Abstract
Objective
Investigate significant new morbidities associated with pediatric critical care.
Design
Randomly selected, prospective cohort
Setting
PICU patients from 8 Medical and Cardiac PICUs.
Patients
This was a randomly selected, prospective cohort of PICU patients from 8 Medical and Cardiac PICUs.
Measurements and Main Results
The main outcomes measures were hospital discharge functional status measured by Functional Status Scale (FSS) scores and new morbidity defined as an increase in the FSS of ≥ 3. Of the 5017 patients, there were 242 new morbidities (4.8%), 99 PICU deaths (2.0%) and 120 hospital deaths (2.4%). Both morbidity and mortality rates differed (p<.001) among the sites. The worst functional status profile was on PICU discharge and improved on hospital discharge. On hospital discharge, the good category decreased from a baseline of 72% to 63%, mild abnormality increased from 10% to 15%, moderate abnormality status increased from 13% to 14%, severe status increased from 4% to 5% and very severe was unchanged at 1%. The highest new morbidity rates were in the neurological diagnoses (7.3%), acquired cardiovascular disease (5.9%), cancer (5.3%) and congenital cardiovascular disease (4.9%). New morbidities occurred in all ages with more in those under 12 months. New morbidities involved all FSS domains with the highest proportions involving respiratory, motor, and feeding dysfunction.
Conclusions
The incidence of new morbidity was 4.8%, twice the mortality rate, and occurred in essentially all types of patients, in relatively equal proportions, and involved all aspects of function. Compared to historical data, it is possible that pediatric critical care has exchanged improved mortality rates for increased morbidity rates.
Keywords: morbidity, functional status, functional status score, pediatrics, outcome prediction, critical care, pediatric critical care, intensive care, pediatric intensive care
Introduction
The development of new morbidities from pediatric intensive care illnesses and therapies is a fundamental yet relatively unexplored outcome measure of pediatric intensive care. It is generally believed that many illnesses requiring admission to the pediatric intensive care unit (PICU) and their therapies result in new morbidity. While there is some condition-specific information on new morbidities associated with PICU illnesses,1–5 there is surprisingly little general PICU population information on the development of new morbidities and these data are over a decade old.6,7 For example, little is known about the diagnoses, operative status, and ages at greatest risk for the development of new morbidities. Fiser et al. in the 1990’s tabulated the disability status (Pediatric Overall and Cerebral Performance Categories (POPC, PCPC)) of admissions and discharges from the PICU. She found that there was a 7.7% increase of at least 2 POPC categories, including a 4.6% death rate, equating to a significant new morbidity rate of 3.1%.7
The aims of this report are to investigate the baseline and hospital discharge functional status of children admitted to the PICU and to describe the general characteristics of patients who developed a new morbidity. Recently, the Collaborative Pediatric Critical Care Research Network (CPCCRN) developed and validated the Functional Status Scale (FSS) to measure the development of new morbidities.8 The FSS was developed to add objectivity, increase granularity and improve quantification of morbidities and is particularly designed for use in large scale studies.9
Methodology
The current investigation was performed at the 7 sites (8 PICUS) in the CPCCRN. These sites have approximately 17,000 PICU admissions per year.10 The details of patient selection and data collection have been published.9,11 In brief, only the first PICU admission was included. Patients ranging in ages from newborn to less than 18 years were randomly selected from both the General/Medical PICUs and Cardiac/Cardiovascular PICUs. There were no separate general surgical or neurological PICUs. This report includes the initial 5017 patients from a larger data collection and included all enrolled patients from the first day of the study (December 4, 2011) to the day when the 5000th patient was enrolled (August 2, 2012). The protocol was approved by the Institutional Review Boards at all participating institutions.
Data for this analysis included diagnostic and demographic data and FSS scores determined at PICU admission to assess baseline (pre-hospital admission) status and status at PICU and hospital discharge. Baseline FSS status was determined from the medical records supplemented by caretaker knowledge as needed to reflect chronic functional status prior to the acute illness. Researchers, research coordinators, and research assistants were trained in data collection with in-person training on multiple occasions and conducted bi-weekly teleconference calls. Diagnoses were classified by the system of dysfunction accounting for the primary reason for PICU admission. Since a previous publication on this sample,9 we are able to better categorize some of the miscellaneous classifications resulting in small changes in the numbers of diagnoses. Operative status included both operating room and interventional catheterization procedures but not diagnostic catheterization procedures.
