Abstract
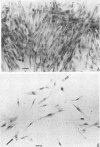
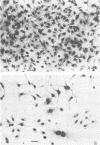
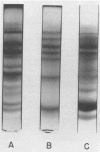
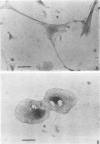
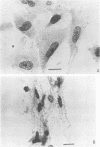
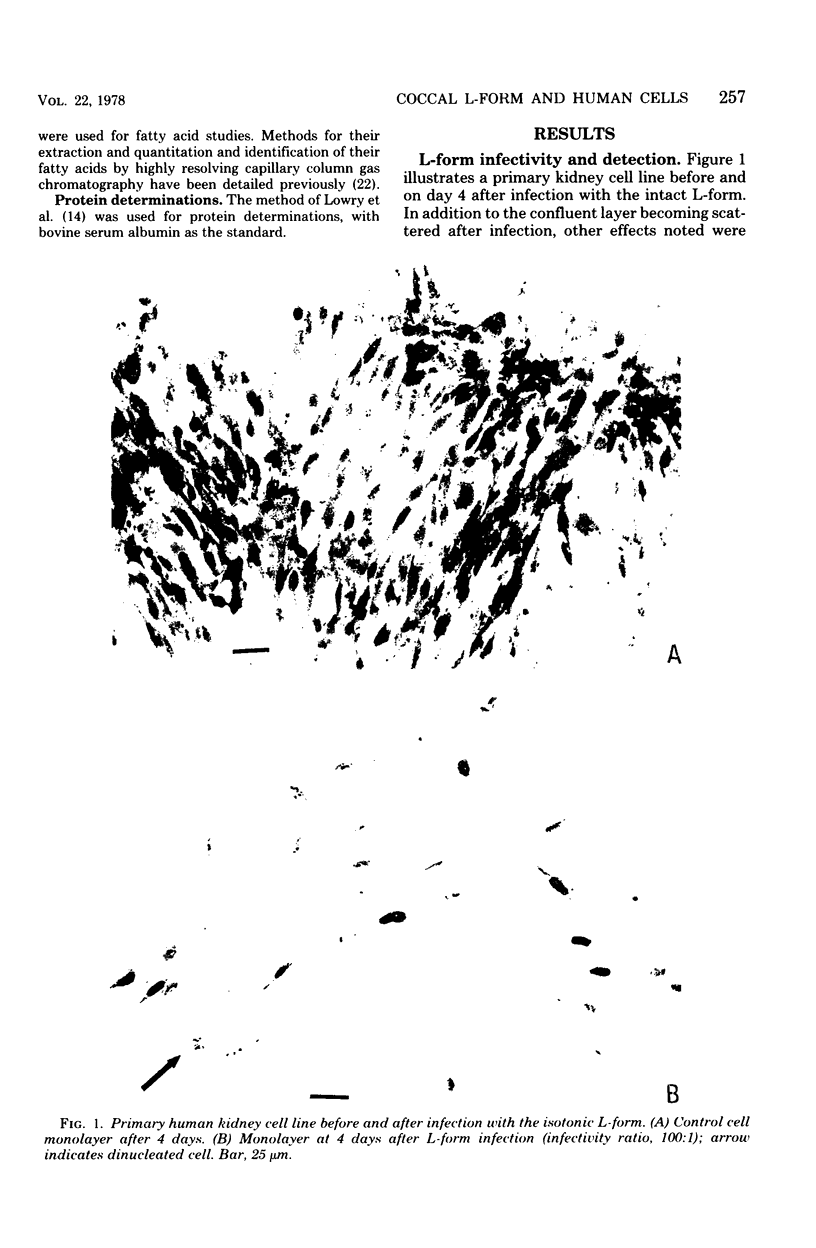
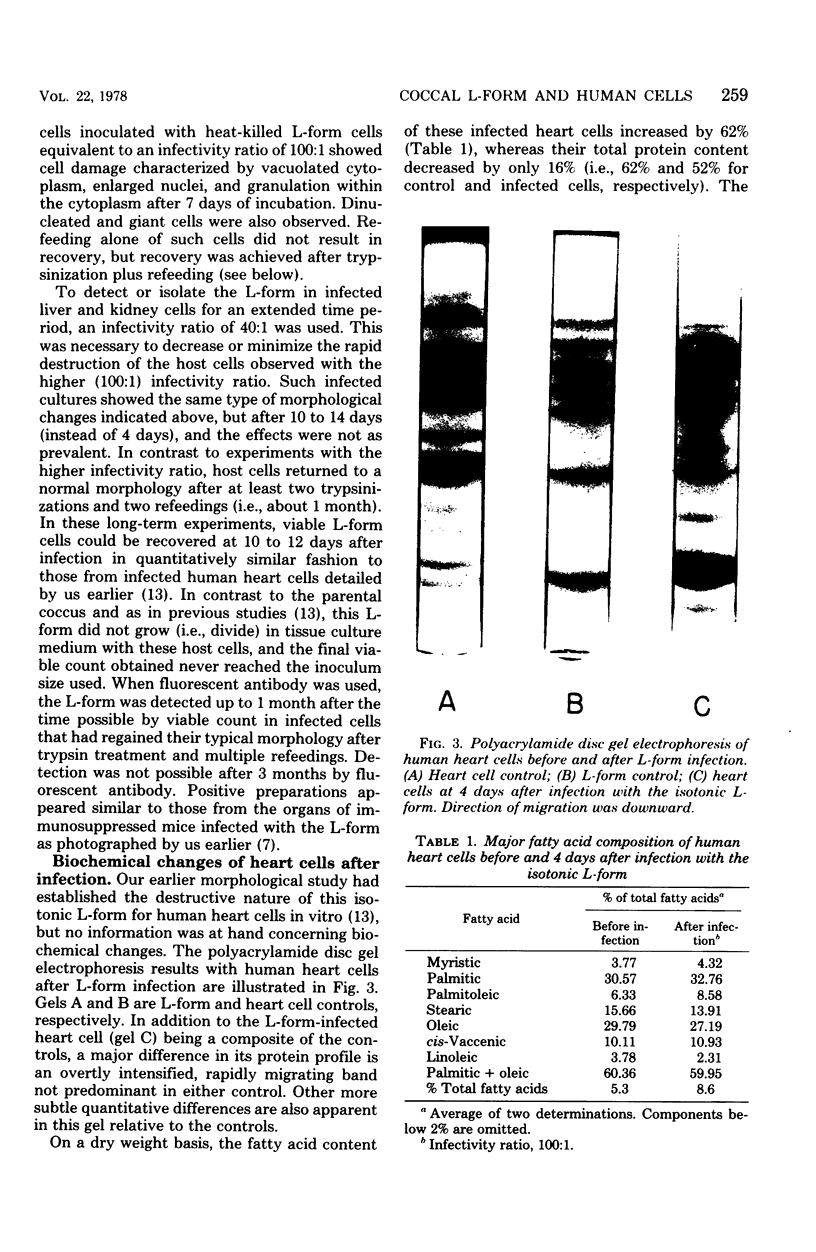
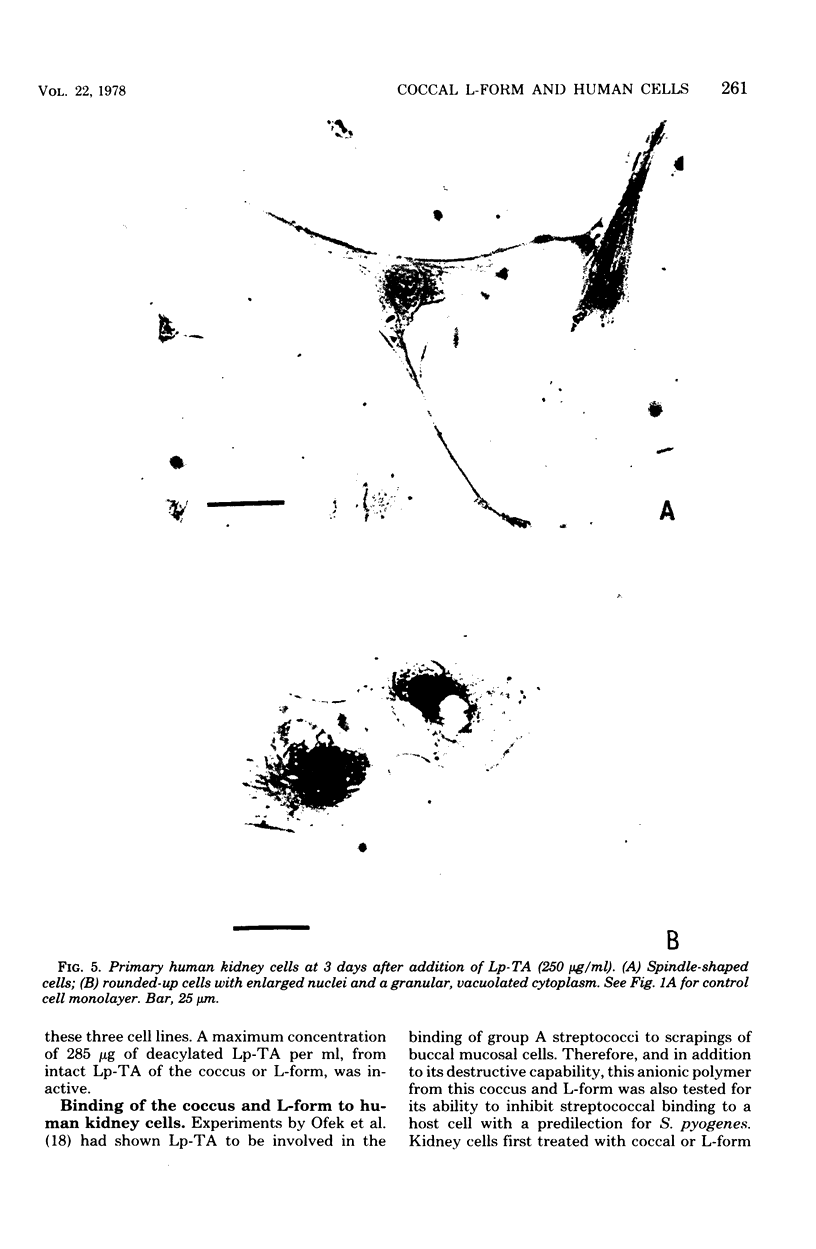
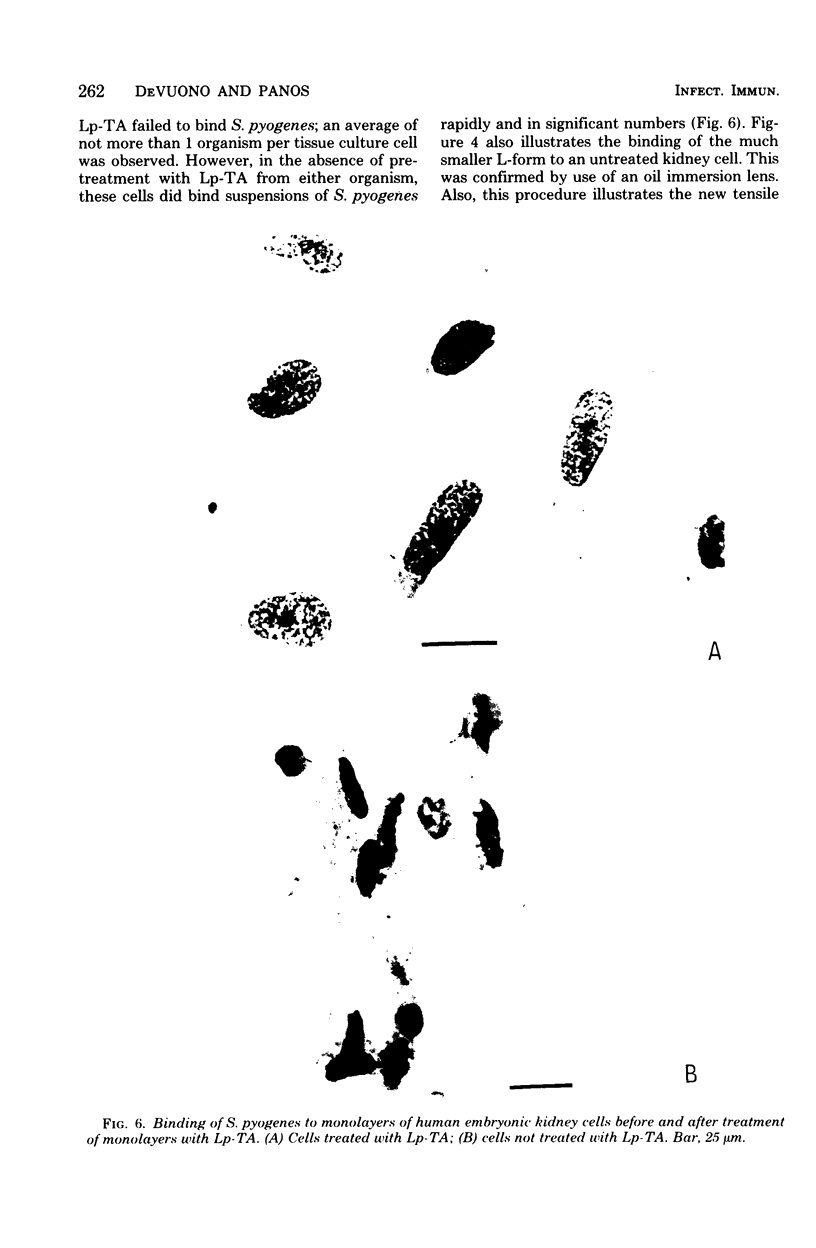
These studies showed the destruction of growing primary and established human cell lines with a predilection for the group A streptococci by an L-form of Streptococcus pyogenes adapted to grow in isotonic media. Also, this L-form was detected by fluorescent antibody for longer periods of time than by viable count in infected but recovered tissue culture monolayers. Additional studies with human heart cells showed changes in their protein profile and fatty acid content (but not composition) after L-form infection. This report is the first to show that the morphological changes and death of human kidney cells by this viable L-form were mimicked by the structurally different lipoteichoic acids from this organism and its parental streptococcus. These lipoteichoic acids were