Abstract
BACKGROUND
Despite targeted antiemetics, data support an unmet need related to the management of delayed nausea and vomiting (NV). Promising pilot data informed this phase III trial evaluating gabapentin for delayed NV from highly emetogenic chemotherapy (HEC).
METHODS
Participants were randomized to receive prophylactic treatment with 20 mg of dexamethasone and a 5HT3 receptor antagonist (RA) on the day of chemotherapy, followed by gabapentin 300 mg twice a day and dexamethasone (dex) or placebo and dex after HEC. Gabapentin/placebo was started the day of chemotherapy and continued through day 5 for the first chemotherapy cycle, whereas dex was titrated down on days 2–4. The primary end point was complete response (CR), defined as no emesis and no use of rescue medications on days 2–6, using an NV diary. The percentages of those in each group with a CR were compared by Fisher’s exact test.
RESULTS
Four hundred thirty patients were enrolled in this study. Forty-seven percent of patients in the gabapentin arm and 41% in the placebo arm had a CR (P = .23). Mean number of emesis episodes was <0.5 daily, and mean nausea severity was <2 (mild). In both arms, patient satisfaction with NV control was greater than 8 (with 10 being perfectly satisfied). There were no significant differences in unwanted side effects.
CONCLUSIONS
In this study, gabapentin did not significantly improve delayed NV. Patients were satisfied with the control of their nausea and vomiting irrespective of arm. The use of a 5HT3 RA and dexamethasone provided good control of nausea and vomiting for most patients.
Keywords: gabapentin, delayed nausea, delayed vomiting, antiemetic therapy, randomized controlled trial
INTRODUCTION
Nausea and Vomiting in Chemotherapy
Nausea and vomiting have been long-standing, dreaded ill effects of chemotherapy. Chemotherapy-induced nausea and vomiting (CINV) has been commonly cited by patients as among the “most unpleasant and distressing” side effects associated with chemotherapy.1 Consequences of CINV include dehydration, anorexia, weight loss, and fluid/electrolyte disturbances, some of which can be potentially life-threatening. These side effects can lead not only to significant decreases in cancer patients’ quality of life, but also to dose reductions, treatment delays or discontinuation of treatment, threatening the potential for a good outcome. In addition to the impact on individual patients, there remains a significant medical economic burden because of unplanned office or emergency visits and rescue therapies required as a result of uncontrolled CINV.2–5
Delayed Chemotherapy-Induced Nausea and Vomiting
Two distinct forms of CINV have been recognized: acute and delayed. Acute nausea and vomiting occurs within the first 24 hours after chemotherapy administration. Delayed CINV has historically been defined as nausea and vomiting that occurs greater than 24 hours after chemotherapy, lasting up to 5 days after chemotherapy administration.6 With the evolution of 5HT3 receptor antagonists, control of acute CINV has significantly improved. 5HT3 antagonists in combination with corticosteroids have become the standard treatment for acute CINV for moderately emetogenic chemotherapy (MEC) regimens, and newer agents have become the standard for highly emetogenic chemotherapy (HEC).7,8 Despite this, control of delayed nausea and vomiting remains an important unmet need with more than 50% of patients experiencing delayed nausea after moderately or highly emetogenic chemotherapy and 30%–50% reporting delayed emesis.3,9
The pathophysiology of delayed CINV is poorly understood. The binding of substance P to NK-1 receptors has been implicated in animal studies 10,11. Therefore, NK-1 receptor antagonists, such as aprepitant and casopitant, were developed and have shown improvement in rates of nausea and emesis control.12–16
Current American Society of Clinical Oncology (ASCO) guidelines recommend dexamethasone plus a 5HT3 receptor antagonist and an NK-1 receptor antagonist for the prevention of CINV in chemotherapeutic regimens of high emetogenic potential.17 However, there are 2 significant issues that may limit the use of NK-1 receptor antagonists as widespread standard therapy. First, there is a significant cost to the medication, which can pose a financial burden to those without prescription drug coverage. Second, aprepitant is metabolized by the P450 system, specifically CYP3A4, potentially causing significant interactions with a variety of common medications metabolized through the same pathway. Thus, other effective options for delayed nausea and vomiting warrant further investigation.
