Abstract
BACKGROUND
Age-period-cohort (APC) analysis can inform registry-based studies of cancer incidence and mortality, but concerns about statistical identifiability and interpretability, as well as the learning curves of statistical software packages, have limited its uptake.
METHODS
We implemented a panel of easy-to-interpret estimable APC functions and corresponding Wald tests in R code that can be accessed through a user-friendly web tool.
RESULTS
Input data for the web tool consist of age-specific numbers of events and person-years over time, in the form of a rate matrix of paired columns. Output functions include model-based estimators of cross-sectional and longitudinal age-specific rates; period and cohort rate ratios that incorporate the overall annual percentage change (net drift); and estimators of the age-specific annual percentage change (local drifts). The web tool includes built-in examples for teaching and demonstration. User data can be input from a Microsoft Excel worksheet or by uploading a comma-separated-value (csv) file. Model outputs can be saved in a variety of formats including R and Excel.
CONCLUSIONS
APC methodology can now be carried out through a freely-available user-friendly web tool. The tool can be accessed at http://analysistools.nci.nih.gov/apc/.
IMPACT
The web tool can help cancer surveillance researchers make important discoveries about emerging cancer trends and patterns.
Keywords: Models/Statistical, Population Surveillance/Methods, Registries, Age Factors, Cohort Studies, United States/epidemiology
INTRODUCTION
Cancer rates are monitored world-wide to assess the burden of cancer and track cancer trends in populations (1–3). Standard statistical methods include examination of plots of disease rates over time (4, 5) and analysis of directly age-standardized rates (ASRs) and estimated annual percentage changes (EAPCs) of the ASRs (6, 7). These approaches are descriptive, agnostic, and non-parametric.
Cancer rates are also examined to reveal clues about cancer etiology (8–17), natural history (18–21), and mortality (9, 22–27). Parametric statistical models often play a more prominent role in these studies, especially the age-period-cohort (APC) model (5, 28–33). Nonetheless, many studies have not taken advantage of the APC framework (34). One basic problem is concern about statistical identifiability and corresponding uncertainty about how to interpret APC parameters (especially the so-called deviations). However, we have suggested these issues reflect a fundamental uncertainty principle intrinsic to all cohort studies, rather than a problem specific to the APC model (34). We and others (5) have also pointed out close connections between estimable functions of APC parameters and standard plots of age-standardized and age-specific disease rates over time. In this regard, estimable APC functions provide a useful parametric framework that complements standard non-parametric descriptive methods.
Several APC functions have proven useful in cancer applications. Anderson et al. (35) introduced the longitudinal age curve in their investigation of breast cancer Black-White racial disparity. The longitudinal age curve ‘stiches together’ observed cohort-specific age-specific rates, thereby providing a smooth summary curve. Speaks et al. (36) considered testicular germ cell tumors and Yang et al. (37) studied ovary cancer using a period rate ratio curve. This function describes the relative rate of cancer in any given calendar period versus a referent period, adjusted for age and non-linear cohort effects. Jemal et al. (24) studied lung cancer mortality, Ma et al. (38) pancreas cancer mortality, and Rosenberg et al. (39) leukemia incidence using a cohort rate ratio curve. This function describes the relative rate of cancer in any given birth cohort versus a referent cohort, adjusted for age and non-linear period effects. Mbulaiteye et al. (40) investigated Burkitt lymphoma and Chaturvedi et al. (41)} examined oral cavity and oropharyngeal squamous cell carcinoma rates using a quantity that we call local drifts. Local drifts provide a model-based EAPC value for each age group. Anderson et al. used all of these functions in their study of breast cancer heterogeneity in Denmark (42).
