Abstract
Background
Diaphragmatic resection (DR) during CRS/ HIPEC exposes the thoracic cavity to direct contamination from the peritoneal cavity. The effect of thoracic chemoperfusion in combination with HIPEC in these patients is unknown.
Methods
A prospective database of 1,077 procedures was analyzed. Type of malignancy, thoracic perfusion, resection status, comorbidities, morbidity, mortality, and overall survival were reviewed.
Results
DR was a component of 102 CRS/HIPEC procedures performed for 57 (55.9 %) appendiceal and 22 (21.6 %) colon primary lesions. DR was associated with higher volume of disease as evidenced by more organ resections (3.7 vs. 2.8, p < 0.001) and increased rates of incomplete cytoreduction (67 vs. 52 %, p = 0.004). Patients with and without DR had similar 30-day major morbidity (23.5 vs. 16.8 %, p = 0.1) and worse 90-day mortality (12.8 % vs. 6.12 %, p = 0.03), respectively. Multivariate analysis showed DR (p = 0.01) and diabetes (p = 0.005) to be associated with worse mortality. Nineteen (20 %) DR patients underwent synchronous abdominal and thoracic chemoperfusion. Intrathoracic recurrence following DR with thoracic perfusion was 17 % (3/18) vs. 2.3 % (2/85) without perfusion (p = 0.04). Median survival following complete cytoreduction was similar for patients with lowgrade appendiceal (LGA) (not reached with DR and 175 months without DR, p = 0.17) and colorectal cancer (23 months with and 31 months without DR, p = 0.76).
Conclusions
Diaphragmatic resection during CRS/HIPEC is an independent predictor of surgical mortality. Intrapleural perfusion was associated with more thoracic recurrence; however, complete cytoreduction with or without DR can achieve similar survival for patients with LGA and colorectal primary lesions. DR should be performed only if careful inspection deems all peritoneal disease resectable.
Cytoreductive surgery with heated intraperitoneal chemotherapy (CRS/HIPEC) has been associated with improved survival in peritoneal surface disease (PSD) from a variety of epithelial malignancies.1–4 These patients frequently demonstrate tumor deposits on the peritoneum overlying the diaphragm. As complete cytoreduction represents a major determinant of survival in patients with peritoneal carcinomatosis, attempts to achieve a complete cytoreduction often involve full-thickness diaphragmatic resection (DR). The implication of this in terms of tumor spread to the pleural cavity and survival is unknown. It is logical to assume that full-thickness diaphragmatic involvement increases the risk of dissemination of malignant cells into the pleural cavity. Additionally, DR may result in iatrogenic contamination of the pleural cavity during cytoreduction. Many PSD centers have adopted simultaneous intraperitoneal and intrapleural chemoperfusion following full-thickness DR in an effort to minimize the risk of intrapleural recurrence.5 Even though intrapleural chemotherapy has a defined role for thoracic mesothelioma as well as refractory malignant pleural effusion, there are no standardized guidelines regarding the use of heated intrapleural chemoperfusion after DR for peritoneal carcinomatosis.6,7
The primary purpose of our study was to assess the impact of DR on the morbidity and mortality of CRS/HIPEC procedures. The secondary goal was to evaluate the impact of DR on survival and thoracic recurrence rates of patients with PSD arising from low-grade appendiceal (LGA) and colon cancers.