The Role of Gabapentin in Delayed CINV
Gabapentin is structurally related to the neurotransmitter gamma-aminobutyric acid (GABA) but does not bind GABA-A or GABA-B receptors and may exert its effect via the modulation of release of excitatory neurotransmitters. The potential role of gabapentin in reducing delayed CINV was first noted anecdotally. Together with a case report, Guttuso et al18 published an open-label proof-of-concept study of 9 women being treated for breast cancer with doxorubicin-based chemotherapy for 4 cycles.18 Guttuso et al theorized that gabapentin may mitigate tachykinin neurotransmitter activity and lead to physiologic activity similar to that seen with NK-1 receptor antagonists, making it a potential intervention for delayed nausea and vomiting. In fact, animal studies provide data to support the role of both central and peripheral mechanisms involved in vomiting, and the relation of substance P and tachykinin receptor involvement in this physiology.10,19 Although the precise mechanism of action of gabapentin remains elusive, some basic scientists concluded that gabapentin binding sites may be both pre- and postsynaptic and are shared by NK-1 receptors.19 That hypothesis made gabapentin an interesting and promising agent to evaluate for CINV. Three pilot studies20–22 provided data to support further evaluation of gabapentin to reduce delayed nausea and vomiting, with only 1 study being a randomized, placebo-controlled trial.20
Based on these promising pilot trials, we endeavored to test gabapentin in a large, phase III trial for delayed CINV. It could be argued that the best trial of gabapentin’s efficacy for preventing delayed nausea and vomiting would be a noninferiority design against aprepitant. However, this design would be cost-prohibitive because of the price of aprepitant. In addition, at the time this study was developed, aprepitant was not widely used in the community setting. Therefore, we used a phase III, randomized, double-blind, placebo-controlled study design to evaluate the efficacy of gabapentin to reduce delayed CINV, to assess its tolerability, and to study its effects on quality-of-life-related variables in patients undergoing chemotherapy.
MATERIALS AND METHODS
Eligibility Criteria
Patients eligible for this study included adults with a performance status of 0–2 who were scheduled to receive HEC. Participants must have been chemotherapy-naive for highly and moderately emetogenic agents. Participants needed to be able to provide written informed consent and be able to swallow pills. Chemotherapy regimens were required to include at least 7 days between cycles of highly emetogenic chemotherapy administration; multiple-day chemotherapy was permitted as long as the chemotherapy drugs given on subsequent days had mild or no emetogenic potential.
Exclusion criteria included current and prior use of or sensitivity to gabapentin or other anticonvulsants and current or planned use of lorazepam, diphenhydramine, eszopiclone, and/or dronabinol during the 6 days of this study, except for treatment of breakthrough nausea and vomiting. In addition, previoius or planned use of aprepitant or any other NK-1 receptor antagonist was prohibited during the study. Patients were also excluded for any of the following: having received pelvic or abdominal radiotherapy within a week of randomization, having had nausea or vomiting or having used antiemetics within 3 days prior to randomization, and having gastrointestinal obstruction, active peptic ulcer disease, uncontrolled heartburn, or a history of nausea and vomiting related to any chemotherapy.
Random Assignment
Participants were stratified according to several variables that have been shown to be associated with the risk of nausea and vomiting.23,24 This included type of chemotherapy regimen (cisplatin based versus other), sex, age (younger than 50 versus 50 or older), or a history of alcoholism, motion sickness, or pregnancy-induced nausea/vomiting. Patients were randomized to either gabapentin or an identical placebo capsule, using the Pocock and Simon dynamic allocation procedure, which balances the marginal distribution of the stratification factors between the treatment groups.25 Both participants and medical personnel were blinded to treatment assignments. Each participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines. This phase III therapeutic trial was monitored at least twice annually by the North Central Cancer Treatment Group, now the Alliance, Data and Safety Monitoring Board, a standing committee composed of individuals from within and outside the cooperative group. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following alliance policies. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
Treatment Protocol
Patients took 1 tablet (gabapentin 300 mg or placebo) beginning on the evening of day 1 of their first chemotherapy cycle. On days 2 and 3, the dose was increased to 1 tablet gabapentin/placebo twice a day. On days 4 and 5, patients could be titrated up to 1 tablet 3 times a day (gabapentin/placebo) if the patient was not having the response they desired. This dose was based on the positive study by Sarkar and colleagues.21 All patients were treated prophylactically for acute nausea and vomiting with a 5HT3 receptor antagonist of the provider/patient’s choice and dexamethasone 20 mg on day 1. On days 2 through 4, participants were prophylactically treated with decreasing doses of dexamethasone (8 mg by mouth twice a day on days 2 and 3, and 4 mg by mouth twice a day on day 4) with or without a 5HT3 receptor antagonist per physician preference for delayed nausea and vomiting, in accordance with ASCO guidelines. At the time of this protocol, ASCO guidelines did not recommend prophylactic treatment with a 5HT3 receptor antagonist for delayed nausea and vomiting.17,26
The study encompassed the first cycle of chemotherapy only. The rationale for this was that if a patient developed delayed nausea and vomiting, there were other Food and Drug Administration–approved treatments for delayed CINV, and it would not be ethical to keep the patient on something that was not helpful for an additional chemotherapy cycle when he/she could be switched to another potentially helpful option.