Since each of these new functions has proven useful in recent studies, we were motivated to make them readily available. However, many investigators with interesting hypotheses about cancer rates are not experts in statistics or familiar with statistical software packages. Therefore, we developed a freely-available user-friendly web tool that be accessed at http://analysistools.nci.nih.gov/apc/. The web tool provides all of the APC functions described above, along with associated statistical hypothesis tests. In this report we summarize the web tool and highlight how it can help identify interesting signals in cancer rates using three illustrative examples from the literature (29, 32, 43). The example data are available through the web tool, and we encourage potential users to work through the examples online.
MATERIALS AND METHODS
Overview of the Web Tool
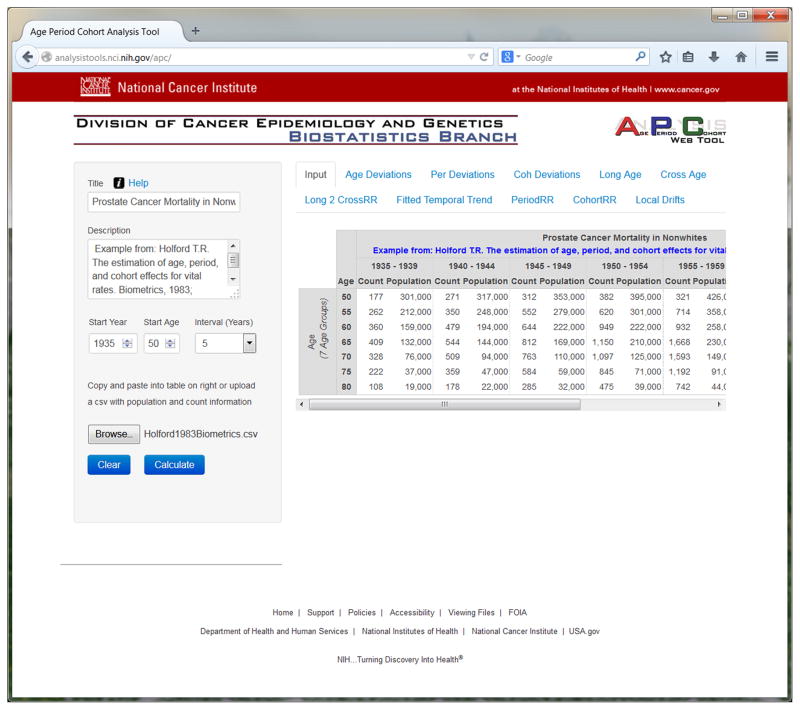
Online Help is available (click on Help in the web tool, or open http://analysistools.nci.nih.gov/apc/help.html). Input data for the web tool consist of age-specific numbers of events and person-years over time, in the form of a rate matrix of paired columns. Three sample datasets that describe prostate (43), lung (29), and breast cancer (32) mortality are linked to the web tool (click on Help, then Sample Data, or open http://analysistools.nci.nih.gov/apc/help.html#example). The input page is shown in Figure 1 for the prostate cancer mortality data (example 1). In general, user data can be input by copy-and-paste from an Excel worksheet or file upload of a comma-separated-values (csv) file. As shown in Figure 1, age groups correspond to rows and calendar periods to columns. The rates are defined by adjacent pairs of columns: the first column of each pair lists the numbers of events by age for a given calendar period, and the second column lists the corresponding persons-years. The age and period intervals must all be equal (44), i.e. if 5-year age groups are used then 5-year calendar periods must also be used. The intervals can range from 1 through 10 years inclusive. Data in this format can easily be obtained from publicly-available data resources with cancer case and population data, such as the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (http://www.seer.cancer.gov) and Cancer Incidence in Five Continents (CI5) of the International Agency for Research on Cancer (http://ci5.iarc.fr).
