Abstract
Activation of the p53 pathway has been considered a therapeutic strategy to target cancers. We have previously identified several p53 activating small molecules in a cell-based screen. Two of the compounds activated p53 by causing DNA damage, but this modality was absent in the other four. We recently showed that one of these, BMH-21, inhibits RNA polymerase I (Pol I) transcription, causes the degradation of Pol I catalytic subunit RPA194 and has potent anticancer activity. We show here that three remaining compounds in this screen, BMH-9, BMH-22 and BMH-23, cause reorganization of nucleolar marker proteins consistent with segregation of the nucleolus, a hallmark of Pol I transcription stress. Further, the compounds destabilize RPA194 in a proteasome-dependent manner and inhibit nascent rRNA synthesis and expression of the 45S rRNA precursor. BMH-9 and BMH-22 –mediated nucleolar stress was detected in ex vivo-cultured human prostate tissues indicating good tissue bioactivity. Testing of closely related analogs showed that their activities were chemically constrained. Viability screen for BMH-9, BMH-22 and BMH-23 in the NCI60 cancer cell lines showed potent anticancer activity across many tumor types. Finally we show that the Pol I transcription stress by BMH-9, BMH-22 and BMH-23 is independent of p53 function. These results highlight the dominant impact of Pol I transcription stress on p53 pathway activation and bring forward chemically novel lead molecules for Pol I inhibition, and potentially, cancer targeting.
Keywords: small molecule, transcription, RNA polymerase I, nucleolus, p53
Introduction
Small molecule chemical library screens have extensively been used to identify chemical entities activating the p53 tumor suppressor protein (1, 2). The rationale has been straightforward – activation of wild type or mutant p53 protein could provide a powerful tool to launch the anti-proliferative, pro-apoptotic and anti-tumor activities of p53 to control tumor growth. Few of these lead molecules have entered clinical trials, such as PRIMA-1 (3, 4) and Nutlin-3 like molecules (5–7). Lead molecules arising from large unbiased screens have a diversity of action mechanisms including inhibition of sirtuins, and regulation of p53 folding and binding (1, 2). We conducted a cell-based high-throughput imaging screen to identify small molecule p53-activators and discovered and validated six lead compounds as activators of p53 based on their ability to stabilize p53, activate p53 reporter and p53 target genes and transcriptional profiling consistent with p53 responses (8). Characterization of their mechanism of p53 activation revealed that two of the compounds caused DNA-damage and mediated p53 stabilization through activation of ATM-signaling pathway. Strikingly, four compounds (BMH-9, BMH-21, BMH-22 and BMH-23) were devoid of DNA-damaging activity as analyzed by several DNA damage markers, and their mechanism of p53 activation remained unresolved. Recently, we showed that one of these compounds, BMH-21, inhibits RNA polymerase I (Pol I) transcription (9).
RNA polymerase I (Pol I) transcription is an emerging tractable process for cancer therapeutics (10–12). The transcription is mediated by a dedicated RNA polymerase holocomplex composed of multisubunit preinitiation and polymerase complexes (13). The transcription is initiated by assembly of the preinitation complex to the rDNA promoter, followed by stochastic assembly of the Pol I complex (13, 14). Once initiated, Pol I transcription is highly processive, and typically, a single Pol I complex transcribes the entire 13 kb coding region (14). Preinitiation complex assembly is instigated by posttranslational modifications of the preinitiation complex proteins (13). These modifications are effected by cyclin-CDK kinases, providing cell cycle-dependence to the transcription, and by cellular signaling and survival pathways, ERK, Akt/PKB, mTOR, Her2/Neu, and Myc (10, 15).
Pol I transcription is compartmentalized to the nucleolus (16–18). The nucleolus is divided into distinct compartments, namely fibrillar center, dense fibrillar component and granular component, encompassing defined functions that support Pol I transcription, rRNA processing and maturation of the rRNAs and ribosome assembly, respectively (17, 18). These subcompartments are marked by distinct localization of nucleolar proteins that participate in the respective rRNA biogenesis processes (18). Stresses that cause Pol I transcription blocks cause rapid and dynamic reorganization of the nucleolar structures and proteins (18–21). For example, proteins of the fibrillar center and dense fibrillar component relocalize to nucleolar cap structures at the nucleolar periphery while granular component proteins typically translocate to the nucleoplasm (18, 22).