Endpoints and Outcome Measures
Patients were instructed to keep a record of each emetic episode as it occurred, a practice consistent with a majority of the seminal trials evaluating antiemetics.27–30 The nausea and vomiting diary began the day of chemotherapy and continued through day 6. The primary end point was the percentage of those with a complete response (CR), defined as no emesis and no rescue medications days 2–6 using a daily diary for nausea and vomiting. In addition, participants recorded their average and worst levels of nausea severity on a 0- to 10-point scale (with 10 being the worst) as well as any rescue medication use, daily. Daily rankings of treatment satisfaction and distress were also recorded using questions with numeric analog scales ranging from 0 to 10 (with 10 being high distress, high satisfaction).
Secondary end points included the percentage of complete responders, defined differently as 1) having no emetic episodes and 2) no more than mild nausea (≤2.5 on a 0 to 10-point scale),30 and 3) no rescue medication use on days 2–6, as captured using the daily diary. The secondary analysis also included adverse effects and tolerability, which were assessed using 2 measures, a self-report numeric analog scale and NCI Common Terminology Criteria for Adverse Events (CTCAE v3.0). Self-reported side effects on questionnaires included drowsiness, impaired concentration, diarrhea, fatigue, mood swings, and loss of appetite, rated on a scale of 0 to 10 (with 10 being the most severe). These were completed on days 1 and 6. This scale was converted to a 0–100 scale for the analysis, with higher numbers indicating better health-related quality of life. Providers graded dizziness, somnolence, ataxia, and edema using the CTCAE v3.0 on days 1, 2, 3, 4, and 6.
An additional secondary end point evaluated function with the Functional Living Index-Emesis (FLIE).31 This is a self-report scale to evaluate the impact of nausea and vomiting on the patient’s daily function that has been used in numerous trials evaluating antiemetic therapy. There are 2 subscales, one for nausea and one for vomiting. The subscales can also be combined for a total score. Total scores range from 18 to 126, with higher scores indicating better health-related quality of life. Internal consistency is reported with a Cronbach’s alpha of .79. Construct validity is reported with correlations of items within the nausea subscale of 0.84 to 0.95. These correlations are a bit weaker in the vomiting domain, ranging from 0.52 to 0.76. Distress from nausea and vomiting control was evaluated as part of the daily diary with numeric analog scales with responses ranging from 0 (none) to 10 (as bad as it can be). Satisfaction with nausea and vomiting control was assessed similarly, from 0 (not at all satisfied) to 10 (totally satisfied).
Statistical Analysis
The primary analysis was intent to treat and used Fisher’s exact test to compare the percentage of complete responders in the treatment groups (dexamethasone [dex]/gabapentin vs dex placebo). Patients who did not complete the study or did not provide complete data were assumed to be nonresponders. The study was powered to detect a 15% difference in responder rates17 between the treatment groups using a 2-tailed alternative, a 5% type I error rate, and achieving 80% power. This required a minimum of 378 patients, (189 per treatment arm). Target accrual was inflated by 10% to account for ineligible and canceled cases, thus making the total target accrual 416 patients.
For the secondary end points, total and subscale scores of the FLIE were calculated as well as satisfaction ratings for nausea and vomiting. Mean values of groups were compared using Kruskal-Wallis testing. Analysis of differences with side effects on the self-report measure involved calculating mean scores for each side effect and comparing differences between groups using Kruskal-Wallis testing. The incidence of toxicities rated using the CTCAE v.3 were compared between groups using Fisher’s exact tests for incidence. All tests were 2-sided tests with 5% type 1 error rate using SAS version 9.2.
RESULTS
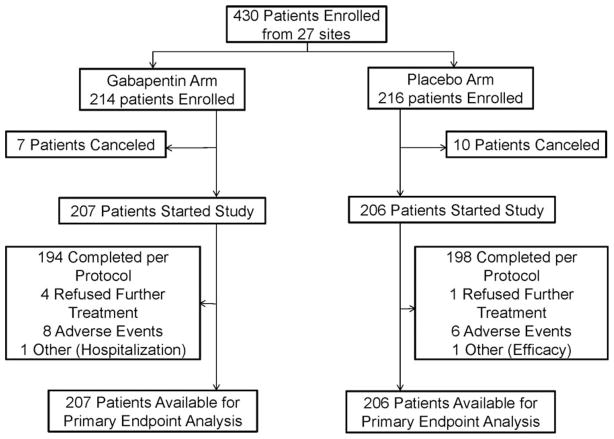
Between May 1, 2009, and February 4, 2011, 430 patients were enrolled at 27 participating clinical sites, primarily community cancer centers. Data were frozen for analysis on August 7, 2011. Patient participation and flow are depicted in a CONSORT diagram (Fig. 1). Baseline patient characteristics are shown in Table 1, illustrating that all baseline factors were evenly distributed between the 2 study arms.
Figure 1.
CONSORT diagram.