Figure 1.
screen shot of input page
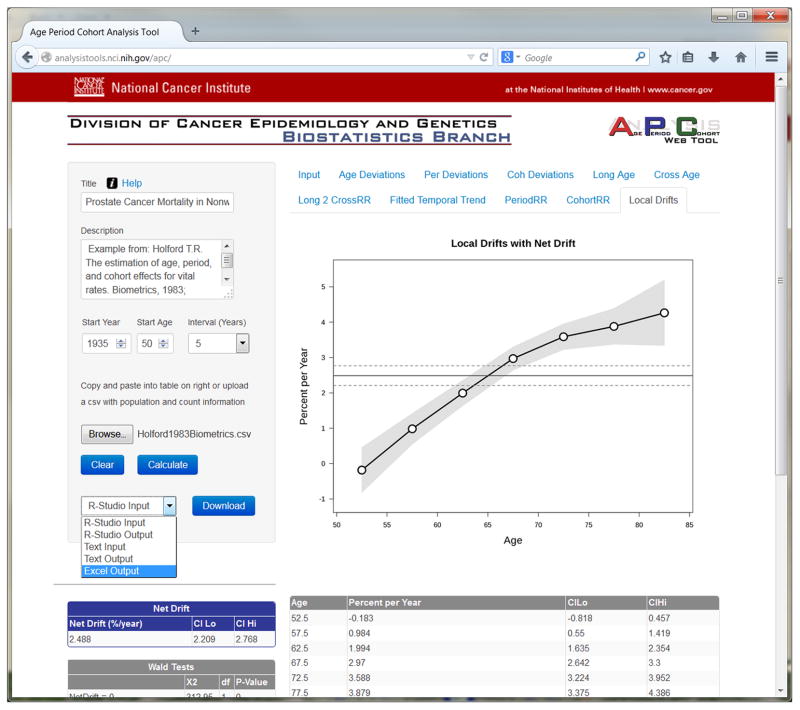
The web tool fits the APC model and calculates parameters and estimable functions summarized in Table 1. On the web site, each function is presented in its own tab in graphical and tabular format, as illustrated in Figure 2. A number of key hypothesis tests are also provided in the ‘Wald Tests’ tab located in the sidebar on the left-hand side of the web page. These hypothesis tests are summarized in Table 2.
Table 1.
Estimable age-period-cohort (APC) parameters and functions calculated by the web tool.
| Nomenclature | Interpretation |
|---|---|
| Net Drift | APC analogue of the estimated annual percentage change (EAPC) in the age-standardized rate (ASR); log-linear component of FTT(p | a0), PRR(p | p0) and CRR(c | c0) |
| CAT = LAT − Net Drift | Cross-sectional age trend; log-linear trend in CrossAge(a | p0) |
| LAT = CAT + Net Drift | Longitudinal age trend; log-linear trend in LongAge(a | c0) |
| Age deviations, AD(a) | Non-linear age effects incorporated into LongAge(a | c0), CrossAge(a | p0) and Long2CrossRR(a | c0, p0); orthogonal to the linear trend in age |
| Period deviations, PD(p) | Non-linear period effects incorporated into FTT(p | a0) and PRR(p | p0); orthogonal to the linear trend in period |
| Cohort deviations, CD(c) | Non-linear cohort effects incorporated into CRR(c | c0) and LocalDrifts (a); orthogonal to the linear trend in cohort (over the entire rate matrix) |
| Fitted Temporal Trends, FTT(p | a0) | Fitted rates in reference age group a0 adjusted for cohort deviations; APC analogue of the ASR |
| Cross-Sectional Age Curve, CrossAge(a | p0) | Fitted cross-sectional age-specific rates in reference period p0 adjusted for cohort deviations |
| Longitudinal Age Curve, LongAge(a | c0) | Fitted longitudinal age-specific rates in reference cohort c0 adjusted for period deviations |
| Ratio of Longitudinal versus Cross-Sectional Age curves Long2CrossRR(a | c0, p0) | Quantifies influence of Net Drift on age-associated natural history |
| Period Rate Ratios, PRR(p | p0) | Ratio of age-specific rates in period p relative to reference period p0 |
| Cohort Rate Ratios, CRR(c | c0) | Ratio of age-specific rates in cohort c relative to reference cohort c0 |
| Local Drifts, LocalDrifts(a) | Estimated annual percentage change over time specific to age group a |
For APC model defined over A age groups and P calendar periods with equal intervals. In the web tool, the central age group, calendar period, and birth cohort define the reference values a0, p0, c0, respectively. When there is an even number of age, period, or cohort categories, the reference value is the lower of the two central values. In the R package, reference values can be set to any observed age, period and cohort = period-age in the rate matrix.
