Abstract
Autism spectrum disorder (ASD) is a pervasive developmental disorder characterized by deficits in social interaction, language, stereotyped behaviors, and restricted range of interests. In previous studies low frequency repetitive transcranial magnetic stimulation (rTMS) has been used, with positive behavioral and electrophysiological results, for the experimental treatment in ASD. In this study we combined prefrontal rTMS sessions with electroencephalographic (EEG) neurofeedback (NFB) to prolong and reinforce TMS-induced EEG changes. The pilot trial recruited 42 children with ASD (~14.5 yrs). Outcome measures included behavioral evaluations and reaction time test with event-related potential (ERP) recording. For the main goal of this exploratory study we used rTMS-neurofeedback combination (TMS-NFB, N=20) and waitlist (WTL, N=22) groups to examine effects of 18 sessions of integrated rTMS-NFB treatment or wait period) on behavioral responses, stimulus and response-locked ERPs, and other functional and clinical outcomes. The underlying hypothesis was that combined TMS-NFB will improve executive functions in autistic patients as compared to the waitlist group. Behavioral and ERP outcomes were collected in pre- and post-treatment tests in both groups. Results of the study supported our hypothesis by demonstration of positive effects of combined TMS-NFB neurotherapy in active treatment group as compared to control waitlist group, as the TMS-NFB group showed significant improvements in behavioral and functional outcomes as compared to the waitlist group.
Keywords: Autism spectrum disorder, Transcranial Magnetic Stimulation (TMS), neurofeedback, EEG gamma activity, theta/beta ratio, reaction time, visual oddball task, executive functions, repetitive behaviors
Introduction
Autism spectrum disorder (ASD) is featured by severe deficits in social communication, social interaction, and restricted, repetitive patterns of behaviors, interests and activities (APA, 2013). Several major neuropsychological model variants have been proposed to explain the cognitive deficits found in ASD by Baron-Cohen and his colleagues (reviewed in Baron-Cohen, 2004). Deficits in executive functioning skills are the salient feature of another important model of autism (Hill 2004; Ozonoff 1997). These skills fall under the purview of those prefrontal functions that facilitate problem-solving, flexible set-shifting and forward planning in the implementation of goal-directed behavior. Other integrative models of autism mainly focus on impaired functional connectivity (Villalobos et al. 2005; Welchew et al. 2005), and abnormalities of neurodevelopmental processes (Casanova et al. 2002; Courchesne et al. 1989) which manifest as a cognitive deficit affecting the “binding together” of discrete features into a single, coherent object or concept (Brock et al. 2002; Brown et al. 2005; Rippon et al. 2007). One more model of autism emphasizes abnormalities in neural connectivity (Belmonte et al. 2004). The model states that autism might be characterized by functional disconnectivity of cortical networks important for specific aspects of social cognition, emotional and behavioral control. There were suggested also models based on mirror neurons system and imitation deficits in autism (Iacoboni and Dapretto 2006; Oberman, Ramachandran, & Pineda 2008; Oberman et al. 2005).
Recent studies by our group have characterized the neuropathology of autism as that of a minicolumnopathy (Casanova 2005, 2006; Casanova et al. 2002,2003,2006, 2012; Sokhadze et al. 2012). Deficits within the inhibitory elements that surround the cell minicolumn suggest a mechanistic explanation to the inhibitory/excitatory (I/E) imbalance in autism ( Casanova et al. 2002, 2003; Rubenstein and Merzenich 2003; Szentagothai and Arbib 1975). Oscillations and synchronization of pyramidal cells in and across minicolumns are maintained by networks of inhibitory GABAergic interneurons (Mann and Paulsen, 2007). Local I/E interactions shape neuronal representations of sensory, motor and cognitive variables, and produce local electroencephalographic (EEG) gamma oscillations. The I/E bias caused by faulty pyramidal cell-interneuronal diads provides a receptive scenario to gamma frequency abnormalities in autism, and can be considered as a neurophysiological biomarker of autism.