The full extent of Pol I deregulation in human cancers is not known. Pol I transcription rates are not being measured, and 18S and 28S mature rRNAs conventionally used for normalization of RNA loading in various experimental approaches have long half lives and poorly reflect changes in the transcription rates. Furthermore, the absence of the multilocus rDNA gene from the human genome assembly obliterates recording of changes in the rRNAs in genome wide approaches. However, it is notable that key oncogenic pathways are ones that increase Pol I transcription rates (10–12). On the other hand, tumor suppressors p53, RB1, ARF, PTEN and GSK3β have been shown to restrict Pol I and ribosome biogenesis (10, 12). Conversely, Pol I transcription inhibition is a profound signal activating p53 (23–26). Mutations of nucleolar and ribosomal proteins drive tumorigenesis and the nucleolus contains proteins associated with cell cycle regulation, DNA repair and oncogenesis (19, 26, 27). Increased size of the nucleolus and staining of nucleolar organizing regions with silver is a frequent pathognomonic feature of cancer cells (28). These are indications for increased ribosynthetic activity of cancer cells to meet their demands for increased protein synthesis, and provide compelling reasoning to employ targeting of Pol I transcription as a therapeutic strategy.
The present study focused on characterization of the mechanism of action of compounds BMH-9, BMH-22 and BMH-23 arising from our initial high-throughput screen for p53 activators (8). We show here that these compounds function as Pol I inhibitors and similarly to BMH-21, cause proteasome-dependent degradation of RPA194. We further show that the molecules act in a p53-independent manner and that Pol I inhibition occurs upstream of p53 activation. The study defines new lead molecules for targeting Pol I transcription.
Materials and Methods
Cells and compounds
A375 melanoma (CRL-1619), U2OS (HTB96) and SaOS-2 (HTB85) osteosarcoma and HCT116 (CCl-247) cells were from American Type Culture Collection that verifies their identity using genomic fingerprinting. The cells were used at passages < 10 after thawing. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. A375 cells were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS) and U2OS and SaOS-2 cells in DMEM supplemented with 15% FBS. Compounds were obtained as follows: BMH-9, BMH-22, BMH-23, BMH-9_A1, BMH-9_A2 and BMH-22_A1 were obtained from ChemDiv and ChemBridge, verified for purity using LC/MS mass spectrometry and 1H-NMR. Actinomycin D was from Sigma, Nutlin-3 was obtained from Alexis Biochemicals and Sigma, and MG132 from Biomol International LP.
Viability assay
Cells were incubated in the presence of the compounds for the indicated times and viability was determined using WST-1 cell proliferation reagent according to manufacturers instructions (Roche Diagnostics).
Flow cytometry
Cell cycle distribution and cell death were analyzed with flow cytometry. Cells were harvested and fixed in 70% ethanol at −20°C followed by RNaseA treatment and stained with propidium iodide. A total of 10,000 counts were collected (LSR, Becton Dickinson) and cell cycle distribution was analyzed using ModFit LT 3.1 software (Verity Software House). Cells present in sub-G1 population were analyzed using the acquisition software (CellQuest).
rRNA synthesis assays
rRNA synthesis assays were conducted essentially as in refs. (9) and (29). Cells were labeled with 1 mM 5-FUrd (Sigma) using hypotonic shift and fixed with ice-cold methanol and acetone. Cells were blocked in 3% BSA and FUrd was detected using anti-5-BrdU antibody. DNA was stained with DAPI. Metabolic labeling of rRNA was conducted as in refs. (9) and (29). Cells were labeled with [5, 6-3H]-uridine (Perkin Elmer) at 2–3 μCi/ml. Total RNA was isolated and 3–5 μg of RNA was separated on a 0.8% agarose-formaldehyde gel. RNA was transferred to Hybond-N+ filter (Amersham), crosslinked, treated with Enhancer (Perkin Elmer) and exposed to film.
Immunofluorescence and image analysis
Immunostaining was performed essentially as in ref. (9). Cells grown on coverslips were fixed in 3.5% paraformaldehyde, permeabilized with 0.5% NP-40 and blocked in 3% BSA. The following primary antibodies were used: UBF (H-300) and RPA194 (C-1) (Santa Cruz Biotechnology), NCL (4E2, Abcam), NPM (FC-61991, Invitrogen), FBL (ab582, Abcam), γH2AX (Upstate), KAP-1 (BD Transduction Laboratories) and p53 (7F5, Cell Signaling Technologies). Secondary Alexa488 and Alexa594-conjugated anti-mouse and anti-rabbit antibodies were from Invitrogen. DNA was stained with DAPI. Images were captured using Axioplan2 fluorescence microscope (Zeiss) equipped with AxioCam HRc CCD-camera and AxioVision 4.5 software using EC Plan-Neofluar objectives (Zeiss). Image analysis was conducted using FrIDA designed for the analysis of RGB color image datasets as in ref. (9). Hue saturation and brightness ranges for green and red fluorescence channel and DNA (blue) were defined for each image set. Image intensities were determined as the fraction of positive cells divided total nuclear area as defined by DNA staining. An average of 100 cells was quantified from two fields for each sample.