Methods
The current study represents a retrospective analysis of a prospectively maintained database of 1,077 CRS/HIPEC procedures performed between 1992 and 2013. Institutional review board approval was obtained. Data relevant to the study included type of primary cancer, age, Eastern Cooperative Oncology Group (ECOG) performance status, comorbidities, extent of cytoreduction, number of organs resected, delivery of thoracic chemoperfusion, morbidity, mortality, and thoracic recurrence. Surgical morbidity and mortality was classified according to Clavien-Dindo classification and considered minor if grades I–II and major if grades III–IV.8
All patients had a complete history and physical examination, tumor markers, and CT of the chest, abdomen, and pelvis before CRS/HIPEC. CRS/HIPEC was performed with the closed technique as previously described by our group.9 DR was performed when tumor involvement was either full thickness through the diaphragm or when peritonectomy of the diaphragmatic peritoneum resulted in a full-thickness diaphragmatic defect. Patients whose disease was successfully stripped from the diaphragmatic peritoneum without creating a fullthickness defect were not included in the study. Thoracic chemoperfusion was performed based on surgeon's discretion and the presence of disease on the diaphragmatic pleura. When performed, a separate cannula was inserted through the diaphragmatic defect and placed in continuity with the remainder of the perfusion circuit. The perfusate volume was adjusted to maintain flow of at least a liter per minute. The chemotherapeutic agent was selected based on type of primary tumor as well as patient's previous response to chemotherapy. Mitomycin C concentration was adjusted by adding an additional 10 mg per additional liter of perfusate. The diaphragmatic defect was closed primarily with a nonabsorbable suture in all but one patient who had diaphragmatic reconstruction with biologic mesh. Resultant pneumothorax was addressed with a tube thoracostomy.
R0 and R1 resections were grouped together as complete cytoreductions. Cytoreductions with residual macroscopic disease were characterized as R2 and subdivided based on the size of residual disease (R2a ≤ 5 mm, R2b ≤ 2 cm, R2c > 2 cm). Recurrence was characterized only in cases where a complete macroscopic cytoreduction was achieved and not for cases with incomplete resections. Recurrence was identified with post CRS/HIPEC surveillance CT imaging at 3-month intervals.
Descriptive statistics included frequency and percentage for categorical variables and mean and standard deviation for continuous variables. Fisher's exact tests and Wilcoxon rank-sum tests were used to compare categorical and continuous variables, respectively. To assess the relationship between independent measures and mortality, binary models, using a generalized estimating equation (GEE), were fit using a binomial distribution and logit link function. As some subjects had more than one procedure, it was necessary to use a technique that adjusts for the correlation within subjects who have multiple procedures.
Overall survival (OS) was estimated using the Kaplan– Meier (product-limit) method from the date of CRS/HIPEC (or first CRS/HIPEC in cases where a patient underwent more than one procedure) to the date of death or last follow-up. The log-rank test compared survival between groups. Statistical significance was defined as a p value < 0.05. All analyses were performed using SAS 9.3, Cary, NC.
Results
A total of 102 patients underwent DR as a component of CRS/HIPEC and 18 (17.6 %) of these had simultaneous thoracic chemoperfusion. Median follow-up was 52 months. Median age for patients undergoing diaphragmatic resection was 51.5 years. Of the 102 CRS/HIPEC cases with synchronous DR, 57 (55.9 %) were performed for appendiceal primary lesions, 22 (21.6 %) for colon cancer, and 12 (11.8 %) for mesothelioma. Demographic characteristics of patients with and without DR are included in Table 1.
Table 1. Patient demographics and procedure characteristics for patients undergoing CRS/HIPEC with and without diaphragm resection.
| Characteristic | Diaphragm resection | No diaphragm resection | p |
|---|---|---|---|
| Age, mean (range), year | 51.5 | 52.7 | 0.27 |
| ECOG performancestatus, n (%) (n = 1,058) |
0.88 | ||
| 0/1 | 86 (85.1 %) | 804 (84 %) | |
| 2 | 15 (14.9 %) | 153 (16 %) | |
| Primary, n (%) (n = 1,077) |
0.01 | ||
| Appendiceal | 57 (55.9%) | 461 (47.3%) | |
| Colon | 22 (21.6%) | 212 (21.7%) | |
| Mesothelioma | 12 (11.8%) | 72 (7.4%) | |
| Ovarian | 1 (1%) | 72 (7.4%) | |
| Unknown | 10 (9.8) | 158 (16.2) | |
| Pre-HIPEC chemotherapy (n = 980), (%) |
47 (51.7) | 483 (54.3) | 0.66 |
| Total # organs resected, mean (range) | 3.7 | 2.8 | <0.001 |
| Resection status, n (%) (n = 1,069) |
0.004 | ||
| R0/1 | 33 (33 %) | 465 (48 %) | |
| R2+ | 67 (67 %) | 504 (52 %) | |
Bold values denote statistical significance
Extent of Cytoreduction
In the DR cohort, 47 (51.7 %) patients had preoperative chemotherapy compared with 483 (54.3 %) patients in the non-DR cohort (p = 0.66). A greater tumor burden and more extensive cytoreduction was observed in the group of patients undergoing a DR as indicated by the number of organs resected (3.7 vs. 2.8; p < 0.001). Possibly due to greater disease burden, incomplete cytoreduction was more frequently observed in this cohort (67 % with DR vs. 52 % without DR; p = 0.004).