TABLE 1.
Baseline Patient Characteristics
| Characteristic | Gabapentin (n = 207) | Placebo (n = 206) | P |
|---|---|---|---|
| Age group | |||
| ≥50 | 150 (73%) | 148 (72%) | .89 |
| Sex | |||
| Female | 145 (70%) | 145 (70%) | .94 |
| Dominant disease status | .77 | ||
| Breast | 117 (57%) | 116 (57%) | |
| Lung | 30 (15%) | 35 (17%) | |
| Colorectal | 1 (1%) | 0 (0%) | |
| Gynecologic | 1 (1%) | 0 (0%) | |
| Hematologic | 5 (2%) | 4 (2%) | |
| Other | 52 (25%) | 50 (24%) | |
| Chemotherapy | .81 | ||
| Cisplatin-based | 79 (38%) | 81 (39%) | |
| Use of palonosetron on day 1 | .95 | ||
| Yes | 121 (59%) | 121 (59%) | |
| Use of 5HT3 antagonist after day 1 | .89 | ||
| Yes | 74 (36%) | 75 (36%) | |
| Number of emetic agents high | 1.00 | ||
| 1–2 | 202 (99%) | 201 (99%) | |
| 3–4 | 3 (2%) | 3 (2%) | |
| Number of emetic agents moderate | .31 | ||
| 0 | 146 (75%) | 151 (80%) | |
| 1–2 | 49 (25%) | 38 (20%) | |
| 3–4 | 1 (1%) | 0 (0%) | |
| History of alcoholism | .71 | ||
| No | 192 (93%) | 193 (94%) | |
| History of motion sickness/pregnancy-induced emesis | .94 | ||
| No | 140 (68%) | 140 (68%) | |
Primary End Point
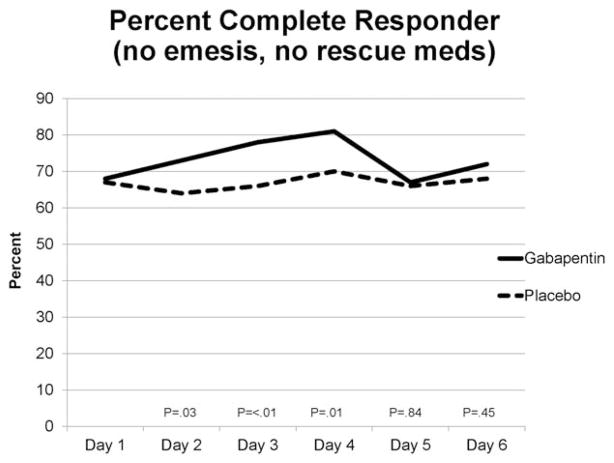
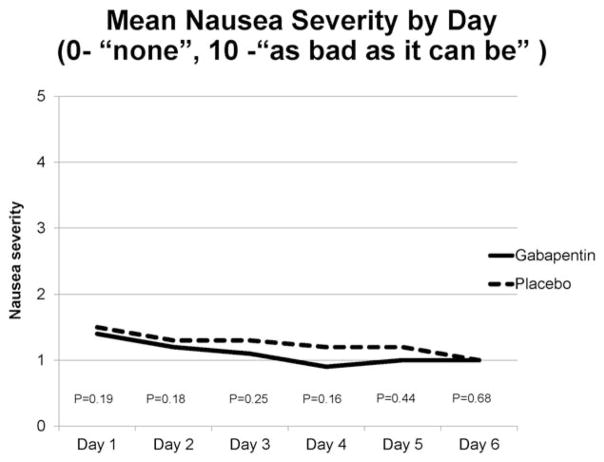
Ninety-seven of 207 patients (47%) in the gabapentin arm and 84 of 206 patients (41%) in the placebo arm had a complete response (CR; P = .23). The percent of responders by day is shown in Figure 2. When nausea severity ≤2.5 was included as part of the secondary analysis, 91 of those on gabapentin (44%) and 81 of those on placebo (39%) had a CR (P = .37). At some time during the study, 62 (30%) in both the gabapentin and placebo arms experienced emesis (P = 1.00), and 93 (45%) on gabapentin and 109 (53%) on placebo took rescue medication (P = .12). However, the mean number of emesis episodes by day was quite low, with a daily mean for either arm <0.5 (Table 2). Mean nausea severity by day was also quite low, with a daily mean for either arm ≤1.5 (Fig. 3).
Figure 2.
Percentage of patients in gabapentin and placebo arms who had a complete response on the indicated day of study. P values obtained via independent t tests for each day.
TABLE 2.