Immunoblotting
Cells were lysed in 0.5% NP-40 buffer (25 mM Tris-HCl, pH 8.0, 120 mM NaCl, 0.5% NP-40, 4 mM NaF, 100 μM Na3VO4, 100 KIU/ml aprotinin, 10 μg/ml leupeptin) or RIPA lysis buffer. Proteins were separated on SDS-PAGE, blotted, probed for respective proteins and detected using ECL (Amersham). The primary antibodies used for detection were NCL (4E2), RPA194 (C-1), TIF-IA (Rrn3, C-20, Santa Cruz Biotechnology), TAFI110 (C-18, Santa Cruz Biotechnology). HRP-conjugated secondary antibodies and were from DAKO or Santa Cruz Biotechnology, HRP-conjugated streptavidin was from DAKO.
Treatment of surgery-derived prostate tissue ex vivo
Prostate tissues were isolated from patients undergoing prostatectomy at the Helsinki University Central Hospital with informed written consent and approval by Ethics Committee (#390/E6/06). A cylinder of 8 mm in diameter was cored out of the peripheral region of the prostate, and the tissues were sliced and cultured as detailed in refs. (30) and (31). All experimental treatments of the cultures were performed on days 1–2. The tissues were fixed, embedded in paraffin, and the blocks were sectioned and processed for immunohistochemistry as detailed in refs. (30) and (31) using NPM antibody (Zymed). Secondary anti-mouse conjugated Alexa-488 antibody was from Molecular Probes. The tissues were counterstained with Hoechst 33342 (Molecular Probes). The specimens were imaged using LSM 510 Meta confocal microscope, Plan-Neofluar 40x/1.3 Oil DIC objective and captured with LSM 3.2 software (Zeiss).
Statistical analysis
Statistical analysis was performed by Student’s t test. Differences were considered statistically significant at P<0.05.
Results
BMH-9, BMH-22 and BMH-23 cause segregation of the nucleolus and proteasome-dependent degradation of RPA194
Segregation of the nucleolus and nucleolar protein translocations are a hallmark of Pol I transcription stress (18, 20). To assess whether BMH-9, BMH-22 and BMH-23 (Fig. 1A) affect the integrity of the nucleolus, we treated A375 melanoma cells with these compounds at 10 μM concentration for 3 hours after which the cells were fixed and stained for fibrillar center and dense fibrillar component proteins UBF and FBL (Fig. 1B) and granular component proteins NPM and NCL (Fig. 1C). In all above assays we used Actinomycin D (ActD) as control at concentrations at which it inhibits Pol I (50 ng/ml).
Figure 1.
BMH-9, BMH-22 and BMH-23 cause nucleolar segregation and RPA194 degradation. A, Chemical structures of BMH-9, BMH-22 and BMH-23. B and C, A375 melanoma cells were incubated for 3 hours with BMH-9, BMH-22 and BMH-23 (10 μM), and ActD (50 ng/ml), fixed and stained for B, UBF and FBL and C, NPM and NCL. Representative single cell images of N = 5 experiments are shown. D, Cells were incubated for 3 hours with BMH-9, BMH-22 and BMH-23 (10 μM), and ActD (50 ng/ml), or were pretreated with proteasome inhibitor MG132 (10 μM) for 30 minutes, fixed and stained for RPA194. Representative images of N = 3 experiments are shown. Scale bars, 10 μm. E, Cells were treated as in D, and additionally, with Nutlin-3 (10 μM). Cell lysates were analyzed by Western blotting for RPA194, TAFI110 and TIF-IA. Representative experiment of N = 4 is shown. F, A375 cells were treated with cycloheximide (CHX) in the presence or absence of the BMH-molecules (10 μM) for the indicated times, analyzed by western blotting and quantified. RPA194 signals were normalized to NCL used as a loading control.
BMH-9, BMH-22 and BMH-23 caused segregation of UBF and FBL into nucleolar caps, as did Act D (Fig. 1B). NPM and NCL translocated from the nucleolus into nucleoplasm (Fig. 1C). These phenotypes are consistent with nucleolar stress.
We then analyzed the effect of BMH-9, BMH-22 and BMH-23 on Pol I complex catalytic core subunit RPA194. As analyzed by immunostaining and western blotting, BMH-9, BMH-22 and BMH-23 caused a marked decrease in RPA194 protein, BMH-22 and BMH-23 being most effective in this regard (Fig. 1D and E). ActD caused RPA194 nucleolar cap formation, and did not affect RPA194 protein (Fig. 1D and E). To assess whether the compounds caused RPA194 destabilization, the cells were incubated in the presence of the compounds and cycloheximide, and the turnover was determined. The results showed that BMH-9, BMH-22 and BMH-23 caused RPA194 destabilization in a manner similar to BMH-21 (Fig. 1F and Supplementary Fig. S1). Consistent with our earlier report on BMH-21 (9), the downregulation of RPA194 was dependent on proteasome activity, as its inhibition by MG132 rescued the decrease in RPA194 (Fig. 1D and E). The deregulation of protein stability by BMH-9, BMH-22 and BMH-23 was selective for RPA194 as Pol I preinitiation complex protein TAFI110 and a transcription initiation factor TIF-IA were unaffected (Fig. 1E). Nutlin-3, a small molecule MDM2 inhibitor that causes p53 stabilization and activation (5) did not affect RPA194 (Fig. 1E).