Diaphragmatic Resection and Surgical Outcomes
Surgical outcomes were compared between patients undergoing CRS/HIPEC with and without DR. When all procedures were compared, there was no difference in the length of operation (8.6 h with DR vs. 8.5 h without DR; p = 0.24). However, procedures with a DR were associated with longer ICU stay (5.6 vs. 3 days; p = 0.14) and significantly longer hospital stay (17.3 vs. 13.8 days; p = 0.01) compared with procedures that did not include a DR.
There were no statistically significant differences in 30-day minor (Clavien-Dindo grade I–II morbidity; 20.6 % with DR vs. 21.2 % without DR; p = 1.0) and major (Clavien-Dindo grade III-IV morbidity; 23.5 % with DR vs. 16.8 % without DR; p = 0.1) morbidity rates between patients with and without DR. Similarly, no significant difference was observed in the 30-day readmission rates for both groups (Table 2). Although the overall morbidity was similar in the two cohorts, the 30-day complication pattern differed between the two groups (Table 3). More specifically, patients undergoing diaphragmatic resection were more likely to develop pulmonary effusion requiring thoracostomy tubes (8.82 vs. 2.56 %; p = 0.003) and respiratory failure requiring prolonged intubation (10.8 vs. 4.9 %; p = 0.02). In addition, the incidence of myocardial infarction (1.96 vs. 0.21 %; p = 0.05), wound dehiscence (5.88 vs. 2.46 %; p = 0.05), and blood transfusion (23.5 vs. 13.6 %; p = 0.01) was higher in the DR group.
Table 2. Morbidity and mortality following CRS/HIPEC with diaphragm resection.
| Diaphragm resection (n = 102) |
No diaphragm resection (n = 975) |
p | ||
|---|---|---|---|---|
| Minor | 30 day | 21 (20.6 %) | 207 (21.2 %) | 1.0 |
| morbidity,n (%) | 31–90 day | 0 | 13 (1.33 %) | 0.62 |
| Major | 30 day | 24 (23.5 %) | 164 (16.8 %) | 0.1 |
| morbidity,n (%) | 31–90 day | 1 (0.98 %) | 11 (1.13 %) | 1.0 |
| Morality, n (%) | Overall | 12 (12.8 %) | 56 (6.12 %) | 0.03 |
| 30 day | 5 (5.75 %) | 20 (2.28 %) | 0.07 | |
| 31–90 day | 3 (3.5 %) | 15 (1.7 %) | 0.2 | |
| Readmission, n (%) | 30 day | 10 (10.9 %) | 137 (15.2 %) | 0.35 |
| 31–90 day | 11 (12.1 %) | 94 (10.5 %) | 0.6 | |
| Mean operation time (hours) | 8.6 | 8.5 | 0.24 | |
| Median hospitalization(days) | 17.3 | 13.8 | 0.01 | |
| Median ICU stay (days) | 5.6 | 3.0 | 0.14 | |
Bold values denote statistical significance
Table 3. 30-day complication pattern following CRS/HIPEC.