Mean Number of Emeses by Day
| Gabapentin (n = 207) | Placebo (n = 206) | P | |
|---|---|---|---|
| Day 2 | .33 | ||
| Mean (SD) | 0.3 (1.1) | 0.2 (0.9) | |
| Day 3 | .20 | ||
| Mean (SD) | 0.1 (0.8) | 0.2 (0.8) | |
| Day 4 | .23 | ||
| Mean (SD) | 0.1 (0.5) | 0.1 (0.4) | |
| Day 5 | .54 | ||
| Mean (SD) | 0.2 (0.8) | 0.1 (0.5) | |
| Day 6 | .15 | ||
| Mean (SD) | 0.1 (0.5) | 0.1 (0.7) |
Figure 3.
Mean severity of nausea on the indicated day of study in both the gabapentin and placebo arms. P values obtained via independent t tests for each.
Secondary End Points
Side effects
The overall toxicity incidence demonstrated no significant difference between arms for the required adverse events as measured by CTCAE v.3. Adverse events relatively common in both groups included dizziness and somnolence. There were no significant differences in toxicities by CTCAE provider grading. Self-reported side effects improved in both groups over the course of the study with one exception: anxiety/nervousness increased over the course of the study, but this did not differ between placebo and gabapentin. There were 2 areas that significantly differed between groups: negative mood swings and appetite loss, favoring the placebo. However, as both of these symptoms improved during the study, the differences are not meaningful. Mean (standard deviation) scores from the Side Effect Experience Diary are shown in Table 3.
TABLE 3.
Self-Reported Side Effects
| Gabapentin (n = 207) | Placebo (n = 206) | Pa | |
|---|---|---|---|
| Drowsiness | .51 | ||
| Mean (SD) | 1.4 (2.5) | 1.6 (2.6) | |
| Concentration | .48 | ||
| Mean (SD) | 1.0 (2.6) | 1.1 (2.5) | |
| Diarrhea | .46 | ||
| Mean (SD) | 0.5 (2.0) | 0.4 (2.0) | |
| Skin irritation | .30 | ||
| Mean (SD) | 0.1 (1.7) | 0.1 (1.6) | |
| Swelling of hands/feet | .78 | ||
| Mean (SD) | 0. 1 (1.6) | 0.0 (1.2) | |
| Dry mouth | .20 | ||
| Mean (SD) | 1.6 (2.4) | 1.2 (2.4) | |
| Dizziness | .25 | ||
| Mean (SD) | 1.3 (2.4) | 0.9 (2.1) | |
| Constipation | .96 | ||
| Mean (SD) | 2.0 (3.0) | 2.0 (3.0) | |
| Tiredness | .11 | ||
| Mean (SD) | 2.3 (3.0) | 2.8 (3.0) | |
| Headache | .42 | ||
| Mean (SD) | 1.3 (2.6) | 1.3 (2.5) | |
| Indigestion/heartburn | .71 | ||
| Mean (SD) | 2.0 (3.0) | 1.9 (2.9) | |
| Anxiety/nervousness | .19 | ||
| Mean (SD) | −0.8 (2.9) | −0.4 (2.9) | |
| Weight gain | .45 | ||
| Mean (SD) | 0.3 (1.9) | 0.1 (1.4) | |
| Appetite loss | .04 | ||
| Mean (SD) | 1.3 (2.7) | 1.9 (3.3) | |
| Loss coordination | |||
| Mean (SD) | 0.4 (1.5) | 0.4 (1.6) | .50 |
| Negative mood swings | |||
| Mean (SD) | 0.3 (2.2) | 0.9 (2.4) | < .01 |
| Vision changes | |||
| Mean (SD) | 0.7 (1.8) | 0.5 (1.3) | .96 |
Change from baseline, Positive numbers indicate improvement.
Kruskal Wallis test.
FLIE
The overall mean score on the FLIE was 108.3 for those receiving gabapentin and 108.5 for placebo (P = .73). The subscale scores also did not significantly differ. For nausea, the mean scores were 51.6 for the gabapentin and 51.2 for the placebo arm (P = .98); for vomiting the mean was 57.0 in the gabapentin arm and 57.3 in the placebo arm (P = .56). Means and medians for individual items are in Table 4.
TABLE 4.