In the present study we investigated effects of novel combined neurotherapy where repetitive transcranial magnetic stimulation (rTMS) over prefrontal areas was followed by prefrontal neurofeedback (NFB) aimed to upregulate gamma oscillations and operantly condition them in children with ASD. The study also explored neural mechanisms of this innovative neuromodulatory intervention approach that targets the core symptoms of the condition without any side effects. Gamma frequencies (30-80 Hz, especially in 35-45 Hz range (or in other words, so called 40 Hz-centered gamma oscillations) in EEG are closely associated with sensory processing, working memory, attention and many other cognitive domains (Donner and Siegel 2011; Gruber et al. 1999;Jensen et al. 2007; Kahana 2006; Keil et al. 1999; Tallon-Baudry et al. 2005; Ward 2003). The pervasive nature of abnormalities ingrained in this oscillatory activity bears significant analogy to the cognitive deficits observed in autism. It is therefore unsurprising that gamma oscillations have been claimed to be directly related to the pathophysiology of autism. To the authors’ knowledge every study on gamma frequencies in autism has been abnormal (Brock et al. 2002; Brown 2005; Brown et al. 2005; Sohal 2012; Sokhadze et al. 2009). Disrupted patterns of coordinated oscillatory output in distributed minicolumnar networks might be associated with cortical “disconnection” in autism. More specifically, altered oscillatory activity in developing cortical circuits may contribute to impaired development of intra-areal and transcortical connections giving rise to a bias in short vs. long cortico-cortical projections (Belmonte and Yurgelun-Todd 2003; Belmonte et al. 2004; Casanova et al. 2003, 2006, 2012).
Previous studies by our group have used evoked and induced gamma activity as outcome measures for slow rTMS in autism (Baruth et al. 2010; Sokhadze et al. 2009b). The use of rTMS was meant to increase the inhibitory tone of cellular elements surrounding the minicolumns of autistic individuals. We found increased evoked gamma to non-target items during oddball task in autism group as compared and reported enhanced gamma responses to targets following 12 sessions of prefrontal rTMS (Baruth et al. 2010). Similar effects were observed in children with ASD enrolled in 18 sessions of the prefrontal rTMS (Hensley et al. 2014).
In the present study we planned to use low frequency rTMS over frontal cortex as a probe to modulate gamma oscillations and operantly condition this high frequency activity. The design of the study included monitoring of post-TMS EEG activity at the prefrontal site, and immediately after rTMS session provide gamma activity feedback training with the goal of instrumentally condition post-TMS gamma activity changes. This neuromodulatory intervention integrated with post-TMS neurofeedback is finely woven with the neuropathological underpinnings of autism previously described in the authors’ laboratory. The studies of neurophysiological mechanism and neurobiology of potential neuromodulatory intervention that targets the core symptoms of the autism condition may have significant clinical impact. We proposed that neurofeedback-based operant conditioning of prefrontal EEG activity immediately post-rTMS sessions will result in more pronounced improvements of functional outcomes as compared control waitlist group of children with ASD, as we hypothesized that rTMS over the dorsolateral prefrontal cortex (DLPFC) improves I/E ratio and enhances gamma activity. Effects of rTMS followed by operant conditioning of TMS-induced gamma modulation are proposed to be more profound that each intervention arm alone, thus emphasizing the primary role of TMS in neuromodulation of EEG gamma. It remains an important goal to select electrocortical measures that could serve as reliable biomarkers of functional outcomes of this novel applied neuroscience based intervention that combines TMS and neurofeedback.