The FSS was developed to provide assessment of functional status suitable for large studies. It is composed of 6 domains (mental status, sensory, communication, motor function, feeding, respiratory) with domain scores ranging from 1 (normal) to 5 (very severe dysfunction). Therefore, total scores may range from 6 to 30 with lower scores indicating better function. The operational definitions and manual for the classifications have been published.8 The FSS validation consisted of comparison to the ABAS II, a validated measure of pediatric adaptive behavior, and comparison to the pediatric performance scales, the Pediatric Cerebral and Overall Performance Categories (PCPC/POPC).8,9 For this analysis, we categorized FSS scores of 6–7 as good, 8–9 as mildly abnormal, 10–15 as moderately abnormal, 16–21 as severely abnormal, and > 21 as very severely abnormal. These category ranges were chosen based on the dysfunction reflected in the score and to be the approximately equivalent FSS score range that corresponded to the POPC categories.9 Newborns who had never achieved a stable baseline of function were assigned an FSS = 6; this was operationalized by assigning a baseline FSS score of 6 to all infant admissions from 0–2 days of age and to transfers from another facility for infants from 3–6 days of age. Significant, new morbidity was defined as worsening of FSS of 3 or greater from baseline to hospital discharge. This definition was based on a consensus perception of the importance of the change(s), and this was the change in mean FSS scores between the normal and moderate disability categories of the POPC.9 Since this was the initial use of the FSS to define new morbidities, we evaluated the change in individual FSS domains and the magnitude of that change for both patients with a worsening FSS of 3 or greater and 2 or less.
Data are expressed as mean +/− standard deviation. Comparison of data across categories utilized the Pearson chi-square test and the Mantel-Haenszel chi-square test. The assessment of association between morbidity and mortality rates utilized the Pearson correlation.
Results
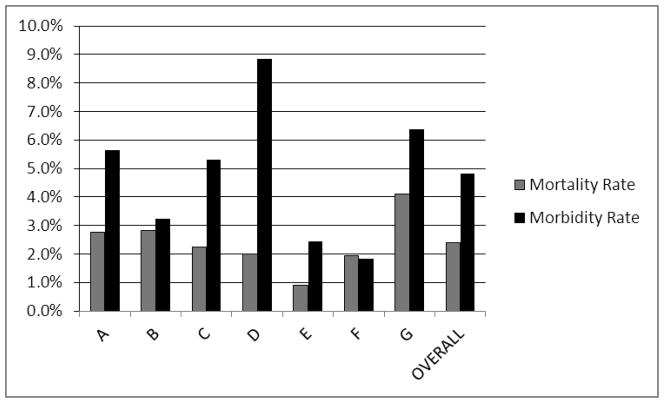
There were 5017 patients and sites contributed from 619 (12%) to 808 (16%) of the sample. Figure 1 shows the morbidity and mortality rates by site and overall. Significant new morbidity occurred in 242 (4.8%). There were 99 PICU deaths (2.0% PICU mortality rate) and 120 hospital deaths (2.4% hospital mortality rate). There was a significant difference in both morbidity p<.0001) and mortality (p=.009) rates among the sites; these rates differed by over 300% between the lowest and the highest sites and did not significantly correlate (r = .38, p=.40). Overall, significant new morbidity occurred in all FSS baseline categories (Figure 2). There was no significant difference among the rates of new morbidities for survivors admitted in the various baseline FSS categories (p > .8). Patients had a median age of 3.7 years (25th, 75th quartile 0.8, 10.9) and stayed in the PICU a median of 2.0 days (25th, 75th quartile 1.0, 4.8).
Figure 1. Morbidity and Mortality Rates by Site.
There was a significant difference among the sites for both morbidity (p<.0001) and mortality (p=.009) rates.
Figure 2. New Morbidities (FSS ≥ 3) as a Percentage of Baseline FSS Categories.