FLIE Subscale Individual Items
| Nausea
|
Vomiting
|
|||||
|---|---|---|---|---|---|---|
| Gabapentin (n = 207) | Placebo (n = 206) | Pa | Gabapentin (n = 207) | Placebo (n = 206) | Pa | |
| How much nausea | .76 | |||||
| Mean (SD) | 5.7 (1.7) | 5.5 (1.9) | .30 | 6.5 (1.3) | 6.2 (1.2) | |
| Median | 6.0 | 6.0 | 7.0 | 7.0 | ||
| Recreation leisure activities | .73 | |||||
| Mean (SD) | 6.0 (1.7) | 5.9 (1.8) | .37 | 6.3 (1.7) | 6.2 (1.8) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Meals or minor housework | .93 | |||||
| Mean (SD) | 5.3 (2.3) | 5.4 (2.2) | .72 | 6.6 (1.2) | 6.6 (1.2) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Enjoy meals | .79 | |||||
| Mean (SD) | 5.8 (1.9) | 5.7 (1.9) | .49 | 6.5 (1.3) | 6.5 (1.4) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Enjoy drinking liquids | .60 | |||||
| Mean (SD) | 5.9 (1.9) | 5.8 (1.8) | .40 | 6.5 (1.4) | 6.6 (1.3) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Time with family/friends | .54 | |||||
| Mean (SD) | 5.6 (2.1) | 5.7 (2.0) | .97 | 6.5 (1.5) | 6.4 (1.5) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Daily functioning | .62 | |||||
| Mean (SD) | 6.0 (1.6) | 5.9 (1.7) | .94 | 6.6 (1.2) | 6.6 (1.1) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Personal hardship | .58 | |||||
| Mean (SD) | 6.0 (1.6) | 6.0 (1.6) | .88 | 6.6 (1.2) | 6.6 (1.1) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Hardship others | .58 | |||||
| Mean (SD) | 6.1 (1.6) | 6.1 (1.5) | .62 | 6.3 (1.8) | 6.4 (1.5) | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | ||
From 1 = a great deal to 7 = not at all.
Kruskal-Wallis test.
Distress and satisfaction
Mean satisfaction scores measured with the diary demonstrated no significant differences between arms, with high levels of satisfaction in both arms (Table 5). For distress related to nausea, distress levels were low and not significantly different between arms, though there was a trend toward less distress in the gabapentin arm (P = .06; Table 5).
TABLE 5.
Distress and Satisfaction (Range, 0–10)
| Gabapentin (n = 200) | Placebo (n = 197) | Pa | |
|---|---|---|---|
| NVD nausea/vomiting satisfaction days 2–6b | .13 | ||
| Mean (SD) | 8.7 (2.1) | 8.5 (2.2) | |
| Median | 10 | 9.6 | |
| Satisfaction nausea controlb | .33 | ||
| Mean (SD) | 8.3 (2.8) | 8.1 (2.9) | |
| Median | 10.0 | 10.0 | |
| Satisfaction vomiting controlb | .92 | ||
| Mean (SD) | 9.1 (2.2) | 9.1 (2.3) | |
| Median | 10.0 | 10.0 | |
| Distress for nauseac | .06 | ||
| Mean (SD) | 0.9 (1.6) | 1.1 (1.5) | |
| Median | 0.0 | 0.2 |
Kruskal-Wallis test.
Higher is better.
Lower is better.
Dosing
The protocol allowed for titration of the study drug from twice to 3 times per day, beginning on day 4, for inadequate control of nausea or vomiting. On day 4, 8% of those in the gabapentin arm and 9% in the placebo arm increased their daily dose. On day 5, 4% of the gabapentin arm and 6% of the placebo arm reported taking study drug 3 times per day. This was not statistically significantly different between arms.
DISCUSSION
The present study is the first large placebo-controlled double-blind, randomized trial investigating the efficacy of gabapentin in controlling delayed CINV. Despite promising pilot study data and a rational physiologic hypothesis, the results of the current study did not provide support for the use of gabapentin to reduce delayed CINV beyond what is possible with dexamethasone.
Complete response rates were lower than what was anticipated based on published literature. In the placebo/corticosteroid group, a complete response rate of 50% was expected based on the ASCO guideline summary of studies using dexamethasone alone as the control arm.17 The complete response in clinical trials for aprepitant with dexamethasone (defined as no emesis and no rescue agent) is reported to be about 65% (average of 55% and 72% CR).17 Curiously, however, mean nausea severity was low, daily emesis was low, distress was low, satisfaction was high, and the results for the FLIE indicate that nausea and vomiting in this study did not negatively affect functioning. It is puzzling that fewer than one-third of the sample experienced emesis, but a high number of patients (45% and 53%) took rescue medication despite an overall low mean nausea severity. One hypothesis as to why this occurred is that patients might have taken rescue medications more prophylactically than for actual “rescue” of moderate nausea and vomiting. Although the treatment for CINV has improved drastically, it is still a side effect dreaded by patients. It is possible that the fear of experiencing nausea and vomiting may trump all, causing patients to take medications “just in case.”
It is important to highlight that according to published literature,3,32 the results of the FLIE indicate that patients in this study did not experience a negative impact on their daily functioning from nausea and vomiting. Overall, scores of 108 or higher are indicative of no problems, and item scores of 6 or more indicate no problems. For both groups, overall mean scores exceeded 108, and as shown in Table 4, each question in the vomiting subscale had means and medians greater than 6. In the nausea sub-scale, 5 of 9 items had means slightly less than 6, but medians were 6–7. The lack of problems with nausea and vomiting is further supported by the very low distress rates and high satisfaction with the control of nausea and vomiting. The use of evidence-based clinical guidelines for prophylaxis of nausea and vomiting provides strong clinical guidance for effective treatment and clearly leads to good patient outcomes.