We considered it feasible to use for this purpose electrocortical responses to sensory stimulation in a reaction time task with illusory figures, because autistic individuals usually present excessive reactions to the complex sensory environment (e.g., aversive reactions to visual, auditory, and tactile stimuli, etc.). Perception and sensory reactivity abnormalities are found in majority of subjects with ASD affecting their ability to effectively process information (Gomes et al. 2008). In a series of electrophysiological studies conducted by our group we explored specifics of event-related potentials (i.e., ERP) reflecting information processing during performance on reaction time tasks in children with ASD ( Baruth et al. 2010c ; Casanova et al. 2012; Sokhadze et al. 2009a,b, 2012b, 2013a ). Our studies were aimed to explore the manifestations of the impaired functional connectivity, excessive cortical excitation/inhibition ratio (i.e., E/I), and deficient executive functioning in ASD by analyzing behavioral performance on attention tasks with dense-array ERP recording. Analysis of the selected ERP components is an informative dynamic method of investigation of information processing stages in the human brain due to the high temporal resolution of this technique. Amplitude and latency of ERP waves at specific topographies reflect both early sensory perception processes and higher-level processing including attention, cortical inhibition, memory update, as well as other cognitive activity processes (Polich 2007). ERPs provide a valuable methodology to study chronometry of information processing stages in neurodevelopmental disorders such as ASD. In addition cognitive tests that use specific ERP components could be used as reliable functional outcomes of bio-behavioral interventions and experimental treatment procedures aimed to treat autism symptoms.
We consider that among the newly emerging neuromodulation techniques rTMS and EEG biofeedback (i.e., neurofeedback [NFB]) are most promising for the treatment of core symptoms in autism. TMS offers a non-invasive method for altering excitability of the neural circuits and induction of a short-term functional reorganization in the human cortex, that is manifested also in observable EEG pattern alterations. TMS is a suitable tool for investigation and modulation of neural plasticity due to its ability to not only stimulate the target cortex, but also induce functional changes in cortical areas anatomically and functionally associated with the stimulated regions. Several current reviews describe current state-of-the-art of rTMS application in autism treatment and research (Oberman et al. 2013, Sokhadze et al., 2013b). Neurofeedback is a form of operant conditioning of EEG in which subjects are trained to enhance desired electrocortical activity, while suppressing undesirable activity. Existing literature support the possibility of using neurofeedback as treatment for some of the symptoms of autism.
Neurofeedback for treatment of autism spectrum disorder is gaining certain popularity and is reviewed in several current papers (Coben 2008, 2013; Coben and Padolsky 2007; Coben and Myers 2010; Coben, Linden & Myers 2010; Kouijers et al. 2009a,b, Linden and Gunkelman 2013; Thompson et al. 2010). While there are only few published systematic studies of neurofeedback treatment of autism using standard neurofeedback protocols (Coben 2008; Coben et al. 2010), several recent reports of NFB for autism based on quantitative EEG (qEEG) findings have been presented (Coben 2013; Coben et al. 2010). This technique involves the use of qEEG to identify patterns of EEG that deviate from standardized norms, and individualized protocols to correct them.
Application of neurofeedback for ADHD in children and adolescents has recently been used extensively reviewed (Lubar 2003; Monastra 2005, 2008) and well supported by the literature (Gevensleben et al. 2009; Arns et al. 2009; Sherlin et al. 2010). This led some researchers to believe that neurofeedback protocols successfully applied for treatment of ADHD may also be efficacious for the treatment of children with autism (Jarusiewicz 2002; Kouijers et al. 2009a,b; Sichel et al. 1995). However, neurofeedback strategies commonly used in ADHD treatment (suppression of the frontal theta, enhancement of the sensorimotor rhythm [SMR] or slow beta band) cannot be transferred to ASD treatment in a manner of a treatment that that fits all conditions. Coben (2013) point at the preference of individualized protocols which targets not only few pre-selected topography (e.g., F3 and C3 in SMR/theta) and not only one or two specific EEG band, but rather use qEEG-guided intervention that do not limit treatment to enhancement/ suppression of specific rhythms. In particular most successful approach in autism treatment using operant conditioning uses coherence training which may result in better functional connectivity.