BMH-9, BMH-22 and BMH-23 inhibit RNA polymerase I transcription
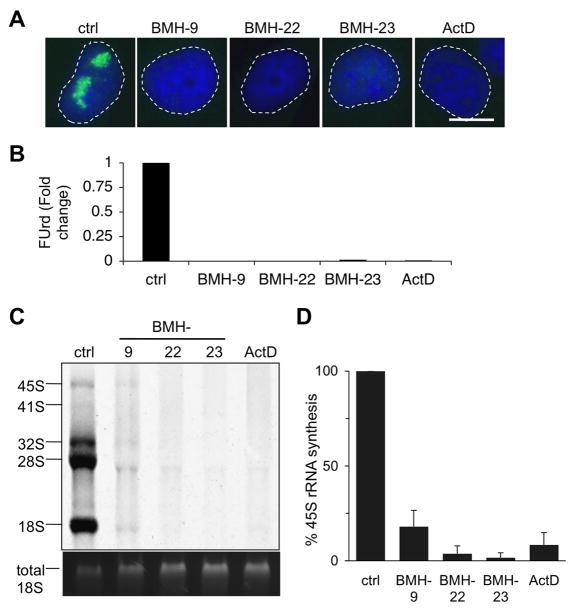
Segregation of the nucleolus reflects Pol I transcription stress. To assess the effects of BMH-9, BMH-22 and BMH-23 on Pol I activity we first analyzed de novo rRNA synthesis using 5-fluorouridine (5-FUrd) incorporation. The BMH-compounds caused potent inhibition of 5-FUrd incorporation (Fig. 2A and B). Further analysis using 3H-uridine metabolic labeling showed that BMH-9, BMH-22 and BMH-23 prominently inhibited the synthesis of the 45S precursor rRNA (Fig. 2C and D). Quantification of two independent experiments showed that the 45S precursor rRNA synthesis was inhibited by over 80% (Fig. 2D). These results demonstrate that BMH-9, BMH-22 and BMH-23 cause robust inhibition of Pol I transcription.
Figure 2.
BMH-9, BMH-22 and BMH-23 inhibit RNA polymerase I transcription. A, A375 cells were incubated for 3 hours with the indicated BMH-compounds (10 μM) and ActD (50 ng/ml) and de novo rRNA synthesis was detected by labeling the cells with FUrd for the last 30 minutes. B, Quantitative image analysis of FUrd incorporation. Fold change as compared to control set as 1 is shown. Representative experiment of N = 4 is shown. C, Metabolic labeling of the cells using 3H-uridine. A375 cells were treated with the compounds at concentrations indicated in A for 4 hours and labeled with 3H-uridine for the last 2 hours. Total RNA was isolated and mature and precursor rRNA forms were separated by electrophoresis. 45S form represents the precursor rRNA transcript. Total 18S is shown as loading control. D, Quantification of 45S rRNA precursor of N = 2 independent experiments.
Growth inhibitory activity of BMH-9, BMH-22 and BMH-23 in the NCI60 cancer cell lines
Our initial analysis of BMH-22 in a mouse model of B cell lymphoma showed its marked anti-tumorigenic potential, and that BMH-9, BMH-22 and BMH-23 decreased the viability of several cancer cell lines (8). Furthermore, testing for hematopoietic progenitor colony formation showed that BMH-9 and BMH-22 had negligible toxicity in this regard (8). In order to gain information of the anticancer properties of the compounds in a larger panel of tumor cell lines, we submitted BMH-9, BMH-22 and BMH-23 to the NCI Developmental Therapeutics Program NCI60 screen (32). The compounds demonstrated cytotoxic and cytostatic responses across the NCI60 cell panel with median growth inhibitory concentrations (GI50) of 4.1 μM, 4.3 μM and 2.0 μM for BMH-9, BMH-22 and BMH-23, respectively (Fig. 3A). Comparison of the effects of BMH-9 and BMH-22 in the NCI60 cancer cells to normal cells we analyzed previously (8) indicated better tolerance in the normal cells (Fig. 3B). However, BMH-23 had substantially more toxicity in normal cells indicating its less optimal properties (not shown). However, BMH-23 did not activate the DNA damage response as assessed by Ser139 H2AX and Ser824 KAP1 phosphorylation (Supplementary Fig. S2) and was in this regard similar to BMH-9, BMH-21 and BMH-22 (8).