It is noteworthy that the daily complete response rates were significantly different, initially favoring gabapentin, on days 2 through 4, but then converging on days 5 and 6. Day 4 was the last day dexamethasone was given. This finding raises the question of whether there may be synergy between dexamethasone and gabapentin.
One limitation of this study was the initial twice-a-day dosing. Although this was based on a previously positive study,21 other studies used 3-times-a-day dosing.20,22 Although this protocol allowed titration up to 3 times per day on day 4, and only 9% of patients increased their dose overall, it cannot be ruled out that starting with 3-times-a-day dosing may have produced different results. A second limitation is that the best study design for this trial might have been a noninferiority design.
In summary, gabapentin did not improve delayed chemotherapy-induced nausea and vomiting beyond what prophylactic dexamethasone provided. Overall, despite lower than expected complete response rates, the number of emetic events was low, and nausea severity was mild. Patients were quite satisfied with the control of their nausea and vomiting irrespective of arm. The use of standard prophylactic guidelines that included a 5HT3 receptor agonist and dexamethasone provided good control of nausea and vomiting for most patients. Although it is acknowledged that delayed nausea and vomiting is less well controlled than acute, these results support that it is a minority of patients who have significant issues with nausea and vomiting during chemotherapy if standard guidelines are followed. Future studies should therefore focus on identifying the approximately 25% of patients who have moderate to severe nausea and emesis that affects their functioning despite evidence-based prophylactic treatment. Rather than designing studies for “all comers” with respect to HEC, future studies might select for and focus on this smaller subset who remain in critical need of better management.
Acknowledgments
FUNDING SUPPORT
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35103, CA-35272, CA-37417, CA-35113, CA-63848, CA-35195, CA-35269, CA-35267, CA-35119, CA-35415, CA-35431, CA-35103, CA-63849, and CA-35101. The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, MD, Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, PhD, CA33601).
Additional participating institutions include Siouxland Hematology-Oncology Associates, Sioux City, Iowa (Donald Wender, MD); Toledo Community Hospital Oncology Program (Rex B. Mowat, MD); Medical College of Georgia, Augusta, Georgia (Anand P. Jillella, MD); Iowa Oncology Research Association CCOP, Des Moines, Iowa (Robert J. Behrens, MD); Michigan Cancer Research Consortium, Ann Arbor, Michigan (Philip J. Stella, MD); Colorado Cancer Research Program, Denver, Colorado (Eduardo R. Pajon, Jr., MD); Mayo Clinic Arizona, Scottsdale, Arizona (Michele Y. Halyard, MD); Medcenter One Health Systems, Bismarck, North Dakota (Edward J. Wos, DO); Carle Cancer Center CCOP, Urbana, Illinois (Kendrith M. Rowland, Jr, MD); Essentia Duluth CCOP, Duluth, Minnesota (Daniel A. Nikcevich, MD); Marshfield Clinical Research Foundation, Minocqua, Wisconsin (Matthias Weiss, MD); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, Minnesota (Patrick J. Flynn, MD); Missouri Valley Cancer Consortium, Omaha, Nebraska (Gamini S. Soori, MD); University of New Mexico, Albuquerque, New Mexico (Zoneddy R. Dayao, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, Indiana (Robin T. Zon, MD); Illinois Oncology Resarch Assn. CCOP, Peoria, Illinois (John W. Kugler, MD); Upstate Carolina CCOP, Spartanburg, South Carolina (James D. Bearden, III, MD); Mayo Clinic Florida, Jacksonville, Florida (Kurt A. Jaeckle, MD); Wichita Community Clinical Oncology Program, Wichita, Kansas (Shaker R. Dakhil, MD)
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
CONFLICT OF INTEREST DISCLOSURES
Debra L. Barton reports grants from the National Cancer Institute. Jyotsna Fuloria reports grants from the National Cancer Institute, Lisa A. Kottschade reports being on the nurse advisory board of Genentech.