Our own approach for neurofeedback application treatment that we discussed for various psychophathologies in our prior reviews ( for example in a case of treatment of substance used disorders and patients with dual diagnosis, see Sokhadze, Trudeau, & Cannon 2008; Sokhadze, Hollifield & Stewart 2007) outlines preference of application of neurofeedback in combination with other, already established treatments arms, or following treatments known to induce specific and well characterized EEG profile changes. In this particular application of neurofeedback training it is conceived as a secondary, adjunct neurotherapy, as we consider low frequency rTMS treatment as an intervention that is proven to change EEG in a positive way in ASD, while neurofeedback is positioned as a treatment arm aimed to reinforce and operantly condition post-TMS electrocortical activity changes, specifically at the prefrontal topography.
The study follows suggestions that autism reflects a global processing neurodevelopmental defect produced by an excessive local connectivity and deficient distal connectivity resulting in functional disconnectivity of networks important in behavior and social cognition. The hypothesis is that EEG biofeedback training combined with rTMS (i.e., in a mode when neurofeedback session follows each rTMS session) will result in an improvement of multiple functions in ASD, and that this integrated neuromodulation effects may help in understanding mechanisms of neuropathology underlying deficits present in autism. The cognitive test with multichannel ERP recording in this study included a visual reaction time task with illusory figures as stimuli. Quantitative EEG (qEEG) was used to describe changes in relative power of selected EEG bands and their ratios during each neurofeedback session. We proposed that if the outcomes of this pilot study will show beneficial effects in autism population, then complementing rTMS with neurofeedback-based operant conditioning may advance neuromodulation approaches in other psychiatric and neurological disorders as well.
Methods
Participants
Participants with ASD (age range 10 to 21 years) were recruited through the University of Louisville Weisskopf Child Evaluation Center (WCEC). Diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA, 2000) and further ascertained with the Autism Diagnostic Interview – Revised (ADI-R) (Le Couter et al., 2003). At the time when this pilot study was launched, DSM-5 was not yet introduced in the WCEC routine diagnostic practice, therefore diagnosis was made using existing DSM-IV-TR classification. Participants with ASD also had a medical evaluation by a developmental pediatrician. All subjects had normal hearing based on past hearing screens. Participants with a history of seizure disorder, significant hearing or visual impairment, a brain abnormality conclusive from imaging studies or an identified genetic disorder were excluded. Thirty eight participants were high-functioning persons with autism diagnosis and 4 had Asperger Syndrome. All had full-scale IQ > 80 assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV, [Wechsler, 2003]) or (for adolescents) the Wechsler Abbreviated Scale of Intelligence (WASI, [Wechsler, 1999]).
We enrolled 42 autistic patients, 34 males and 8 females, with a mean age of 14.6 ± 3.1 years. Twenty of them were assigned to active 1.0 Hz TMS-NFB treatment (TMS-NFB group), while 22 were assigned to the waiting-list group (WTL group). Since this pilot study had exploratory character and was not a truly randomized clinical trial (RCT), assignment of participants was not exactly random, because the WTL group assignment was partially determined by their treatment option preferences and parents/guardians availability to start one of several experimental treatment protocols offered by the Cognitive Neuroscience laboratory (i.e., 12 sessions of 1.0 Hz TMS without neurofeedback, 18 sessions of 0.5 Hz TMS without neurofeedback, 18 sessions of neurofeedback without TMS, Berard's Auditory Integration Training, etc.). Mean age of subjects in the TMS-NFB group was 14.7 ± 3.3 years, and 14.2 ± 2.8 years in the waiting-list group. There was no significant difference in either age or full-scale IQ between the TMS-NFB and the WTL groups.