| Complication | Non-DR(n = 975) | DR(n = 102) | p |
|---|---|---|---|
| Reexploration (all cause), n (%) | 71 (7.3 %) | 9 (8.8 %) | 0.55 |
| IR-guided drain placement, n (%) | 57 (5.85 %) | 11 (10.8 %) | 0.08 |
| Respiratory failure requiring mechanical ventilation, n (%) | 48 (4.92 %) | 11 (10.78 %) | 0.02 |
| Pneumonia, n (%) | 51 (5.23 %) | 9 (8.82 %) | 0.17 |
| Pulmonary effusion requiring thoracentesis, n (%) | 15 (1.54 %) | 1 (0.98 %) | 1.0 |
| Pulmonary effusion requiring thoracostomy, n (%) | 25 (2.56 %) | 9 (8.82 %) | 0.003 |
| Pneumothorax, n (%) | 11 (1.13 %) | 2 (1.96 %) | 0.35 |
| Deep vein thrombosis, n (%) | 17 (1.74 %) | 4 (3.92 %) | 0.13 |
| Pulmonary embolus, n (%) | 9 (0.92 %) | 0 | 1.0 |
| Myocardial infarction, n (%) | 2 (0.21 %) | 2 (1.96 %) | 0.05 |
| Enteric leak, managed nonoperatively, n (%) | 8 (0.82 %) | 1 (0.98 %) | 0.59 |
| Enterocutaneous fistula, n (%) | 12 (1.23 %) | 2 (1.96 %) | 0.63 |
| Abscess, treated with antibiotics, n (%) | 27 (2.77 %) | 6 (5.88 %) | 0.12 |
| Acute renal failure requiring dialysis, n (%) | 15 (1.54 %) | 4 (3.92 %) | 0.1 |
| Anemia requiring transfusion, (%) | 133 (13.6 %) | 24 (23.5 %) | 0.01 |
| Pericardial effusion requiring thoracotomy, n (%) | 1 (0.1 %) | 0 | 1.0 |
| Bacterial peritonitis, n (%) | 8 (0.82 %) | 2 (1.96 %) | 0.24 |
Bold values denote statistical significance
The 90-day mortality rate was significantly higher in the cohort of patients undergoing DR (12.8 vs. 6.12 %; p = 0.03). To delineate if the increased mortality was related to the extent of CRS or the diaphragmatic resection itself, univariate and multivariate analyses were performed comparing the DR and non-DR groups (Table 4). On univariate analysis, number of comorbidities (p = 0.005), diabetes (p = 0.01), nutritional status (p = 0.02), and R status of resection (p = 0.02) predicted mortality. On multivariate analysis, however, only diaphragmatic resection [p = 0.03, hazard ratio (HR) 3.1, 95 % confidence interval (CI) 1.1– 8.6], and diabetes (p = 0.006, HR 3.9, 95 % CI 1.5–10.4) were independent predictors of mortality.
Table 4. Univariate/multivariate analysis to evaluate the higher mortality associated with diaphragmatic resection.
| Characteristic | Univariate p | Multivariate p |
|---|---|---|
| Diaphragm resection | 0.07 | 0.01 |
| Type of primary (appendiceal/colon) | <0.001 | |
| Diabetes | 0.01 | 0.005 |
| Heart disease | 0.42 | |
| Lung disease | 0.57 | |
| Smoking | 0.08 | |
| ECOG | 0.08 | |
| R status | 0.02 | |
| Albumin | 0.02 | |
| Total organs | 0.53 | |
| Number of comorbidities | 0.005 |
Bold values denote statistical significance
Thoracic Chemoperfusion
Of the 103 patients undergoing DR, 18 had synchronous intraperitoneal and intrapleural chemoperfusion. Failure in the thorax in patients with DR followed by thoracic perfusion was 17 % (3/18) versus 2.3 % (2/85) in patients with DR and no thoracic perfusion (p = 0.04). Following intrapleural chemoperfusion, thoracic recurrence was observed in two colon cancers with recurrence at 6 and 7 months, respectively and a mesothelioma patient with recurrence at a year, whereas thoracic recurrence following DR without thoracic chemoperfusion was observed in lowgrade appendiceal tumor patients 3 years after the operation and high-grade appendiceal primary within 4 months from the time of operation. All patients with thoracic recurrence after pleural chemoperfusion had no evidence of pleural disease at the time of diaphragmatic resection.