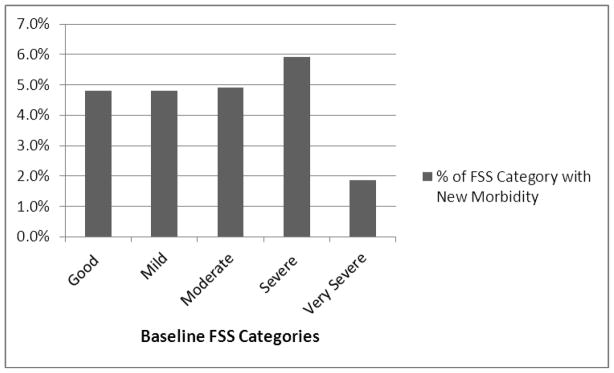
There was no significant difference among the rates of new morbidities for survivors admitted in the baseline FSS categories (p>.8).
Functional status categories at baseline and hospital discharge are shown in Figure 3. The worst functional status profile was on discharge from the PICU but improved on hospital discharge. On hospital discharge, the good category decreased from a baseline of 72% to 63%, mild abnormality increased from a baseline of 10% to 15%, moderate abnormality status increased from 13% to 14%, severe status increased from 4% to 5% and very severe was unchanged at 1%.
Figure 3. FSS Categories at Baseline, PICU Discharge, and Hospital Discharge.
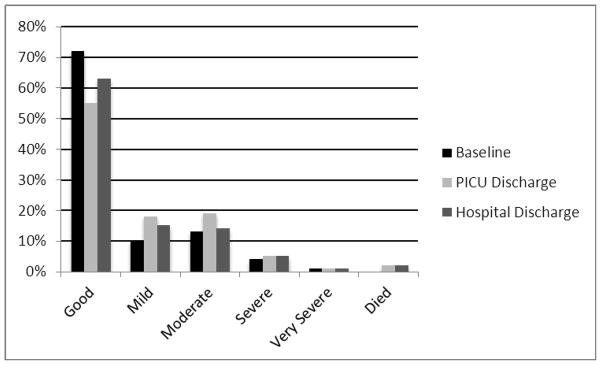
See text for details.
Of the patients classified with new morbidities, 109 (45.0%) had a worsening of 3 levels or more FSS levels in at least one FSS domain, 122 (50.4%) had a worsening of 2 FSS levels in 1 or more FSS domains but no change of 3 or more, and only 11 (4.5%) had a worsening of only 1 level in 3 or more FSS domains. Table 1 shows the new morbidities by diagnoses classified by the physiological system of primary dysfunction. Overall, 31% of patients were admitted with respiratory disease, 21% with neurological disease, and 25% with acquired and congenital heart disease. There were significantly different morbidity rates among the diagnoses (p=.0005) with the highest new morbidity rates in the neurological diagnoses (7.3%), acquired cardiovascular disease (5.9%), cancer (5.3%) and congenital cardiovascular disease (4.9%).
Table 1. New Morbidities by Admission Diagnosis.
Admission diagnoses are classified by system of primary dysfunction. The new morbidity rates among the diagnoses were significantly different (p < .0001).
| System of Primary Dysfunction | N (% of Sample) | New Morbidity (n) | New Morbidity (% of Diagnosis) |
|---|---|---|---|
| Respiratory | 1563 (31%) | 67 | 4.3% |
| Neurological | 1031 (21%) | 75 | 7.3% |
| Cardiovascular Disease - Congenital | 955 (19%) | 47 | 4.9% |
| Cardiovascular Disease - Acquired | 323 (6%) | 19 | 5.9% |
| Cancer | 247 (5%) | 13 | 5.3% |
| Musculoskeletal Condition | 219 (4%) | 7 | 3.2% |
| Gastrointestinal disorder | 183 (4%) | 6 | 3.3% |
| Endocrine | 146 (3%) | 0 | 0% |
| Renal | 52 (1%) | 1 | 1.9% |
| Miscellaneous | 298 (6%) | 7 | 2.3% |
The operative categories are shown in Table 2. A total of 40% of the sample were operative patients with a rate of new morbidities of 3.5%. The new morbidities rates were significantly different (p =< .003) with the highest rates of new morbidities occurring in the non-operative patients (5.7%) and general surgery patients (5.7%) followed by cardiac surgery (4.5%). Neurosurgical patients had an incidence of new morbidities of only 3.1%. New morbidities occurred in all age categories with more in those under 12 months than in those over 12 months of age (Table 3) and these rates were also significantly different among the age categories (p < .0001).