The study complied with all relevant national regulations and institutional policies and has been approved by the local Institutional Review Board (IRB). Participating subjects and their parents (or legal guardians) were provided with full information about the study including the purpose, requirements, responsibilities, reimbursement, risks, benefits, alternatives, and role of the local IRB. The subjects were reimbursed only for participation in two ERP tests ($25/per test). The consent and assent forms approved by the IRB were reviewed and explained to all subjects who expressed interest to participate. All questions were answered before consent signature was requested. If the individual agreed to participate, both she/he and parent/guardian signed and dated the consent or assent form and received a copy countersigned by the investigator who obtained consent.
Procedures
ERP test: Three-stimuli oddball task with Kanizsa figures
The stimuli employed in the test were Kanizsa square (target), Kanizsa triangle (non-target), non-Kanizsa square, and non-Kanizsa triangle (standards) (Kanizsa, 1976). The task represents a classic three-stimuli oddball with infrequent illusory Kanizsa target (square, 25%) and infrequent Kanizsa distracter (triangle, 25% ) figures presented for 250 ms among frequent non-Kanizsa stimuli (so called standards, 50%) with inter-trial interval in 1,100-1,300 ms range. Totally 240 trials were presented following a brief practice block. The practice block had 20 trials only with the experimenter present in the room to make sure that subject correctly understands test conditions and recognizes target stimuli. The total time of the test including sensor application and practice was about 25 min. For better habituation and adaptation to experimental setting, the participants were encouraged to have at least one session for conditioning to brainwave sensor net (without performing task) and getting familiar with laboratory environment.
Event-related Potential acquisition and processing
Electroencephalographic (EEG) signals from 128 sites were recorded with a dense-array EGI system (Electrical Geodesics, Inc, Eugene, Oregon). Subjects were placed in electrically and acoustically isolated chamber from the Industrial Acoustics Co. (Bronx, NY). Stimulus presentation and motor response collection was controlled using E-prime (PST, Inc., Pittsburg, PA). Visual stimuli were presented on a flat monitor located in 45-50 cm from the subject, and motor responses were registered with a keypad (Serial Box, PST, Inc). Sampling rate of EEG was 500 Hz, and analog Notch (60 Hz, IIR) and analog elliptical bandpass filters were set at 0.1 - 200 Hz. Impedances were under 40 KΩ. Stimulus-locked EEG data were segmented off-line into 200 ms pre-stimulus baseline to 800 ms epoch post-stimulus. EEG recordings were screened for artifacts and trials with eye blinks, gross movements etc were removed using EGI software artifact rejection tools (Fletcher et al. 1996, Luu et al. 2001, Perrin et al. 1987, Srinivasan et al. 1998). The remaining artifact-free EEG data for trials with correct responses was then digitally filtered using 60 Hz Notch filter and 0.3-20 Hz bandpass filter. Averaged ERP data was baseline corrected (200 ms) and ERPs after averaging and baseline correction were re-referenced into an average reference frame. Response-locked EEGs were segmented into 500 ms pre-response to 500 ms post-response (i.e., commission error). More detailed account for experimental procedure and EEG data acquisition and processing can be found in our prior publications that used similar methodology (Baruth et al. 2010a,b; Casanova et al. 2012; Sokhadze et al. 2012a,b, 2013a).
Event-Related Potentials (ERP): Dependent variables
Stimulus-locked dependent ERP variables
Dependent variables for the frontal and fronto-central region-of-interest (ROI) were N100 (80-180 ms), N200 (220– 350 ms), P2a (180-320 ms), and P3a (300–600 ms), and for the parietal and parieto-occipital ROI were P200 (180-220 ms), N200 (200-320 ms) and P3b (320–600 ms) ERP waves. For P2d component (i.e., differences wave of frontal P2a) we calculated difference wave (P2a to targets minus P2a to non-targets) to detect mean difference between two conditions both in amplitude and latency within 180-320 ms post-stimulus window.
