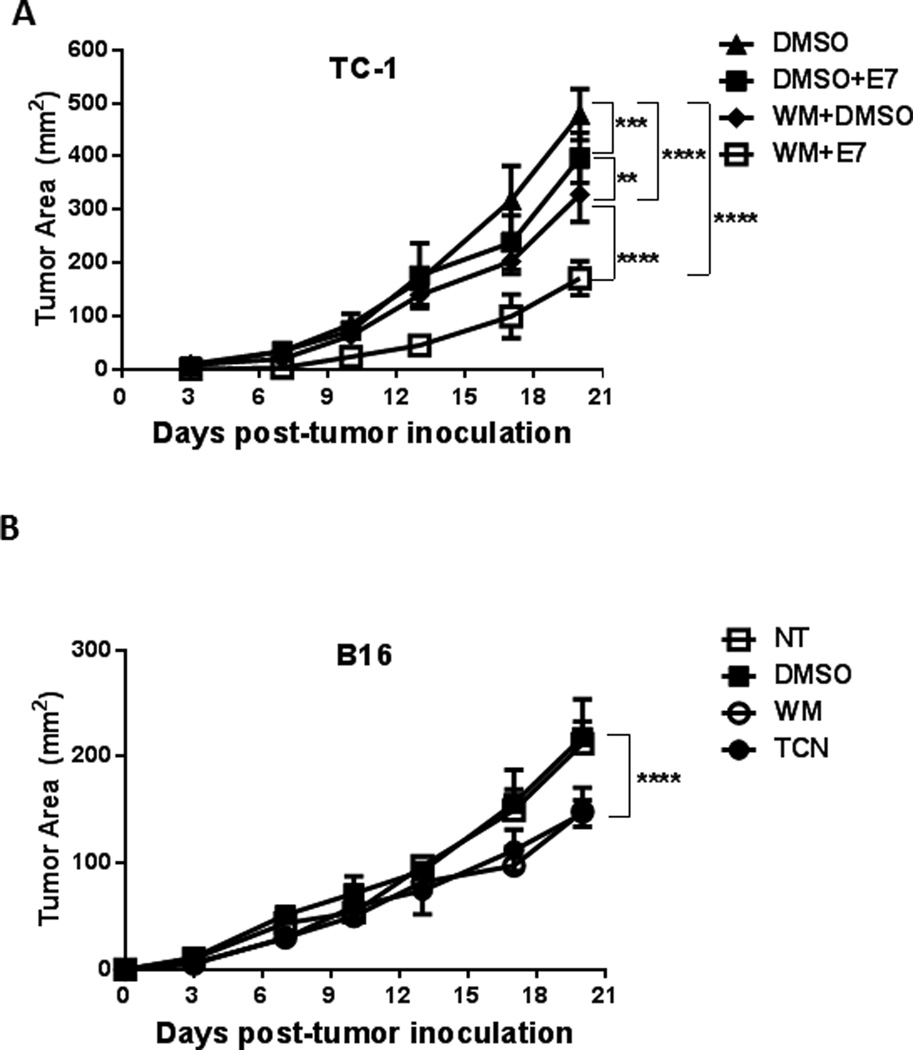
The depletion of Treg results in enhanced antitumor immune responses (23). Our data so far show that PI3K and Akt inhibition results in selective in vitro inhibition of Treg and enhanced immune response to peptide vaccination. We therefore tested the utility of targeting the PI3K-Akt pathway for tumor treatment. Mice implanted s.c. with TC-1 tumor cells were treated with WM or DMSO for one week after palpable tumors were detected. Mice were then vaccinated with the E7 vaccine as described above and tumor growth was monitored. Although both E7 vaccination and WM treatment significantly inhibited TC-1 tumor growth (p<0.05 and p<0.001 respectively), the greatest impairment was achieved with the combination of WM treatment and E7 vaccination (p<0.0001)(Figure 4A).
Figure 4.
- TC-1 tumor-bearing mice were treated with WM or DMSO for one week after palpable tumors were detected. Mice were then vaccinated with E7 peptide and tumor growth was monitored. Both E7 vaccination (p<0.05) and WM treatment (p<0.001) significantly inhibited TC-1 tumor growth, yet the greatest impairment was achieved with the combination of WM treatment and E7 vaccination (p<0.0001).
- Mice were prophylactically treated with WM, TCN or DMSO prior to s.c. B16 tumor inoculation and tumor growth was monitored. Tumor growth was significantly inhibited by WM and TCN compared to DMSO-treated animals (p<0.0001).