Table 2. New Morbidities by Operative Category.
The new morbidity rates among the operative systems were significantly different (p < .005).
| Operative System | N (%) | New Morbidities (n (% of Category)) |
|---|---|---|
| No Operation | 3025 (60%) | 173 (5.7%) |
| Cardiac | 755 (15%) | 34 (4.5%) |
| Neurosurgery | 353 (7%) | 11 (3.1%) |
| ENT | 285 (6%) | 3 (1.1%) |
| Orthopedic | 181 (4%) | 5 (2.8%) |
| General surgery | 176 (4%) | 10 (5.7%) |
| Interventional catheterization | 72 (1%) | 2 (2.8%) |
| Other | 170 (3%) | 4 (2.4%) |
Table 3. New Morbidities by Age Categories.
The new morbidity rates among the age categories were significantly different (p < .0001).
| Age at PICU Admission | n (%) | New Morbidities (n (% of Category)) |
|---|---|---|
| 0 day to < 7 days | 167 (3%) | 15 (9.0%) |
| 7 days to <14 days | 92 (2%) | 10 (10.9) |
| 14 days to < 1 month | 71 (1%) | 4 (5.6%) |
| 1 month to < 12 months | 1060 (21%) | 68 (6.4%) |
| 12 months to < 60 months | 1429 (28%) | 62 (4.3%) |
| 60 months to < 144 months | 1094 (22%) | 42 (3.8%) |
| >= 144 months | 1104 (22%) | 41 (3.7%) |
New morbidities occurred in all of the FSS domains. Table 4 shows the domains and numbers of patients where there was a worsening in domain scores of 3 levels or more, 2 and 1 for those patients with new morbidities, and those patients whose FSS scores worsened by 2 but were not classified with new morbidities. For the patients with new morbidities and an increase of 3 or more in the domain scores, the largest numbers occurred in the respiratory (21.1%) and motor (14.5%) domains. For patients with new morbidities and domain increases of 2, the largest numbers occurred in the feeding (47.9%) and motor (34.3%) domains. Only 6 patients (0.1%) had increases in domain scores of 3 or more but were not classified with new morbidities because of improvements in another domain. Of the patients that had worsening of 2 levels in their domain score but were not classified with a new morbidity, most occurred in the feeding and motor domains. A total of 106 patients improved their FSS by 2 or more and improved in a single domain by 2 or more. Most improvement occurred in the feeding, motor, and respiratory domains.
Table 4.
Functional Status Scale (FSS) Domain Changes in Patients with Increasing FSS Scores.
| Domain Change ≥ 3 Levels | Domain Change = 2 Levels | Domain Change = 1 Level | |
|---|---|---|---|
| Patients With New Morbidities (FSS ≥ 3, n = 242) | |||
| FSS Domain Mental Status | 19 (7.9%) | 21 (8.7%) | 59 (24.4%) |
| FSS Domain Motor | 35 (14.5%) | 83 (34.3%) | 23 (9.5%) |
| FSS Domain Sensory | 16 (6.6%) | 14 (5.8%) | 34 (14.1%) |
| FSS Domain Respiratory | 51 (21.1%) | 14 (5.8%) | 45 (18.6%) |
| FSS Domain Feeding | 22 (9.1%) | 116 (47.9%) | 18 (7.4%) |
| FSS Domain Communication | 16 (6.6%) | 21 (8.7%) | 72 (29.8%) |
| Patients Without New Morbidities But FSS Increase (n = 660) | |||
| FSS Domain Mental Status | 0 | 5 (0.8%) | 55 (8.3%) |
| FSS Domain Motor | 0 | 92 (13.9%) | 106 (16.1%) |
| FSS Domain Sensory | 0 | 5 (0.8%) | 41 (6.2%) |
| FSS Domain Respiratory | 3 (0.5%) | 19 (2.9%) | 88 (13.3%) |
| FSS Domain Feeding | 2 (0.3%) | 188 (28.5%) | 63 (9.6%) |
| FSS Domain Communication | 1 (0.2%) | 4 (0.6%) | 46 (7.0%) |
Discussion
New, significant morbidities resulting from the illnesses and therapies in the PICU are common and occur in essentially all types of patients in relatively equal proportions. The incidence of new morbidity was 4.8%, twice the mortality rate. The rate of new morbidity was 4.5% to 6% in patients with good, mildly abnormal, moderately abnormal and severely abnormal baseline status. While the incidence of new morbidity was only 1.9% in very severely abnormal children at baseline, this lower incidence likely was observed because these patients already had very severe dysfunction. New morbidities developed in all common diagnostic groups with the highest rates in neurological and acquired cardiovascular disease. While new morbidities developed in non-operative patients more than operative patients (5.7% vs 3.5%), they also occurred in almost all operative groups with the highest rates in cardiac surgery and general surgery, and in only 3.1% of neurosurgical patients. Finally, while new morbidities occurred more often in infants, they occurred in all age groups.