References
- 1.Morrow GR, Hickok JT, Burish TG, et al. Frequency and clinical implications of delayed nausea and delayed emesis. Am J Clin Oncol. 1996;19:199–203. doi: 10.1097/00000421-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Ihbe-Heffinger A, Ehlken B, Bernard R, et al. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15:526–536. doi: 10.1093/annonc/mdh110. [DOI] [PubMed] [Google Scholar]
- 3.Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 4.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 5.Tina Shih YC, Xu Y, Elting LS. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer. 2007;110:678–685. doi: 10.1002/cncr.22823. [DOI] [PubMed] [Google Scholar]
- 6.Roila F, Donati D, Tamberi S, et al. Delayed emesis: incidence, pattern, prognostic factors and optimal treatment. Support Care Cancer. 2002;10:88–95. doi: 10.1007/s005200100295. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Practice Guidelines in Oncology. 2004 [Google Scholar]
- 8.Loprinzi CL, Christensen B, Grendahl B, et al. Standard Antiemetic Order Guidelines. 2000 [Google Scholar]
- 9.Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–2268. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 10.Watson J, Gonsalves S, Fossa A, et al. The anti-emetic effects of CP-99, 994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol. 1995;115:84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesketh PJ. Potential role of the NK1 receptor antagonists in chemotherapy-induced nausea and vomiting. Support Care Cancer. 2001;9:350–354. doi: 10.1007/s005200000199. [DOI] [PubMed] [Google Scholar]
- 12.Campos D, Pereira JR, Reinhardt RR, et al. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol. 2001;19:1759–1767. doi: 10.1200/JCO.2001.19.6.1759. [DOI] [PubMed] [Google Scholar]
- 13.Hesketh PJ, Gralla RJ, Webb RT, et al. Randomized phase II study of the neurokinin 1 receptor antagonist CJ-11,974 in the control of cisplatin-induced emesis. J Clin Oncol. 1999;17:338–343. doi: 10.1200/JCO.1999.17.1.338. [DOI] [PubMed] [Google Scholar]
- 14.Navari RM, Reinhardt RR, Gralla RJ, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med. 1999;340:190–195. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- 15.Van Belle S, Lichinitser MR, Navari RM, et al. Prevention of cisplatin-induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869. Cancer. 2002;94:3032–3041. doi: 10.1002/cncr.10516. [DOI] [PubMed] [Google Scholar]
- 16.Grote T, Hajdenberg J, Cartmell A, et al. Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitant. J Support Oncol. 2006;4:403–408. [PubMed] [Google Scholar]
- 17.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society for Clinical Oncology clinical practice update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guttuso T, Jr, Roscoe J, Griggs J. Effect of gabapentin on nausea induced by chemotherapy in patients with breast cancer. Lancet. 2003;361:1703–1705. doi: 10.1016/S0140-6736(03)13365-X. [DOI] [PubMed] [Google Scholar]
- 19.Maneuf YP, Blake R, Andrews NA, et al. Reduction by gabapentin of K+-evoked release of [3H]-glutamate from the caudal trigeminal nucleus of the streptozotocin-treated rat. Br J Pharmacol. 2004;141:574–579. doi: 10.1038/sj.bjp.0705579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz FM, de Iracema Gomes Cubero D, Taranto P, et al. Gabapentin for the prevention of chemotherapy- induced nausea and vomiting: a pilot study. Support Care Cancer. 2012;20:601–606. doi: 10.1007/s00520-011-1138-4. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar S, Saha S, Patra N, et al. Role of gabapentin in chemotherapy induced delayed emesis-an Indian experience. Support Care Cancer. 2007;15:688. [Google Scholar]
- 22.Pacheco A, Verschraegen C, Mangalik A, et al. A randomized open-label comparison of aprepitant (A) versus gabapentin (G) in the prevention of the refractory nausea and vomiting associated with moderately and severely emetogenic chemotherapy. J Clin Oncol. 2008;26(Suppl):20509. [Google Scholar]
- 23.Gralla RJ, Clark RA, Kris MG, et al. Methodology in anti-emetic trials. Eur J Cancer. 1991;27(Suppl 1):S5–S8. discussion S22. [PubMed] [Google Scholar]
- 24.Tonato M, Roila F, Del Favero A. Methodology of antiemetic trials: a review. Ann Oncol. 1991;2:107–114. doi: 10.1093/oxfordjournals.annonc.a057871. [DOI] [PubMed] [Google Scholar]
- 25.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 26.Kris MG, Gralla RJ, Tyson LB, et al. Controlling delayed vomiting: double-blind, randomized trial comparing placebo, dexamethasone alone, and metoclopramide plus dexamethasone in patients receiving cisplatin. J Clin Oncol. 1989;7:108–114. doi: 10.1200/JCO.1989.7.1.108. [DOI] [PubMed] [Google Scholar]
- 27.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 28.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer. 2005;41:1278–1285. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Aapro M, Fabi A, Nole F, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083–1088. doi: 10.1093/annonc/mdp584. [DOI] [PubMed] [Google Scholar]
- 30.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin–the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 31.Martin AR, Pearson JD, Cai B, et al. Assessing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003;11:522–527. doi: 10.1007/s00520-003-0482-4. [DOI] [PubMed] [Google Scholar]
- 32.Haiderali A, Menditto L, Good M, et al. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19:843–851. doi: 10.1007/s00520-010-0915-9. [DOI] [PubMed] [Google Scholar]