To evaluate the mechanism by which PI3K-Akt inhibition reduced tumor growth and to minimize the direct effect of PI3K-Akt inhibitors on the tumors, a prophylactic tumor model was employed using B16 and EL4 tumor cells. Mice were treated with WM, TCN or DMSO on days -7, -4 and -2 prior to s.c. tumor inoculation on day 0 and tumor growth was monitored thereafter. WM and TCN were chosen due to their short half-life (10 minutes and 6 hours, respectively) to ensure a minimal direct cytotoxic effect of the inhibitors on the tumor cells). Tumor growth was significantly inhibited by WM and TCN compared to DMSO in the B16 (p<0.0001) (Figure 4B) and EL4 (data not shown) tumor models.
These results demonstrate the effectiveness of PI3K and Akt inhibitors in suppressing tumor growth even without a tumor-specific vaccine and with minimal direct effect on the tumor cells.
Inhibition of the PI3K-Akt pathway enhances the antitumor effect of tumor-specific vaccines
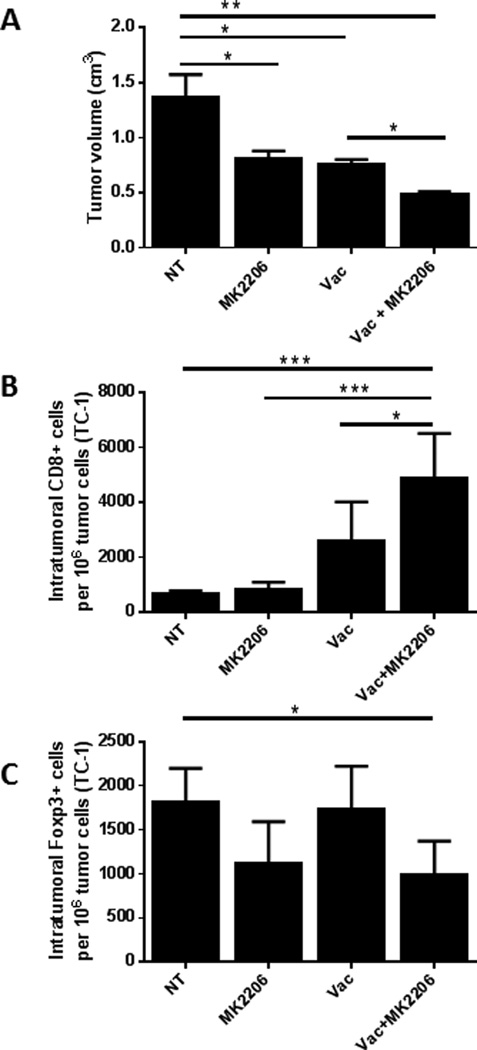
The above results clearly showed an enhanced antitumor effect of a vaccine when combined with PI3K-Akt pathway inhibitors. To further evaluate the effect of PI3K-Akt pathway inhibition on the TME, we tested the effect of combining the E7 vaccine with MK-2206 on tumor growth and immune-cell infiltration in the TC-1 mouse tumor model. MK-2206 is currently in several clinical trials and has a long half-life (60–90 hours), and was therefore chosen to be used to assess the effect of Akt inhibition on the TME. Following tumor implantation (day 0), appropriate groups of mice were injected with the E7 vaccine and/or MK-2206. Tumors were measured and the mice euthanized on day 21, and the intratumoral lymphocytic infiltrate was assessed. Both the vaccine and MK-2206 individually significantly reduced tumor sizes in comparison to the non-treated controls (p<0.05)(Figure 5A); however, when combined, they reduced the tumor volume more profoundly compared to that of non-treated controls (p<0.01) and of single vaccine treatment (p<0.05).
Figure 5.
Akt inhibition by MK-2206 enhances the anti-tumor therapeutic effect of tumor-specific vaccine.
- The combination of MK-2206 with the vaccine had a synergistic effect in the reduction of tumor volume in comparison to the vaccine alone (p<0.05) (combined data from two independent experiments).
- The combination of MK-2206 with the vaccine significantly increased the number of tumor infiltrating CD8+ T cells in comparison to the non-treated control (p<0.001) (representative example).
- The combination of MK-2206 with the vaccine significantly reduced the number of tumor infiltrating CD4+Foxp3+ Treg in comparison to the vaccine alone (p<0.05) (representative example).
The tumor tissues were then processed and the numbers of CD8+ and Foxp3+ T cells per million tumor cells within the tumors were evaluated. In tumors treated with the combination of MK-2206 with the vaccine, a significantly higher number of CD8+ T cells was detected in comparison to that of the non-treated group (p<0.001) (Figure 5B). The group treated with the combination also showed a significant reduction in Treg (Foxp3+)-infiltration in comparison to that of the non-treated group (p<0.05) and of the vaccine alone group (p<0.01) (Figure 5C).