Both morbidity and mortality rates differed by more than 300% among the sites. Mortality rate differences among sites are well known and can be adjusted for by physiological status and other patient descriptors.12,13 Such adjustment has formed the foundation for many comparative and quality studies in critical care. It is not yet known whether morbidity rate differences can be accounted for with similar or different independent variables that will enable us to incorporate morbidity into studies investigating the importance of care factors in patient outcomes or quality studies. In this small sample of sites, morbidity and mortality rates were not strongly correlated indicating that incorporating morbidity into outcome models may uncover new associations and expand our understanding of factors associated with the best outcomes from pediatric critical care.
Morbidity assessments are appropriately becoming a more important aspect of pediatric outcomes research.14–19 We defined morbidity broadly because the effects of acute conditions and their therapies can affect many different organ systems. We chose functional status because it is conceptually similar to adaptive behavior which corresponds to activities of daily living, a commonly used and practical measure in adults.20 Other investigators may focus on different definitions of morbidity depending on their research needs. Recently, the use of health related quality of life instruments have become more common place; these methods are often based in large part on the health burden of functional disabilities so the FSS represents a more proximate measure of a similar outcome.21–23 Notably, there are hurdles to overcome when classifying children. First, functional status assessments that are reliable at the level of the individual are time consuming and require considerable training; therefore they are not practical for most large sample-size studies.24–27 Second, pediatric functional status assessment methods must incorporate the rapidly changing norms of growth and development, making them difficult to design and complex to develop.28,29 The FSS, designed to be used in large sample size studies, performed well with regard to both adaptive behavior methods and the pediatric scales based on the Glasgow Outcome Scale and was successfully implemented in this multi-center study.8,9,30 We chose the FSS categories to be approximately equivalent to the POPC categories which have been used in other large pediatric studies.31,32
The FSS definition of significant morbidity of an increase of 3 or more worked well. A total of 95% of diagnoses had a worsening of at least two levels in at least one FSS domain (good to moderate, mild to severe, moderate to very severe). These changes occurred in all of the FSS domains with a predominance of respiratory and motor for the domains that changed 3 or more levels and feeding and motor for those changing 2 levels.
Our data compared with the historical data suggest that pediatric critical care may have exchanged mortality for morbidity over the last several decades. While it is not possible to precisely compare the rates over time because of the different research methods, data from the 1990’s31 demonstrated a PICU mortality rate of 4.6% and a PICU morbidity rate of 3.1% (based on a 2 or greater POPC change) while our data had a reversal of these percentages with a hospital mortality rate of 2.4% and morbidity rate of 4.8%. Thus, the “morbidity and mortality rate” decreased only from 7.7% to 7.2%. Since these rates are not severity or risk adjusted, the changes in admission criteria as well as other factors which have occurred in the last several decades could also significantly influence this comparison.
Conclusion
New, significant morbidities associated with pediatric critical care are common (4.8%) and occur in essentially all types of patients. Since reducing morbidity as well as mortality is focus of medical initiatives, this rate is an important benchmark. There was significant inter-site variability in the unadjusted morbidity rates. It is possible that further investigation of the differences in morbidity rates could result in advances in the structure and process of pediatric critical care in a manner similar to the advances based on mortality rate differences. Our data compared with the historical data suggests that pediatric critical care may have exchanged mortality for morbidity over the last several decades.