Figure 3.
BMH-9, BMH-22 and BMH-23 activities in the NCI60 cancer cell panel. A, NCI Developmental Therapeutics Program NCI60 screen. Compound activities are presented as 50% growth inhibitory concentration (GI50) using the median GI50 value as y-axis. The respective median GI50 concentrations are shown in the panels. B, Box plot of GI50 values of BMH-9 and BMH-22 in NCI60 cell lines and normal human primary cells (as adopted from ref. 8).
Bioactivity of BMH-9 and BMH-22 in ex vivo-cultured human prostate tissue
We next studied the nucleolar stress response of the compounds in a human prostate tissue model (30, 31). Tissue biopsies obtained from volunteers undergoing radical prostatectomy were sectioned and maintained under culture. The tissues were treated with BMH-9 and BMH-22, fixed and processed for immunohistochemical staining of NPM. BMH-9 and BMH-22 caused prominent translocation of NPM from the nucleolus to nucleoplasm (Fig. 4), in line with that observed in cultured cells (Fig. 1C). This indicates that BMH-9 and BMH-22 are tissue permeable and cause relevant bioactivity reflecting the nucleolar stress response.
Figure 4.

Bioactivity of BMH-9 and BMH-22 in ex vivo cultured human prostate tissue. Ex vivo-cultured tissues were treated with BMH-9 and BMH-22 (20 μM) for 8 hours. Tissues were stained for NPM (green) and DNA (blue). Inset, 3x enlarged field. Scale bar 50 μm. Representative of N = 3 independent prostate tissues.
Chemical constraints of BMH-9 and BMH-22/23 analogs
The small molecule libraries chosen for our initial cell-based p53 activation screen presented high chemical diversity. We subsequently identified two structurally close analogs of BMH-9 (BMH-9_A1, BMH-9_A2) and one for BMH-22 and BMH-23 (BMH-22_A1) (Fig. 5A), and used them to assess chemical characteristics associated with their activity. BMH-9_A1, BMH-9_A2 did not affect the localization or stability of NCL and RPA194 (Fig. 5B), or that of p53 (not shown) indicating that they are inactive. BMH-22_A1, in which a methyl –group was substituted with an ethyl group, retained some activity as compared to the parent molecules BMH-22 and BMH-23 (Fig. 5B–D). These findings suggested that the parent core structures may be relatively tightly constrained.
Figure 5.
RPA194 degradation is a biological response unique to BMH-parent molecules. A, Chemical structures of BMH-9 and BMH-22/23 analogs. B, A375 cells were treated with the parent molecules and their analogs (10 μM) and incubated for 3 hours. Cells were stained for NCL and RPA194. Scale bar 10 μm. C and D, Dose-responses. A375 cells were treated with 2.5 and 10 μM concentrations of the parent molecules and analogs, fixed and stained for NCL and RPA194 followed by quantitative image analysis. Fold change as compared to control, set as 1, is shown.
p53 activator Nutlin-3 does not affect nucleolar integrity and is dispensable for BMH-9, BMH-22 and BMH-23 nucleolar effects
p53 has been shown to inhibit Pol I transcription by interfering with the preinitiation complex formation (33, 34). On the other hand, ample studies show that inhibition of ribosome biogenesis leads to p53 activation, placing p53 activation downstream of Pol I transcription blocks (25, 26). We studied the p53-dependency of the nucleolar stress response utilizing Nutlin-3. Although Nutlin-3 increased p53 protein it did not affect the localization of nucleolar proteins UBF, FBL, NPM and NCL (Supplementary Fig. S3A and S3B), RPA194 (Supplementary Fig. S3C and D), or decrease nascent rRNA synthesis (Supplementary Fig. S3E and F).
To analyze the dependency of the nucleolar responses by BMH-9, BMH-22 and BMH-23 on p53, we monitored these responses in a p53 null cell line SaOS-2. As shown in Fig. 6A, the BMH-compounds caused UBF cap formation, translocation of NCL from the nucleolus and degradation of RPA194 consistent with segregation of the nucleolus. Thus, the nucleolar stress response by the BMH-compounds was evident in the absence of p53.