These data further emphasized the enhancement of the therapeutic efficacy of tumor vaccines by the use of an Akt inhibitor and demonstrated an augmentation of the antitumor immune response when the vaccine was combined with the inhibitor.
PI3K-Akt inhibition reduces tumor growth in a Treg-dependent manner
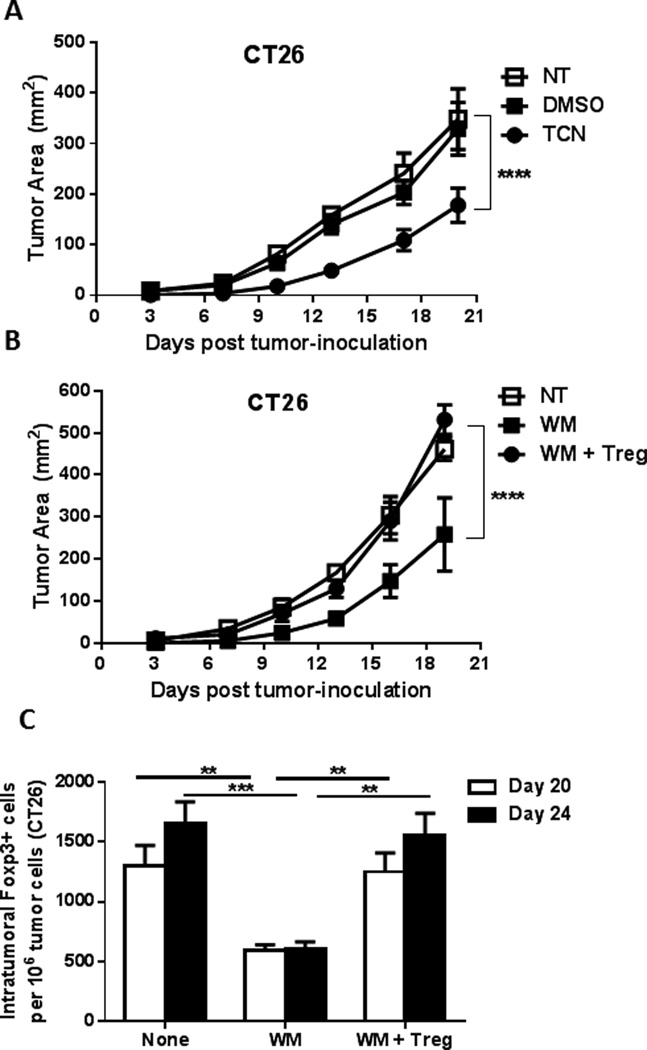
So far, we have shown that inhibiting the PI3K-Akt pathway results in the suppression of tumor growth, enhancement of the therapeutic efficacy of tumor-specific vaccine, and enhancement of the antitumor immune response (increase in intratumoral CD8+ cells and decrease in Treg). To confirm the role of Treg-depletion by PI3K-Akt inhibitors in their antitumor therapeutic effect, the CT26 tumor model was used. Similar to what was observed with the B16 and EL4 tumor models, prophylactic treatment of mice with WM or TCN prior to subcutaneous tumor inoculation resulted in a significant tumor growth inhibition by TCN (p<0.0001) (Figure 6A) and WM (p<0.0001)(Figure 6B) compared to that in DMSO-treated animals. However, this effect was reversed when the WM-treated mice were reconstituted with Treg. Ex-vivo cultured Treg infusion on day 4 resulted in the restoration of tumor growth comparable to that in DMSO-treated mice (Figure 6B). The same effect was observed with the EL4 tumor model (data not shown).
Figure 6.
PI3K-Akt inhibition reduces tumor growth in a Treg-dependent manner.
- Prophylactic treatment of the mice with TCN for one week prior to subcutaneous tumor inoculation resulted in a significant tumor growth inhibition (p<0.0001).
- Prophylactic treatment of the mice with WM for one week prior to s.c. tumor inoculation resulted in a significant tumor growth inhibition WM (p<0.0001). This effect was reversed when Treg were reconstituted by ex-vivo cultured Treg infusion on day 4
- Analysis of FoxP3+ T-cell infiltration into CT26 tumor (days 20 and 24) elucidated a significant decrease in Treg numbers per million tumor cells after WM treatment (p<0.01), which was restored after infusion of ex vivo grown Treg.