Acknowledgments
The Authors wish to acknowledge the contributions of the following individuals:
Teresa Liu, MPH, CCRP; University of Utah
Jean Reardon, MA, BSN, RN; Children’s National Medical Center
Elyse Tomanio, BSN, RN; Children’s National Medical Center
Morella Menicucci, MD, CCRP; Children’s National Medical Center
Fidel Ramos, BA; Children’s National Medical Center
Aimee Labell, MS, RN; Phoenix Children’s Hospital
Jeffrey Terry, MBA; Children’s Hospital Los Angeles
Margaret Villa, RN; Children’s Hospital of Los Angeles and Mattel Children’s Hospital
Jeni Kwok, JD; Children’s Hospital of Los Angeles
Amy Yamakawa, BS; Children’s Hospital of Los Angeles
Ann Pawluszka, BSN, RN; Children’s Hospital of Michigan
Mary Ann DiLiberto, BS, RN, CCRC; Children’s Hospital of Philadelphia
Carolann Twelves, BSN, RN; Children’s Hospital of Philadelphia
Monica S. Weber, RN, BSN, CCRP; University of Michigan
Lauren Conlin, BSN, RN, CCRP; University of Michigan
Alan C. Abraham, BA, CCRC; University of Pittsburgh Medical Center
Jennifer Jones, RN; University of Pittsburgh Medical Center
Jeri Burr, MS, RN-BC, CCRN; University of Utah
Carol Nicholson, MD
Funding Source: Supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: U10HD050096, U10HD049981, U10HD049983, U10HD050012, U10HD063108, U10HD063106, U10HD063114 and U01HD049934.
Abbreviations
- FSS
Functional Status Scale
- POPC
Pediatric Overall Performance Category
- PCPC
Pediatric Cerebral Performance Category
- PICU
Pediatric Intensive Care Unit
Footnotes
Conflict of Interests: There are no conflicts of interest to disclose.
Financial Disclosures: There are no disclosures.
Contributors’ Statement Page
Murray M. Pollack, MD: Dr. Pollack lead in the conceptualization, and design, oversaw the analysis and interpretation, and was primarily responsible for manuscript preparation and approved the final manuscript.
Richard Holubkov, PhD: Dr. Holubkov participated in the conceptualization and design, directed the analysis and interpretation, and participated in manuscript preparation and approved the final manuscript.
Tomohiko Funai, MS; Mr. Funai participated in the conceptualization and design, conducted the analyses, participated in interpretation, and participated in manuscript preparation and approved the final manuscript.
Amy Clark, MS; Ms. Clark participated in the conceptualization and design, conducted the analyses and participated in the interpretation, participated in manuscript preparation and approved the final manuscript.
John T. Berger, MD: Dr. Berger participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript and supervised data collection at one site.
Kathleen Meert, MD: Dr. Meert participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Christopher J. L. Newth, MD, FRCPC: Dr. Newth participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Thomas Shanley, MD: Dr. Shanley participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Frank Moler, MD: Dr. Moler participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Joseph Carcillo, MD;. Dr. Carcillo participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Robert A. Berg, MD: Dr. Berg participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Heidi Dalton, MD: Dr. Dalton participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
David L. Wessel, MD: Dr. Wessel participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Rick E. Harrison, MD: Dr. Harrison participated in the conceptualization and design, analysis and interpretation, manuscript preparation, approved the final manuscript, and supervised data collection at one site.
Allan Doctor, MD: Dr. Doctor participated in the conceptualization and design, analysis and interpretation, manuscript preparation and approved the final manuscript.
J. Michael Dean, MD: Dr. Dean co-lead in the conceptualization, and design, oversaw the analysis, participated in the data interpretation, participated in manuscript preparation and approved the final manuscript.
Tammara L. Jenkins, MSN, RN: Ms. Jenkins participated in the conceptualization and design, analysis and interpretation, manuscript preparation and approved the final manuscript.
Contributor Information
Murray M. Pollack, Department of Child Health, Phoenix Children’s Hospital and University of Arizona College of Medicine-Phoenix, Phoenix, AZ.
Richard Holubkov, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Tomohiko Funai, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Amy Clark, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
John T. Berger, Department of Pediatrics, Children’s National Medical Center, Washington DC.
Kathleen Meert, Department of Pediatrics, Children’s Hospital of Michigan, Detroit, MI.
Christopher J. L. Newth, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital Los Angeles, Los Angeles, CA.