Figure 2.
screen shot of local drifts tab
Table 2.
Wald Chi-Square tests for estimable functions in the age-period-cohort (APC) model*.
| Null Hypothesis | Implications | Degrees of Freedom |
|---|---|---|
| Net drift equals 0 | Fitted temporal trends are stable over time. Fitted longitudinal and cross-sectional age curves are proportional. |
1 |
| All age deviations equal 0 | Fitted longitudinal and cross-sectional age curves are log-linear. | A−2 |
| All period deviations equal 0 | Fitted temporal trends and period rate ratios are log-linear. | P−2 |
| All cohort deviations equal 0 | Cohort rate ratios are log-linear; all local drifts equal the net drift. | C−2 |
| All period rate ratios equal 1 | Net drift is 0 and fitted temporal trends are constant; Cross-sectional age curve describes age incidence pattern in every period. | P−1 |
| All cohort rate ratios equal 1 | Net drift is 0 and all local drifts are 0; Longitudinal age curve describes age incidence in every cohort. | C−1 |
| All local drifts equal the net drift | Temporal trends are the same in every age group. | A − 1 if A = P, A otherwise |
For APC model defined over A age groups, P calendar periods, and C = P + A − 1 birth cohorts.
RESULTS
Interpretation of Estimable Functions
Using outputs from the web tool (Table 1), the user can interpret the observed rates as a product of age, period, and cohort effects. Different combinations of functions summarize the patterns longitudinally or prospectively and cross-sectionally. Longitudinally, the expected rate per 100,000 person years among persons born in year c and followed-up at age a equals R(a | c) = LongAge(a | c0) × CRR(c | c0) × ePD(c+a). Cross-sectionally, the expected rates by age conditional on period equal R(a | p) = CrossAge(a | p0) × PRR(p | p0) × eCD(p−a) and the expected rates by period conditional on age equal .
Formally, if we calculate the log-linear regressions of R(p | a) versus a (one regression for each age group), the Local Drifts are equal to the slopes of the regression lines βa expressed as an estimated annual percentage change 100% × (eβa − 1). From the expression for R(p | a), it follows that any differences between the Local Drifts and the Net Drift are a function of the cohort deviations CD(p − a).
Illustrative Examples
The built-in examples represent qualitatively different rate patterns. For the prostate cancer example (Sample Data 1 online), the cohort rate ratio curve has a striking inverted-V shape. Consequently, the local drifts (Figure 2) are highly significant (Chi-Square = 137.5 on 6 degrees of freedom, P 0); the local drift values increase from around 0 percent per year among men ages 50 – 54 years, to around 4 percent per year among men ages 80 – 84 years. In contrast, for the lung cancer example (Sample Data 2 online), the cohort deviations are not statistically significant; hence the cross-sectional age-specific rates over time are more or less proportional. Furthermore, because the period deviations are also not statistically significant, the common secular pattern is more or less log-linear.
For the breast cancer example (Sample Data 3 online), the overall net drift of −0.289 percent per year is quite modest. Nonetheless, the cohort rate ratio curve identifies a remarkable moderation in breast cancer mortality among women born after 1926. This cohort trend is responsible for the striking pattern of local drifts: mortality is stable or significantly decreasing over time among women ages 58 – 59 years and younger, but is significantly increasing over time, by as much as 1 percent per year, among women ages 60 – 61 years and older.