Analysis of FoxP3+ T-cell infiltration into CT26 tumors elucidated a significant decrease in Treg numbers after WM treatment that was restored after infusion of ex vivo grown Treg four days after tumor inoculation (Figure 6C).
Taken together, these data demonstrated that the Treg dependence on PI3K-Akt signaling can be exploited to selectively deplete suppressive CD4 Treg, resulting in an enhanced capacity to elicit an antitumor immune response when combined with vaccine.
Discussion
CD4 T cells differentiate into a panoply of effector cells with an array of diverse functions in the immune response. Preclinical mouse models have identified inflammatory CD4 T cells that mediate tumor regression and regulatory CD4 T cells that support tumor growth (18).
A correlation of tumor-infiltrating Treg with poor clinical prognosis has been demonstrated (19–22), and the depletion of Treg was found to enhance antitumor immunity and promote tumor regression (23–26). However, there is still a scarceness of efficient and highly selective Treg-depleting clinical reagents for use in tumor immunomodulation.
Several reports have shown that Treg and Tconv display unique signaling signatures downstream of TCR (28–31). The PI3K-Akt signaling pathway is important in the cellular response to TCR stimulation and co-stimulation (32, 33). This pathway plays a critical role in T-cell functions including proliferation, survival, migration, and metabolism (35, 36).
Due to the important role of the PI3K-Akt pathway in T-cell function (35, 36) and the reported differences between Treg and Tconv downstream of TCR (28–31), we investigated the impact of Akt and PI3K inhibition on Treg and Tconv to discern any selective effect that inhibition of the PI3K-Akt pathway might have on these CD4 T-cell subsets.
We demonstrated that several molecular inhibitors that target PI3K and its downstream effector, Akt, selectively inhibit the in vitro proliferation of human and murine Treg when compared to Tconv. This selective decrease in Treg proliferation provided us with a potential strategy to modulate the Treg/Tconv balance in vivo. We found that Akt and PI3K inhibition selectively decreased the number of Treg in vivo both in naïve and tumor-bearing mice. We found that this translated into a therapeutic effect in which the in vivo treatment of tumor-bearing mice with PI3K-Akt pathway inhibitors displayed a significant antitumor therapeutic efficacy. The inhibition of tumor growth was more profound when Akt and PI3K inhibitors were combined with an antitumor vaccine. Analysis of the tumor microenvironment revealed an enhanced antitumor immune response to the vaccine when combined with PI3K-Akt pathway inhibitors. On their own or in combination with vaccines, the antitumor therapeutic effects of Akt and PI3K inhibitors were found to be Treg-dependent as they could be reversed by Treg reconstitution.
It is interesting to point out the apparent difference between the inhibition of Akt and PI3K compared to the downstream inhibition of mTOR. mTOR inhibition with rapamycin has been shown to exhibit the opposite effect as it supports the proliferation and survival of Treg (data not shown and (40–43)) and is therefore used as an immunosuppressant. The opposing effects of PI3K-Akt versus mTOR inhibition on Treg may be explained by a feedback loop in which mTOR Inhibition results in PI3K-dependent Akt activation, and which, in turn, sustains signaling through mTOR (44).
It is also important to highlight the opposite role that the PI3K-Akt pathway plays in de novo differentiation of mouse Treg. Active Akt signaling has been shown to be a potent suppressor of differentiation of human (45) and mouse CD4 Treg (46).
Here, we report that PI3K-Akt inhibitors reduced tumor growth in several mouse models, and we have shown that this is due to the selective inhibition of Treg. However, the antitumor effect due to the inhibition of PI3K-Akt can be exhibited through other mechanisms such as the direct inhibition of tumor growth and the enhanced survival of CD8 T cells (Akt signaling drives CD8 T-cell differentiation and limits survival and memory formation (47)). To reduce the direct effect of the inhibitors on tumor cells, mice were treated with the inhibitors prior to their inoculation with tumor cells, and the inhibitors WM and TCN were chosen for in vivo treatment due to their short half-life. To further demonstrate the role of selective inhibition of Treg as opposed to enhanced CD8 effector function, mice were infused with Treg, which restored tumor growth to a rate identical to that of the control animals. Together with the selective inhibition of in vitro proliferation in Treg, these data strongly implicate the disruption of Treg homeostasis as a mechanism of PI3K-Akt inhibitor-mediated antitumor function.