Amongst the DR cohort, there was no significant difference in age, ECOG status, and preoperative chemotherapy status between patients who underwent chest chemoperfusion versus those who did not. However, thoracic chemoperfusion patients more frequently obtained an R2 cytoreduction (p = 0.04). Furthermore, the DR subgroup that did receive thoracic chemoperfusion consisted of a larger proportion of patients with colon cancer (31.6 vs. 21.4 %) and mesothelioma (31.6 vs. 8.6 %) and a smaller proportion of patients with appendiceal primary lesions (36.8 vs. 68.6 %; p = 0.02).
Diaphragmatic Resection and Survival
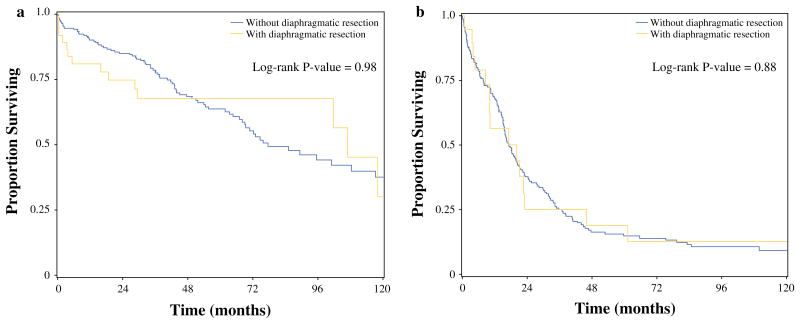
There were no statistically significant differences in median OS for patients following CRS/HIPEC with and without diaphragmatic resection for LGA cancer (106.9 vs. 77.3 months, p = 0.98) or colon cancer (17.0 vs. 17.3 months, p = 0.88), respectively (Fig. 1). When looking exclusively at those patients who received a complete cytoreduction, the same held true. Median OS in patients with LGA cancer receiving complete cytoreduction was not reached with DR and 175 months without DR (p = 0.17). Median OS for patients with colorectal cancer receiving complete cytoreduction was 23 months with DR versus 31 months without DR (p = 0.76).
Fig. 1. Overall survival with and without a diaphragmatic resection as a component of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with a appendiceal and b colon primary lesions.
Discussion
CRS/HIPEC with DR has the theoretical risk of pleural contamination with the possibility of thoracic recurrence. To address this theoretical risk, the pleural space can be incorporated in the perfusion circuit and treated with HIPEC. The role of diaphragmatic resection with thoracic heated chemoperfusion for peritoneal carcinomatosis treated with CRS/HIPEC remains undefined.
The primary purpose of our study was to assess the impact of diaphragmatic resection on morbidity and mortality of CRS/HIPEC procedures. The secondary goal was to evaluate the impact of DR on OS and thoracic recurrence rates of patients with PSD arising from LGA and colon cancers.
The presented data shows that diaphragmatic resection as part of cytoreduction did not contribute to a higher overall morbidity but was associated with significantly higher 90-day mortality. Diaphragmatic resection was associated with more extensive CRS and greater disease burden as indicated by the higher total number of organs resected and incomplete CRS. Although the extent of cytoreduction was predictor of mortality on univariate analysis, only DR and diabetes were predictors of mortality on multivariate analysis. Detailed analysis of the specific complication pattern associated with DR showed that the morbidity and mortality might be closely linked. Higher rate of myocardial infarction and respiratory failure associated with DR in patients with other preexisting multiple comorbidities (Table 4) likely explain the higher mortality rates observed in the DR cohort. The specific complication pattern was not well tolerated especially in diabetic patients, where the chronic cumulative effect of diabetes in several organ systems increased the likelihood of mortality threefold when CRS/HIPEC was combined with DR. Of note, thoracic chemoperfusion itself had no observed increase in mortality in the specific cohort.