Figure 6.
p53 is dispensable for nucleolar stress. A, SaOS-2 cells were treated with BMH-9, BMH-22 and BMH-23 (10 μM) and ActD (50 ng/ml) for 3 hours and stained for UBF, NCL and RPA194. Scale bars 10 μm. B, p53 isogenic HCT116 cells were treated with BMH-9, BMH-22, BMH-23 and Nutlin-3 (5 μM) for the indicated times and cells were counted. N = 2 experiments conducted in triplicate. Fold change to control are shown as mean ± SEM. C, SaOS-2 cells were treated with BMH-9, BMH-22, BMH-23 and Nutlin-3 (10 μM) or irradiated (IR) with 10 Gy and incubated for 72 hours. Cells were fixed and stained with propidium iodine and analyzed by flow cytometry. Cell cycle distribution was analyzed, and is shown in the inset. Separately, the fraction of sub-G1 cells was analyzed and is shown as percentage of cells in the total population. D, A375 cells were treated with BMH-9, BMH-22 and BMH-23 (0.5 μM and 5 μM) and in combination with Nutlin-3 (0.5 μM and 5 μM) and incubated for 24 hours. Viability was determined using the WST-1 assay. N = 2 experiments conducted in triplicate. Combination index (CI) was determined according to Chou-Talalay (35). Dashed line indicates additive effect, and values < 1 represent synergism. E, Schematic drawing of BMH-compound effects.
We have earlier shown using TP53 isogenic HCT116 cells that the compound cytotoxic activities are independent of p53, whereas BMH-9 demonstrated partial dependency (8). This was further tested here in a kinetic study where BMH-compounds were used at their near IC50 doses and cells were counted after 24 h, 72 h and 120 h. As shown in Fig. 6B, BMH-22 and BMH-23 decreased the number of HCT116 p53+/+ and p53−/− cells in a similar manner, whereas BMH-9 and Nutlin-3 were less effective in the p53−/− cells. In addition, we analyzed whether BMH-9 and BMH-22 affect cell cycle in p53 null SaOS-2 cells. Cells were treated with the compounds and incubated for 72 h. In comparison, cells were treated with Nutlin-3 and ionizing radiation (IR). Nutlin-3 had no discernible effect on the cell cycle distribution, whereas BMH-9 and BMH-22 increased the sub-G1 fraction of the cells and altered the distribution of S and G2/M phase cells (Fig. 6C). BMH-22 had more prominent effects in this regard. IR caused a profound G2/M phase arrest, as expected. These findings demonstrated that BMH-22, and BMH-23 in those assays that it was tested, acted in a p53 independent manner. To assess whether activation of p53 by Nutlin-3 synergizes with the BMH-compounds we co-treated the cells with increasing doses of the compounds and Nutlin-3, analyzed cell viability and determined the Chou-Talalay combination index (CI) (35). Synergism was detected between Nutlin-3 and BMH-23 (CI 0.628), and moderately with BMH-22 (0.776) (Fig. 6D).
Discussion
This paper describes novel small molecule lead structures for inhibition of RNA Pol I. BMH-9, a quinolinecarboxylate and BMH-22 and BMH-23, benzonaphthyridins, cause nucleolar stress represented by relocalization of nucleolar proteins, inhibition Pol I transcription and loss of RPA194. These activities are strikingly similar to the structurally distinct pyridoquinazolinecarboxamide BMH-21 that we described as first-in-kind Pol I inhibitor that activates RPA194 destruction (9). All molecules elicit broad anticancer activity across the NCI60 cancer lines. These findings support the notion that Pol I targeting effectively restricts cancer cell growth. The present study concludes the identification of activities of the six p53 activating molecules discovered in our screen (8) and shows that 4 of these act as Pol I inhibitors and share the exceptional property to destabilize RPA194.
Pol I transcription block, such as elicited by BMH-21, causes rapid segregation of the nucleolus and relocalization of nucleolar proteins (9). These changes occur temporally faster than stabilization of p53 (9). p53 is potently activated as a consequence of ribotoxic stress response by ribosomal and other nucleolar proteins (20, 23, 24–26). We show here that p53 is not required for the nucleolar stress responses by BMH-9, BMH-22 and BMH-23. Conversely, activation of p53 by Nutlin-3 did not affect Pol I transcription activity, stability of RPA194 or integrity of the nucleolus. Furthermore, there were marked cell cycle changes and increased apoptosis in p53 defective cells by the compounds. These findings indicate that first, p53 activation occurs downstream of Pol I inhibition (Fig. 6E), and secondly, that p53 provides little contribution to the compound cytotoxic activity in vitro except for BMH-9. However, considering that p53 has a multitude of potential tumor suppressive activities such as modulation of the innate immune system and inhibition of tumor metastasis, some of these activities may provide a significant benefit in the in vivo setting. Interestingly, co-treatment of cells with BMH-9, BMH-22 and BMH-23 and Nutlin-3 showed synergism, especially with BMH-23, suggesting the p53 pathways activated by inhibition of Pol I and MDM2 may not fully overlap.
In our earlier studies we showed that BMH-22 significantly represses B cell lymphoma tumor growth and that BMH-9 and BMH-22 did not alter the histology of normal mouse tissues or reduce colony formation of hematopoietic progenitors (8). We show here that treatment of human prostate tissues derived from radical prostatectomies with BMH-9 and BMH-22 cause efficient nucleoplasmic translocation of NPM, indicating that highly expressed nucleolar proteins, such as NPM and NCL may serve as useful and sensitive biodynamic markers for on-target monitoring. These are good indicators for the BMH-9 and BMH-22 bioavailability, potential tolerance and support the design of preclinical studies testing the compound efficacy and bioactivity. BMH-23, however, was not well tolerated in normal cells.