In the prostate and breast cancer examples, cohort deviations are substantially larger than period deviations. Hence, both sets of rates are well approximated by a multiplicative model in which the longitudinal age curve is modulated up or down according to the values of the cohort rate ratio curve.
Software
The computational methods for our web tool are implemented in R. The R package is freely available through the web tool (click on Help, then FAQ). The R code runs on a back-end server. Python and JavaScript are used on a front-end server to obtain the user’s input, communicate with the R server, and format the results for the user.
The web tool can save the outputs in a text file, Excel Workbook, or R Workspace. After fitting a model, select the desired output format from the drop-down menu (see Figure 2), then click on the Download button. The text output files were generated by the R package and are displayed in tabs in the browser window. Users can copy and paste this output to their own files. Many users may prefer to download the outputs in the form of an Excel Workbook. Each function tab on the web site appears as a function sheet in the workbook, which provides model outputs in tabular and graphical form, precisely as they appear on the web. The table of Wald tests is also included, along with the Net Drift and log-linear APC parameters. The inputs and outputs can also be saved as R Workspaces, which can be opened in R for downstream analyses in the R environment.
DISCUSSION
We developed a user-friendly web tool that makes age-period-cohort (APC) analysis broadly available without the need for any programming. Our R package is also freely available. The functions in the web tool have proven utility in registry-based studies of cancer incidence and mortality. The tool includes three built-in examples for teaching and demonstration, and it can output all of the results in several formats, including Excel.
Net drift is perhaps the single most important parameter in the APC model, so the drift value is prominently displayed by our Web tool. One important yet perhaps under-appreciated etiological implication of drift is made clear by our Web tool: whenever there is drift, the cross-sectional and longitudinal age curves will diverge. The mathematical reason is that the cross-sectional age trend (CAT) in the former is always equal to the longitudinal age trend (LAT) in the latter minus the log-linear net drift coefficient (45) (Table 1). The intuitive reason is the cross-sectional age curve reflects the experience of older cohorts at older ages and younger cohorts at younger ages. When there is a progressive increase in rates from older to younger generations, the cross-sectional age curve gives the false impression of falling rates with advancing age at diagnosis (46), and conversely. Therefore, the cross-sectional age curve should be interpreted cautiously as a surrogate for the longitudinal age-associated natural history unless there is little net drift. The bias can be severe whenever there is substantial drift of say ±1% per year or more, as in the lung cancer example. We prefer to make inferences about age-associated natural history using the longitudinal age curve (35, 40, 47–51), which is now broadly available through our Web tool.
One of the most novel components of our Web tool is the local drifts and associated Wald test for heterogeneity. In our view, after net drift, the test for significance of the local drifts is the second most important number coming out of an APC analysis. It can be shown that the local drifts are determined from the slope (derivative) of the cohort rate ratio curve. In other words, local drifts are a consequence of trends in birth cohort effects. If the local drifts are significant (because of birth cohort effects), an important implication is a single summary age-standardized rate curve and EAPC value cannot adequately describe the time trends in every age group. The web tool for the first time allows the user to test for this situation. In our experience, local drifts are often quite heterogeneous, and striking local drift patterns such as those illustrated by the prostate and breast cancer examples are not uncommon.
Age, period, and cohort deviations are also provided by the web tool. These quantities measure curvature, which describes local changes in trends, independently of the magnitude or direction of the overall trend. There are situations where it is desirable to base inferences on these quantities (36, 38), especially in comparative analysis.
Various investigators have calculated cohort and period rate ratio curves by imposing different constraints on the parameters in the APC model (8, 9, 17, 52). In our web tool both the cohort rate ratio curve as well as the period rate ratio curve incorporate the entire value of the net drift. Importantly, in our software, the cohort deviations are constructed to be orthogonal to the linear trend in cohort over the entire set of observed rates. This definition ensures that various products of age, period, and cohort functions provided by our software are mathematically equivalent to the fitted rates. Since the fitted rates track the observed rates as closely as possible, the estimable functions provided by our software are most consistent with the observed data.