Patients undergoing thoracic chemoperfusion after DR had higher intrathoracic recurrence rates than patients with DR who were not perfused in the thoracic cavity. This paradoxical finding could be at least partially explained by a selection bias as full-thickness diaphragmatic involvement would have been considered more prone to develop intrathoracic recurrence and thus the surgeon would have been more likely to perfuse the thoracic cavity. The higher rates of suboptimal intra-abdominal cytoreduction as well as more aggressive primaries (colon cancer and mesothelioma) in this cohort also would support a selection bias. Yet, the possibility that heated chemotherapy cannot control iatrogenic contamination of the thoracic cavity should be taken into consideration every time that a small diaphragmatic defect is converted purposefully into a bigger defect to facilitate thoracic chemoperfusion.
Patients with PSD from LGA primary lesions who had DR and achieved complete macroscopic cytoreduction had similar OS compared with their counterparts without DR. Colon cancer patients with and without DR who had a complete cytoreduction also showed statistically similar and meaningful OS. Because diaphragmatic involvement indicates higher burden of disease, it is possible that power attenuation precluded the trend towards worse survival for colorectal cancer patients with DR to reach statistical importance.
This study has the classic limitations of a retrospective analysis from a single institution, including evolving selection criteria and individual treatment bias. However, it accurately describes the complication pattern of diaphragmatic resections during CRS/HIPEC procedures and raises into question the adopted practice of perfusing the thoracic cavity concurrently with the peritoneal cavity, which up to this point has not been sufficiently addressed.
Conclusions
Diaphragmatic resection during CRS/HIPEC is an independent predictor of mortality, related to a specific complication pattern that includes respiratory failure. Patients with diabetes are more prone to adverse effects when DR is undertaken. Diaphragmatic involvement indicates increased burden of disease, and DR should be performed only if careful inspection of the peritoneal cavity determines that a complete macroscopic cytoreduction is feasible. Following complete cytoreduction, patients with PSD from LGA and colon cancer receiving DR as a component of CRS/HIPEC experience meaningful and similar OS compared with their non-DR counterparts. The role of concurrent thoracic chemoperfusion in these patients in controlling thoracic contamination and recurrence remains unknown and should be carefully considered on an individual basis until further data documenting a clinical benefit is available.
Acknowledgments
Supported by Wake Forest University Biostatistics shared resource NCI CCSG P30CA012197.
Footnotes
Presented at The Society of Surgical Oncology 67th annual meeting, Phoenix, AZ, March 12-15, 2014.
Disclosures Nothing to disclose.
References
- 1.Levine EA, Stewart JHt, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218(4):573–585. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 3.Elias D, Glehen O, Pocard M, et al. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg. 2010;251:896–901. doi: 10.1097/SLA.0b013e3181d9765d. [DOI] [PubMed] [Google Scholar]
- 4.Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker PH, Chang D, Stuart OA. Hyperthermic intraoperative thoracoabdominal chemotherapy. Gastroenterol Res Pract. 2012:623417. doi: 10.1155/2012/623417. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujoomdar AA, Sugarbaker DJ. Hyperthermic chemoperfusion for the treatment of malignant pleural mesothelioma. Sem Thorac Cardiovasc Surg. 2008;20:298–304. doi: 10.1053/j.semtcvs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Chen WJ, Yuan SF, Yan QY, et al. Intrapleural chemo- and hyperthermotherapies for malignant pleural effusion: a randomized prospective study. Cancer Investig. 2012;30:126–30. doi: 10.3109/07357907.2011.633292. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine EA, Stewart JHt, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–3. doi: 10.1016/j.jamcollsurg.2006.12.048. discussion 953–5. [DOI] [PubMed] [Google Scholar]