also equally effective in preventing attachment of S. pyogenes to human cell monolayers, but their deacylation obviated these two activities. Finally, the attachment of the isotonic L-form, as well as the parental streptococcus, to growing human kidney cells suggested that a rigid cell wall is not a prerequisite for host attachment in vitro.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly R., Shinefield H. I., Strauss W. G., Maibach H. I. Bacterial adherence to nasal mucosal cells. Infect Immun. 1977 Sep;17(3):546–549. doi: 10.1128/iai.17.3.546-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Chiang T. M., Ofek I., Kang A. H. Interaction of lipoteichoic acid of group A streptococci with human platelets. Infect Immun. 1977 May;16(2):649–654. doi: 10.1128/iai.16.2.649-654.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Panos C. Persistence, pathogenesis, and morphology of an L-form of Streptococcus pyogenes adapted to physiological isotonic conditions when in immunosuppressed mice. Infect Immun. 1976 Nov;14(5):1228–1240. doi: 10.1128/iai.14.5.1228-1240.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. Phosphatidylkojibiosyl diglyceride. The covalently linked lipid constituent of the membrane lipoteichoic acid from Streptococcus faecalis (faecium) ATCC 9790. J Biol Chem. 1975 Jan 25;250(2):702–709. [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann E., Lüderitz O., Knox K., Weinfeld N. Structural requirements for bone resorption by endotoxin and lipoteichoic acid. J Dent Res. 1975 Jun;54(SPEC):B94–B99. doi: 10.1177/00220345750540023401. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leon O., Panos C. Adaptation of an osmotically fragile L-form of Streptococcus pyogenes to physiological osmotic conditions and its ability to destroy human heart cells in tissue culture. Infect Immun. 1976 Jan;13(1):252–262. doi: 10.1128/iai.13.1.252-262.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecki N., Becker F., Baca O. G., Paretsky D. Changes in liver and L-cell plasma membranes during infection with Coxiella burnetii. Infect Immun. 1978 Jan;19(1):272–280. doi: 10.1128/iai.19.1.272-280.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eyal F., Morrison J. C. Postnatal development of binding of streptococci and lipoteichoic acid by oral mucosal cells of humans. J Infect Dis. 1977 Feb;135(2):267–274. doi: 10.1093/infdis/135.2.267. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Panos C., Fagan G., Zarkadas C. G. Comparative electrophoretic and amino acid analyses of isolated membranes from Streptococcus pyogenes and stabilized L-form. J Bacteriol. 1972 Oct;112(1):285–290. doi: 10.1128/jb.112.1.285-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos C., Rottem S. Incorporation and elongation of fatty acid isomers by Mycoplasma laidlawii A. Biochemistry. 1970 Jan 20;9(2):407–412. doi: 10.1021/bi00804a030. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Membrane lipoteichoic acid of Streptococcus pyogenes and its stabilized L-form and the effect of two antibiotics upon its cellular content. J Bacteriol. 1976 Aug;127(2):855–862. doi: 10.1128/jb.127.2.855-862.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltersdorff R. L., Fiedel B. A., Jackson R. W. Induction of nephrocalcinosis in rabbit kidneys after long-term exposure to a streptococcal teichoic acid. Infect Immun. 1977 Sep;17(3):665–667. doi: 10.1128/iai.17.3.665-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]