An important caveat is the functions in our web tool were designed to highlight key signals in cancer rates, but it remains up to the investigator to propose plausible explanations. Also, the tool does not produce standard descriptive plots since these can be generated by numerous other software applications including Excel. In the future we hope to develop a companion web tool for comparative analysis of two sets of rates (45).
In summary, our web tool for age-period-cohort analysis provides a suite of age-period-cohort functions and parameters that complement traditional descriptive approaches. It is very simple to cut and paste case and population data into our web tool or upload the data from a csv file. All of the outputs can be downloaded in a number of formats that enable further downstream analysis. Our hope is that our web tool will help cancer surveillance researchers make important discoveries about emerging cancer trends and patterns.
Acknowledgments
GRANT SUPPORT
This research was supported entirely by the Intramural Research Program of the NIH, National Cancer Institute (NCI), Division of Cancer Epidemiology and Genetics.
The web tool was made possible thanks to the expert support of computer scientists at the NCI Center for Biomedical Informatics and Information Technology (CBIIT). We gratefully acknowledge the NCI CBIIT Team that implemented the Age Period Cohort Analysis Web Tool:
Robert Shirley, NCI CBIIT
Sue Pan, NCI CBIIT
Larry Brem, Leidos Biomedical Research, Inc. (NCI CBIIT Dev Team Contractor)
Brent Coffey, Leidos Biomedical Research, Inc. (NCI CBIIT Dev Team Contractor)
Shaun Einolf, Leidos Biomedical Research, Inc. (NCI CBIIT Dev Team Contractor)
Sula Rajapakse, Leidos Biomedical Research, Inc. (NCI CBIIT Dev Team Contractor)
Cuong Nguyen, SRA International, Inc. (NCI CBIIT Systems Team Contractor)
Footnotes
CONFLICTS OF INTEREST
The authors have no potential conflicts of interests.
References
- 1.Curado MP, Edwards B, Shin HR, Ferlay J, Heanue M, Boyle P, et al., editors. Cancer Incidence in Five Continents. IX. Lyon, France: International Agency for Research on Cancer; 2009. [Google Scholar]
- 2.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. American journal of epidemiology. 1995;141(4):300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 5.Robertson C, Boyle P. Age-period-cohort models of chronic disease rates. II: Graphical approaches. Statistics in medicine. 1998;17(12):1325–39. doi: 10.1002/(sici)1097-0258(19980630)17:12<1325::aid-sim854>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847–54. doi: 10.1111/j.1541-0420.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Bergstrom R, Adami HO, Mohner M, Zatonski W, Storm H, Ekbom A, et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. Journal of the National Cancer Institute. 1996;88(11):727–33. doi: 10.1093/jnci/88.11.727. [DOI] [PubMed] [Google Scholar]
- 9.Verhoeven R, Houterman S, Kiemeney B, Koldewijn E, Coebergh JW. Testicular cancer: marked birth cohort effects on incidence and a decline in mortality in southern Netherlands since 1970. Int J Cancer. 2008;122(3):639–42. doi: 10.1002/ijc.23061. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Semenciw R, Waters C, Wen SW, Mery LS, Mao Y. Clues to the aetiological heterogeneity of testicular seminomas and non-seminomas: time trends and age-period-cohort effects. International journal of epidemiology. 2000;29(5):826–31. doi: 10.1093/ije/29.5.826. [DOI] [PubMed] [Google Scholar]
- 11.Bray F, Richiardi L, Ekbom A, Forman D, Pukkala E, Cuninkova M, et al. Do testicular seminoma and nonseminoma share the same etiology? Evidence from an age-period-cohort analysis of incidence trends in eight European countries. Cancer Epidemiol Biomarkers Prev. 2006;15(4):652–8. doi: 10.1158/1055-9965.EPI-05-0565. [DOI] [PubMed] [Google Scholar]