Interestingly, data collected on small molecule bioactivity screens and available in the NCBI PubChem Bioassays show that BMH-22 and BMH-23 have been tested in over 600 assays each, and scored as hits in 55 and 89 assays, respectively. In assays for cancer-relevant pathways, both have frequently scored as hits in assays where the readouts have involved cell viability or DNA binding. However, the compounds have not scored positive when they have progressed to secondary, validation or confirmatory screens. In other words, to the best of our knowledge, BMH-22 and BMH-23 are yet to be ascribed with other properties besides their action as DNA intercalators, cytotoxicity towards cancer cells, and, as shown by our studies, inhibition of Pol I.
BMH-22 and BMH-23 differ chemically by only a single methyl group, and BMH-22_A1 by an ethyl group. Of these, BMH-23 demonstrated higher activity than either BMH-22 or BMH-22_A1, indicating that it is more potent, and also more toxic. BMH-9 represents a chemically distinct molecule but has a dimethylaminopropylamino arm resembling that of the dimethylcarboxamide arm in BMH-21. BMH-9 analogs A_1 and A_2, in which the arm was modified by shortening the carbon linker or by introducing a bulky pyridine ring were both inactive in their ability to activate the nucleolar stress response. This suggests that the arm imparts significant bioactivity of the molecule.
The present study identifies two structurally new molecules, BMH-9 and close homologs BMH-22 and BMH-23 for inhibition of Pol I. Earlier efforts have brought forward the molecule CX-5461, which appears to inhibit Pol I preinitiation complex formation and is structurally and mechanistically distinct from the BMH-compounds (9, 37). It has also shown promising activity in preclinical trials and has entered a phase I clinical trial in Australia (36, 37). In addition, many cancer therapeutics, especially topoisomerase I and II poisons inhibit Pol I transcription by causing torsional stress of the rDNA (38). These activities will be relevant to consider and monitor for their potential therapeutic advantage.
Supplementary Material
Acknowledgments
Financial support: Academy of Finland (251307), Johns Hopkins University start-up funds, NIH P30 CA006973, NIH P50 CA058236 and NIH 1R01 CA172069 (to M. Laiho) and Biomedicum Helsinki Foundation, Cancer Society Finland and Finnish Cultural Foundation (to K. Peltonen).
We thank the NCI Developmental Therapeutics Program for performing the NCI60 cell line screen. Kaisa Penttilä is thanked for excellent technical assistance and Biomedicum Imaging Unit (University of Helsinki) for imaging services.
Footnotes
Disclosure of Potential Conflicts of Interest: All authors declare no potential conflicts of interest.
Authors’ Contributions
Conception and design: K. Peltonen, M. Laiho.
Acquisition of data: K. Peltonen, H. Liu, L. Colis, P. Sirajuddin, S. Jäämaa, Z. Zhang, T. af Hällström, H.M. Moore
Analysis and interpretation of data: K. Peltonen, L. Colis, P. Sirajuddin, M. Laiho
Writing and review of the manuscript: K. Peltonen, M. Laiho
Approval of the final version of the paper: All authors
References
- 1.Wiman KG. Restoration of wild-type p53 function in human tumors: strategies for efficient cancer therapy. Adv Cancer Res. 2007;97:321–38. doi: 10.1016/S0065-230X(06)97014-6. [DOI] [PubMed] [Google Scholar]
- 2.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–73. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 3.Wiman KG. Pharmacological reactivation of mutant p53: from protein structure to the cancer patient. Oncogene. 2010;29:4245–52. doi: 10.1038/onc.2010.188. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann S, Bykov VJ, Ali D, Andrén O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633–9. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 5.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 6.Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73:2587–97. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Bernard D, Wang S. Small molecule inhibitors of MDM2-p53 and MDMX-p53 interactions as new cancer therapeutics. BioDiscovery. 2013;8:4. [Google Scholar]
- 8.Peltonen K, Colis L, Liu H, Jäämaa S, Moore HM, Enbäck J, et al. Identification of novel p53 pathway activating small-molecule compounds reveals unexpected similarities with known therapeutic agents. PLoS One. 2010;5:e12996. doi: 10.1371/journal.pone.0012996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltonen K, Colis L, Liu H, Trivedi R, Moubarek MS, Moore HM, et al. A targeting modality for destruction of RNA Polymerase I that possesses anticancer activity. Cancer Cell. 2014;25:77–90. doi: 10.1016/j.ccr.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grummt I. Wisely chosen paths--regulation of rRNA synthesis. FEBS J. 2010;277:4626–39. doi: 10.1111/j.1742-4658.2010.07892.x. [DOI] [PubMed] [Google Scholar]