In summary, clinical modulation of the tumor immune response has been limited by a lack of highly selective reagents that specifically target Treg. Here we demonstrate that selective inhibition of human and murine Treg proliferation could be achieved using PI3K-Akt molecular inhibitors. PI3K and Akt inhibitors selectively disrupt the homeostasis of Treg and result in a significant antitumor effect that is Treg-dependent. Recent clinical studies have correlated immune dysfunction after immunotherapy with an increase of Treg (48–50). The data presented in this report demonstrated that PI3K-Akt inhibition enhances antitumor immune responses when combined with vaccines by selectively reducing the number of Treg. The selective Treg effect and enhanced therapeutic potential suggests that PI3K-Akt inhibitors could be exploited in the clinic as immune modulators in cancer therapy.
References
- 1.Rosenberg SA. Development of effective immunotherapy for the treatment of patients with cancer. J Am Coll Surg. 2004;198:685–696. doi: 10.1016/j.jamcollsurg.2004.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Kochenderfer JN. Personalized cell transfer immunotherapy for B-cell malignancies and solid cancers. Mol Ther. 2011;19:1928–1930. doi: 10.1038/mt.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 20.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 21.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 22.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Tao H, Zhen Z, Chen H, Chen G, Yang Y. Depletion of CD4+ CD25+ regulatory T cells promotes CCL21-mediated antitumor immunity. PLoS One. 2013;8:e73952. doi: 10.1371/journal.pone.0073952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Jarry U, Donnou S, Vincent M, Jeannin P, Pineau L, Fremaux I, et al. Treg depletion followed by intracerebral CpG-ODN injection induce brain tumor rejection. J Neuroimmunol. 2014;267:35–42. doi: 10.1016/j.jneuroim.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, et al. A Listeria Vaccine and Depletion of T-Regulatory Cells Activate Immunity Against Early Stage Pancreatic Intraepithelial Neoplasms and Prolong Survival of Mice. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reginato E, Mroz P, Chung H, Kawakubo M, Wolf P, Hamblin MR. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. Br J Cancer. 2013;109:2167–2174. doi: 10.1038/bjc.2013.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vries IJ, Castelli C, Huygens C, Jacobs JF, Stockis J, Schuler-Thurner B, et al. Frequency of circulating Tregs with demethylated FOXP3 intron 1 in melanoma patients receiving tumor vaccines and potentially Treg-depleting agents. Clin Cancer Res. 2011;17:841–848. doi: 10.1158/1078-0432.CCR-10-2227. [DOI] [PubMed] [Google Scholar]
- 28.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crellin NK, Garcia RV, Levings MK. Flow cytometry-based methods for studying signaling in human CD4+CD25+FOXP3+ T regulatory cells. J Immunol Methods. 2007;324:92–104. doi: 10.1016/j.jim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 31.Carson BD, Ziegler SF. Impaired T cell receptor signaling in Foxp3+ CD4 T cells. Ann N Y Acad Sci. 2007;1103:167–178. doi: 10.1196/annals.1394.022. [DOI] [PubMed] [Google Scholar]
- 32.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5:239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantrell DA. T-cell antigen receptor signal transduction. Immunology. 2002;105:369–374. doi: 10.1046/j.1365-2567.2002.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 36.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simova J, Bubenik J, Bieblova J, Rosalia RA, Fric J, Reinis M. Depletion of T(reg) cells inhibits minimal residual disease after surgery of HPV16-associated tumours. Int J Oncol. 2006;29:1567–1571. [PubMed] [Google Scholar]
- 38.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Research. 1996;56:21–26. [PubMed] [Google Scholar]
- 39.Ahlers JD, Belyakov IM, Terabe M, Koka R, Donaldson DD, Thomas EK, et al. A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L. Proc Natl Acad Sci U S A. 2002;99:13020–13025. doi: 10.1073/pnas.192251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long SA, Buckner JH. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun. 2008;30:293–302. doi: 10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 42.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 43.Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18:2006–2017. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saitoh A, Narita M, Watanabe N, Tochiki N, Yamahira A, Nakamura T, et al. WT1 peptide vaccination in a CML patient: induction of effective cytotoxic T lymphocytes and significance of peptide administration interval. Med Oncol. 2011;28:219–230. doi: 10.1007/s12032-010-9425-3. [DOI] [PubMed] [Google Scholar]
- 49.Macatangay BJ, Szajnik ME, Whiteside TL, Riddler SA, Rinaldo CR. Regulatory T cell suppression of Gag-specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV-1-infected patients on ART. PLoS One. 2010;5:e9852. doi: 10.1371/journal.pone.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, et al. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS One. 2012;7:e32614. doi: 10.1371/journal.pone.0032614. [DOI] [PMC free article] [PubMed] [Google Scholar]