- 12.Spix C, Eletr D, Blettner M, Kaatsch P. Temporal trends in the incidence rate of childhood cancer in Germany 1987–2004. Int J Cancer. 2008;122(8):1859–67. doi: 10.1002/ijc.23281. [DOI] [PubMed] [Google Scholar]
- 13.McNally RJ, Cairns DP, Eden OB, Kelsey AM, Taylor GM, Birch JM. Examination of temporal trends in the incidence of childhood leukaemias and lymphomas provides aetiological clues. Leukemia. 2001;15(10):1612–8. doi: 10.1038/sj.leu.2402252. [DOI] [PubMed] [Google Scholar]
- 14.Svensson E, Moller B, Tretli S, Barlow L, Engholm G, Pukkala E, et al. Early life events and later risk of colorectal cancer: age-period-cohort modelling in the Nordic countries and Estonia. Cancer Causes Control. 2005;16(3):215–23. doi: 10.1007/s10552-004-3073-x. [DOI] [PubMed] [Google Scholar]
- 15.Chu KC, Tarone RE, Brawley OW. Breast cancer trends of black women compared with white women. Arch Fam Med. 1999;8(6):521–8. doi: 10.1001/archfami.8.6.521. [DOI] [PubMed] [Google Scholar]
- 16.Sim X, Ali RA, Wedren S, Goh DL, Tan CS, Reilly M, et al. Ethnic differences in the time trend of female breast cancer incidence: Singapore, 1968–2002. BMC Cancer. 2006;6:261. doi: 10.1186/1471-2407-6-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. JNatlCancer InstMonogr. 2006;(36):19–25. doi: 10.1093/jncimonographs/lgj016. [DOI] [PubMed] [Google Scholar]
- 18.McNally RJ, Rowland D, Roman E, Cartwright RA. Age and sex distributions of hematological malignancies in the U.K. HematolOncol. 1997;15(4):173–89. doi: 10.1002/(sici)1099-1069(199711)15:4<173::aid-hon610>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;90(11):1502–10. [PubMed] [Google Scholar]
- 20.Tarone RE, Chu KC. The greater impact of menopause on ER− than ER+ breast cancer incidence: a possible explanation (United States) Cancer Causes Control. 2002;13(1):7–14. doi: 10.1023/a:1013960609008. [DOI] [PubMed] [Google Scholar]
- 21.Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer EpidemiolBiomarkers Prev. 2004;13(7):1128–35. [PubMed] [Google Scholar]
- 22.Chu KC, Tarone RE, Chow WH, Hankey BF, Ries LA. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. Journal of the National Cancer Institute. 1994;86(13):997–1006. doi: 10.1093/jnci/86.13.997. [DOI] [PubMed] [Google Scholar]
- 23.Corrao G, Ferrari P, Zambon A, Torchio P, Arico S, Decarli A. Trends of liver cirrhosis mortality in Europe, 1970–1989: age-period-cohort analysis and changing alcohol consumption. International journal of epidemiology. 1997;26(1):100–9. doi: 10.1093/ije/26.1.100. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Ma J, Rosenberg PS, Siegel R, Anderson WF. Increasing Lung Cancer Death Rates Among Young Women in Southern and Midwestern States. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.42.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–26. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 26.Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer. 2006;118(12):3099–111. doi: 10.1002/ijc.21747. [DOI] [PubMed] [Google Scholar]
- 27.Cayuela A, Rodriguez-Dominguez S, Lopez-Campos JL, Vigil E. Age-period-cohort analysis of lung cancer mortality rates in Andalusia, 1975–2004. Lung Cancer. 2007;57(3):261–5. doi: 10.1016/j.lungcan.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Holford TR, Armitage P, Colton T. Encyclopedia of Biostatistics. John Wiley & Sons, Ltd; 2005. Age-Period-Cohort Analysis; pp. 82–99. [Google Scholar]
- 29.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. StatMed. 1987;6(4):449–67. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 30.Clayton D, Kaldor J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics. 1987;43(3):671–81. [PubMed] [Google Scholar]