Response-locked Event-Related Potentials (ERN/Pe)
Response locked dependent variables in this study were amplitude and latency of the Error-related Negativity (ERN peaking within 40-150 ms post-error) and Error-related Positivity (Pe, peaking within 100-300 ms post-error). The ROI for both ERN and Pe components included FCz, sites between FCz and FC3- C1, and between FCz and FC2-C2). Amplitude and latency analysis of ERN/Pe was performed with a custom-made application in Matlab (Clemans et al., 2011a). Validation of correct identification of ERN and Pe waves was further ascertained using another custom Matlab application using wavelet transformation (Clemans et al., 2011b).
Treatment Procedures: Transcranial Magnetic Stimulation (TMS)
Repetitive TMS (rTMS) was administered using a Magstim 220 Rapid device (Magstim Corp., Sheffield, UK) with a 70-mm figure-eight coil. Threshold of motor response (MT) was identified for each hemisphere in all participants with autism by increasing the output of the stimulator by 5% until a 50 μV deflection or a visible twitch in the First Dorsal Interosseous (FDI) muscle was detected in at least 3 trials of stimulation over the motor cortex controlling the contralateral FDI. Electromyographic (EMG) responses were recorded with a C-2 J&J Engineering Inc multichannel physiological monitoring device with Physiodata software (J&J Engineering, Inc. , Bainbridge Island, WA).
The rTMS was administered weekly for 18 weeks with the 1st six treatments were over the left DLPFC, while the next 6 were over the right DLPFC, whereas remaining 6 treatments were done bilaterally over the DLFC (evenly at the left and right DLPFC). The DLPFC site for magnetic stimulation was found by placing the TMS coil 5 cm anterior, and in a parasagital plane, to the site of maximal FDI response. A swimming cap was used to make the TMS coil positioning easier. TMS was administered at 1.0 Hz frequency and 90% MT. There were total of 180 pulses per day session with 9 trains with 20 pulses each. There were 20–30 s between the trains intervals used. Decision to select 90% of the MT was based on the prior publications where rTMS was used for the stimulation of DLPFC in various neuro- and psychiatric disorders ( reviewed in Daskalakis et al. 2002, Gershon et al. 2003, Greenberg 2007, Loo and Mitchell 2005, Oberman et al. 2013; Pascual-Leone et al. 2000; Wassermann and Lisanby 2001).
Neurofeedback Protocol and Data Collection
Immediately after rTMS session subjects completed approximately 20 min long sessions using a “Focus/Neureka!” protocol designed to train so called “Focused Attention” index (FA index) and “40 Hz-centered gamma” index (40Hz index) measures according to the specification of the Peak Performance Trainer (PAT) system (Neurotek, Goshen, KY). The goal of each subject was to enhance so called single-pointed “Focused Attention” index measure throughout the session while maintaining an adequate level of so called “Neureka!” measure (i.e., 40Hz index) within a certain range. All sessions were completed using different fragments of documentary films depicting nature scenes from the BBC “Planet Earth” and “Life” series, and National Geographic DVDs (e.g., “Africa's Wildlife”, “America's Greatest Animals”, “Birds of Paradise”, etc.). Different scenes were utilized to maintain the engagement among the participants. Based on the thresholds set, the subject would receive biofeedback both in the visual and auditory modalities. Visual feedback was arranged in a form of control of brightness, size, and continuation of the video by the “FA index” and “40Hz index” measures. Auditory feedback was used to inform subjects when these measures were under the threshold level, in the case of “FA index”, or outside the acceptable range, in the case of “40Hz index”. All EEG signals and training parameters were measured using 3 electrodes, one active electrode at the prefrontal EEG (FPz) site, the second being a reference on the left ear, and a third sensor serving as a ground and located between above two elctrodes. The sensors were soaked in a potassium chloride solution to enhance conduction.