Thomas Shanley, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
Frank Moler, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
Joseph Carcillo, Department of Critical Care Medicine, Children’s Hospital of Pittsburgh, Pittsburgh, PA.
Robert A. Berg, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA.
Heidi Dalton, Department of Child Health, Phoenix Children’s Hospital and University of Arizona College of Medicine-Phoenix, Phoenix, AZ.
David L. Wessel, Department of Pediatrics, Children’s National Medical Center, Washington DC.
Rick E. Harrison, Department of Pediatrics, University of California at Los Angeles, Los Angeles, CA.
Allan Doctor, Departments of Pediatrics and Biochemistry, Washington University School of Medicine, St. Louis, MO.
J. Michael Dean, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Tammara L. Jenkins, Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), the National Institutes of Health (NIH), Bethesda, MD. for the NICHD Collaborative Pediatric Critical Care Research Network (CPCCRN).
References
- 1.Gabbe BJSP, Sutherland AM, Palmer CS, Williamson OD, Butt W, Bevan C, Cameron PA. Functional and health-related quality of life outcomes after pediatric trauma. Journal of Trauma-Injury Infection & Critical Care. 2011;70(6):1532–1538. doi: 10.1097/TA.0b013e31820e8546. [DOI] [PubMed] [Google Scholar]
- 2.Marino BSTR, Wernofsky G, Drotar D, Newburger JW, Mahony L, Mussatto K, Tong E, Cohen M, Andersen C, Shera D, Khoury PR, Wray J, Gaynor JW, Helfaer MA, Kazak AE, Shea JA Pediatric Cardiac Quality of Life Inventory Testing Study Consortium. Validation fo the pediatric cardiac quality of life inventory. Peditrics. 2010;126(3):498–508. doi: 10.1542/peds.2009-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres A, Jr, Pickert CB, Firestone J, Walker WM, Fiser DH. Long-term functional outcome of inpatient pediatric cardiopulmonary resuscitation. Pediatric Emergency Care. 1997;16(6):369–373. doi: 10.1097/00006565-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahim S, Singh S, Hutchison JS, et al. Adaptive behavior, functional outcomes, and quality of life outcomes of children requiring urgent ICU admission. Pediatr Crit Care Med. 2013 Jan;14(1):10–18. doi: 10.1097/PCC.0b013e31825b64b3. [DOI] [PubMed] [Google Scholar]
- 5.Tilford JM, Aitken ME, Goodman AC, et al. Child health-related quality of life following neurocritical care for traumatic brain injury: an analysis of preference-weighted outcomes. Neurocrit Care. 2007;7(1):64–75. doi: 10.1007/s12028-007-0037-5. [DOI] [PubMed] [Google Scholar]
- 6.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatric. 1992;121:69–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 7.Fiser DH, Tilford JM, Robertson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit. Crit Care Med. 2000;28:1173–1179. doi: 10.1097/00003246-200004000-00043. [DOI] [PubMed] [Google Scholar]
- 8.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009 Jul;124(1):e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack MMHR, Funai T, Clark A, Moler F, Shanley T, Meert K, Newth CJL, Carcillo J, Berger JT, Doctor A, Berg RA, Dalton H, Wessel DL, Harrison RE, Dean JM, Jenkins TL. The Relationship between the Functional Status Scale and the Pediatric Overall and Cerebral Performance Categories. JAMA Pediatrics. 2014;168 doi: 10.1001/jamapediatrics.2013.5316. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willson DF, Dean JM, Meert KL, et al. Collaborative pediatric critical care research network: looking back and moving forward. Pediatr Crit Care Med. 2010;11(1):1–6. doi: 10.1097/PCC.0b013e3181c01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack MMDJ, Butler J, Holubkov R, Doctor A, Meert KI, Newth CJL, Berg RA, Moler F, Dalton H, Wessel DL, Berger J, Harrison RE, Carcillo JA, Shanley TP, Nicholson CE. The ideal time for critical care severity-of-illness assessment. Pediatr Crit Care Med. 2013;14(5):448–453. doi: 10.1097/PCC.0b013e31828a7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack MM, Ruttimann UE, Getson PR. Accurate prediction of the outcome of pediatric intensive care. A new quantitative method. N Engl J Med. 1987 Jan 15;316(3):134–139. doi: 10.1056/NEJM198701153160304. [DOI] [PubMed] [Google Scholar]