- 11.Drygin D, Rice WG, Grummt I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol. 2010;50:131–56. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 12.Bywater MJ, Pearson RB, McArthur GA, Hannan RD. Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nat Rev Cancer. 2013;13:299–314. doi: 10.1038/nrc3496. [DOI] [PubMed] [Google Scholar]
- 13.Russell J, Zomerdijk JC. The RNA polymerase I transcription machinery. Biochem Soc Symp. 2006;73:203–16. doi: 10.1042/bss0730203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorski SA, Snyder SK, John S, Grummt I, Misteli T. Modulation of RNA polymerase assembly dynamics in transcriptional regulation. Mol Cell. 2008;30:486–97. doi: 10.1016/j.molcel.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanovsky V, Langlois F, Gagnon-Kugler T, Rothblum LI, Moss T. Growth factor signaling regulates elongation of RNA polymerase I transcription in mammals via UBF phosphorylation and r-chromatin remodeling. Mol Cell. 2006;21:629–39. doi: 10.1016/j.molcel.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 16.McStay B, Grummt I. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu Rev Cell Dev Biol. 2008;24:131–57. doi: 10.1146/annurev.cellbio.24.110707.175259. [DOI] [PubMed] [Google Scholar]
- 17.Pederson T. The nucleolus. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a000638. pii: a000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Verdun D, Roussel P, Thiry M, Sirri V, Lafontaine DL. The nucleolus: structure/function relationship in RNA metabolism. Wiley Interdiscip Rev RNA. 2010;1:415–31. doi: 10.1002/wrna.39. [DOI] [PubMed] [Google Scholar]
- 19.Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, et al. Nucleolar proteome dynamics. Nature. 2005;433:77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- 20.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–27. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore HM, Bai B, Boisvert FM, Latonen L, Rantanen V, Simpson JC, et al. Quantitative proteomics and dynamic imaging of the nucleolus reveal distinct responses to UV and ionizing radiation. Mol Cell Proteomics. 2011;10:M111.009241. doi: 10.1074/mcp.M111.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shav-Tal Y, Blechman J, Darzacq X, Montagna C, Dye BT, Patton JG, et al. Dynamic sorting of nuclear components into distinct nucleolar caps during transcriptional inhibition. Mol Biol Cell. 2005;16:2395–413. doi: 10.1091/mbc.E04-11-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–77. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurki S, Peltonen K, Latonen L, Kiviharju TM, Ojala PM, Meek D, et al. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell. 2004;5:465–75. doi: 10.1016/s1535-6108(04)00110-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16:369–77. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bursac S, Donati G, Brdovcak MC, Volarevic S. Activation of the tumor suppressor p53 upon impairment of ribosome biogenesis. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.08.014. pii:S0925-4439(13)00306-2. [DOI] [PubMed] [Google Scholar]
- 27.Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- 28.Montanaro L, Treré D, Derenzini M. Nucleolus, ribosomes, and cancer. Am J Pathol. 2008;173:301–10. doi: 10.2353/ajpath.2008.070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore HM, Bai B, Matilainen O, Colis L, Peltonen K, Laiho M. Proteasome activity influences UV-mediated subnuclear localization changes of NPM. PLoS One. 2013;8:e59096. doi: 10.1371/journal.pone.0059096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jäämaa S, Af Hällström TM, Sankila A, Rantanen V, Koistinen H, Stenman UH, et al. DNA damage recognition via activated ATM and p53 pathway in nonproliferating human prostate tissue. Cancer Res. 2010;70:8630–41. doi: 10.1158/0008-5472.CAN-10-0937. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Yang Z, Jäämaa S, Liu H, Pellakuru LG, Iwata T, et al. Differential epithelium DNA damage response to ATM and DNA-PK pathway inhibition in human prostate tissue culture. Cell Cycle. 2011;10:3545–53. doi: 10.4161/cc.10.20.17841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–23. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 33.Budde A, Grummt I. p53 represses ribosomal gene transcription. Oncogene. 1999;18:1119–24. doi: 10.1038/sj.onc.1202402. [DOI] [PubMed] [Google Scholar]
- 34.Zhai W, Comai L. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol Cell Biol. 2000;20:5930–8. doi: 10.1128/mcb.20.16.5930-5938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 36.Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C, et al. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011;71:1418–30. doi: 10.1158/0008-5472.CAN-10-1728. [DOI] [PubMed] [Google Scholar]
- 37.Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burger K, Mühl B, Harasim T, Rohrmoser M, Malamoussi A, Orban M, et al. Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem. 2010;285:12416–25. doi: 10.1074/jbc.M109.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.