- 31.Robertson C, Boyle P. Age-period-cohort analysis of chronic disease rates. I: Modelling approach. Statistics in medicine. 1998;17(12):1305–23. doi: 10.1002/(sici)1097-0258(19980630)17:12<1305::aid-sim853>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Tarone RE, Chu KC. Evaluation of birth cohort patterns in population disease rates. AmJEpidemiol. 1996;143(1):85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]
- 33.Tarone RE, Chu KC. Nonparametric evaluation of birth cohort trends in disease rates. JEpidemiolBiostat. 2000;5(3):177–91. [PubMed] [Google Scholar]
- 34.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20(7):1263–8. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson WF, Rosenberg PS, Menashe I, Mitani I, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. Journal of the National Cancer Institute. 2008;100(24):11. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speaks C, McGlynn KA, Cook MB. Significant calendar period deviations in testicular germ cell tumors indicate that postnatal exposures are etiologically relevant. Cancer Causes Control. 2012;23(10):1593–8. doi: 10.1007/s10552-012-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang HP, Anderson WF, Rosenberg PS, Trabert B, Gierach GL, Wentzensen N, et al. Ovarian cancer incidence trends in relation to changing patterns of menopausal hormone therapy use in the United States. J Clin Oncol. 2013;31(17):2146–51. doi: 10.1200/JCO.2012.45.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, Siegel R, Jemal A. Pancreatic Cancer Death Rates by Race Among US Men and Women, 1970–2009. Journal of the National Cancer Institute. 2013;105(22):1694–700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg PS, Wilson KL, Anderson WF. Are incidence rates of adult leukemia in the United States significantly associated with birth cohort? Cancer Epidemiol Biomarkers Prev. 2012;21(12):2159–66. doi: 10.1158/1055-9965.EPI-12-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer. 2010;126(7):1732–9. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson WF, Rosenberg PS, Petito L, Katki HA, Ejlertsen B, Ewertz DM, et al. Divergent estrogen receptor positive and negative breast cancer trends and etiologic heterogeneity in Denmark. Int J Cancer. 2013 doi: 10.1002/ijc.28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–24. [PubMed] [Google Scholar]
- 44.Holford TR. Approaches to fitting age-period-cohort models with unequal intervals. Statistics in medicine. 2006;25(6):977–93. doi: 10.1002/sim.2253. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg PS, Anderson WF. Proportional hazards models and age-period-cohort analysis of cancer rates. Statistics in medicine. 2010;29(11):1228–38. doi: 10.1002/sim.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 (Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 47.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male Breast Cancer: A Population-Based Comparison With Female Breast Cancer. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer. 2009;115(18):4176–85. doi: 10.1002/cncr.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimley PM, Matsuno RK, Rosenberg PS, Henson DE, Schwartz AM, Anderson WF. Qualitative age interactions between low-grade and high-grade serous ovarian carcinomas. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2256–61. doi: 10.1158/1055-9965.EPI-09-0240. [DOI] [PubMed] [Google Scholar]
- 50.Reimers LL, Anderson WF, Rosenberg PS, Henson DE, Castle PE. Etiologic heterogeneity for cervical carcinoma by histopathologic type, using comparative age-period-cohort models. Cancer Epidemiol Biomarkers Prev. 2009;18(3):792–800. doi: 10.1158/1055-9965.EPI-08-0965. [DOI] [PubMed] [Google Scholar]
- 51.Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1092–100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carstensen B. Age-period-cohort models for the Lexis diagram. Statistics in medicine. 2007;26(15):3018–45. doi: 10.1002/sim.2764. [DOI] [PubMed] [Google Scholar]