- 13.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Critical Care Medicine. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Stevens KJ, Freeman JV. An assessment of the psychometric performance of the Health Utilities Index 2 and 3 in children following discharge from a U.K. pediatric intensive care unit. Pediatr Crit Care Med. 2012 Jul;13(4):387–392. doi: 10.1097/PCC.0b013e318238969a. [DOI] [PubMed] [Google Scholar]
- 15.Rennick JE, Johnston CC, Lambert SD, et al. Measuring psychological outcomes following pediatric intensive care unit hospitalization: psychometric analysis of the Children’s Critical Illness Impact Scale. Pediatr Crit Care Med. 2011 Nov;12(6):635–642. doi: 10.1097/PCC.0b013e3182191bfa. [DOI] [PubMed] [Google Scholar]
- 16.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012 Apr;59(4):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Fernandez Y, Azagra AM-d, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Critical Care Medicine. 2012 Dec;40(12):3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 18.Coleman NE, Slonim AD. Health-related outcomes in children after critical illness. Pediatr Crit Care Med. 2012 Jul;13(4):482–483. doi: 10.1097/PCC.0b013e31824174b3. [DOI] [PubMed] [Google Scholar]
- 19.Burstein DS, Jacobs JP, Li JS, et al. Care models and associated outcomes in congenital heart surgery. Pediatrics. 2011 Jun;127(6):e1482–1489. doi: 10.1542/peds.2010-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buurman BM, van Munster BC, Korevaar JC, de Haan RJ, de Rooij SE. Variability in measuring (instrumental) activities of daily living functioning and functional decline in hospitalized older medical patients: a systematic review. J Clin Epidemiol. 2011 Jun;64(6):619–627. doi: 10.1016/j.jclinepi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Weissberg-Benchell J, Zielinski TE, Rodgers S, et al. Pediatric health-related quality of life: Feasibility, reliability and validity of the PedsQL transplant module. Am J Transplant. 2010 Jul;10(7):1677–1685. doi: 10.1111/j.1600-6143.2010.03149.x. [DOI] [PubMed] [Google Scholar]
- 22.Varni JW, Limbers CA, Neighbors K, et al. The PedsQLTM Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011 Feb;20(1):45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 23.Tahirovic E, Begic H, Tahirovic H, Varni JW. Quality of life in children after cardiac surgery for congenital heart disease. Coll Antropol. 2011 Dec;35(4):1285–1290. [PubMed] [Google Scholar]
- 24.Anderson V, Le Brocque R, Iselin G, et al. Adaptive ability, behavior and quality of life pre and posttraumatic brain injury in childhood. Disabil Rehabil. 2012;34(19):1639–1647. doi: 10.3109/09638288.2012.656789. [DOI] [PubMed] [Google Scholar]
- 25.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma. 2012 Apr 10;29(6):1126–1139. doi: 10.1089/neu.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Archives of Pediatrics & Adolescent Medicine. 2000 Jul;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 27.Sparrow SCD, Balla D. Vineland Adaptive Behaviror Scales - Survey Forms Manual. 2. AGS Publishing; 2005. Vineland-II. [Google Scholar]
- 28.Mangione-Smith R, McGlynn EA. Assessing the quality of healthcare provided to children. Health Serv Res. 1998 Oct;33(4 Pt 2):1059–1090. [PMC free article] [PubMed] [Google Scholar]
- 29.Aylward GP, Aylward BS. The changing yardstick in measurement of cognitive abilities in infancy. J Dev Behav Pediatr. 2011 Jul-Aug;32(6):465–468. doi: 10.1097/DBP.0b013e3182202eb3. [DOI] [PubMed] [Google Scholar]
- 30.Jennett BBM. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 31.Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Critical Care Medicine. 2000 Apr;28(4):1173–1179. doi: 10.1097/00003246-200004000-00043. [DOI] [PubMed] [Google Scholar]
- 32.Langhelle A, Nolan J, Herlitz J, et al. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation. 2005 Sep;66(3):271–283. doi: 10.1016/j.resuscitation.2005.06.005. [DOI] [PubMed] [Google Scholar]