Each subject completed a minimum of 18 weekly neurofeedback sessions, training to increase “Focused Attention” index and “40 Hz Gamma” index using the “Focus/Neureka” PAT protocol. The threshold for both indices was adjusted 4-5 times by experimenter during each session to maintain moderate difficulty. The target length of each session recorded was 15-25 min, with most sessions (85%) reaching the length goal of a 20-min minimum recording of usable EEG data. Eye blink artifacts removal was implemented using a custom made BioExplorer (BioExplorer 1.5, CyberEvolution, WA) application.
The EEG Signal Processing in neurofeedback sessions
Custom-made codes were programmed to effectively analyze and compute all the desired measures using Matlab software (MathWorks, Inc, Massachusetts). The EEG signal in the PAT device was collected and recorded with BioExplorer-based software application. The raw EEG and the separate desired frequency bands of data from each session can be exported in BioReview report, an extension application of BioExplorer. The report in BioReview is designed based on the Visual Basic program. According to what is desired, each filter function can be added to the report block diagram and then the settings of the filter are customized and edited. By configurations, along with the raw EEG signal, the separated delta (2-4 Hz), theta (4-8 Hz), alpha(8-13 Hz), low beta (13-18 Hz), high beta (18-30 Hz), and gamma (30-45 Hz) are also acquired after being exported from BioReview. In this configuration, each data point was exported to a text file in which the different measures were organized into columns and each subsequent row represented the change in time between samples.
After exporting the text file from BioReview, the data was then transferred to be analyzed and processed in Matlab software. For calculation of the relative power, it is necessary to gain the total power of the band from 2 Hz to 45Hz (the whole bands from delta to gamma frequencies). Therefore, a custom band-pass filter application created by the integration of wavelet transformation and a Blackman-Harris window configuration that separates the 2-45Hz portion of the raw signal into its own filtered signal was designed in Matlab. In the study, the sample-rate of the raw signals in Bioexplorer system is 256 Hz. Eighteen sessions of EEG signals from prefrontal site were recorded for each subject and there were 25-30 min data in each session, from which 20 min data (excluding first and last minutes of session) were analyzed to detect changes of EEG during each session. Relative power calculations were completed in Matlab. Only relative power of gamma was used as an individual band of interest. Other measures were ratios of selected EEG bands. The ratios of interest for this study were theta (4-8 Hz) to low beta (13-18 Hz) – theta/low beta ratio, and theta to high beta (18-30 Hz) – theta/high beta ratio.
Clinical social and behavioral evaluation outcomes
For the evaluation of social and behavioral functioning we utilized caregiver reports and clinician ratings of improvement. Every participant was evaluated before TMS course and within 2 weeks following TMS treatment. Aberrant Behavior Checklist (ABC, Aman and Singh 1994, Aman 2004) is a clinician administered rating scale to assess Irritability, Lethargy/Social Withdrawal, Stereotypy, Hyperactivity, and Inappropriate Speech based on parent/caregiver report. Each area contains multiple items receiving a rating from 0 to 3. Items are summed and high scores for each area reflect severity of the problem area. The ABC has been shown to be effective in assessing behavior changes in autism [ (Aman 2004). Specifically, for this study we used the Irritability, Lethargy/Social Withdrawal and Hyperactivity subscales of the ABC as outcome measures, as stereotype behavior is more reliably measured by the RBS questionnaire. Repetitive Behavior Scale—Revised (RBS-R, Bodfish et al., 1999) is a caregiver completed rating scale (ratings from 0 to 3) assessing stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted range (Bodfish et al., 2000). Items from the RBS-R scales are summed to obtain a measure of severity of repetitive behavior. The RBS-R was validated in independent samples and showed high internal consistency and interrater reliability (Lim and Aman 2007). Both questionnaires are well established in autism research and treatment clinics.
Statistical Analysis
The primary model for statistical analyses of subject-averaged ERP and motor response data was the two factor repeated measure ANOVA. Dependent ERP variables were amplitude and latency of ERP at pre-determined ROIs. The within-participant factors were followings: Stimulus (Kanizsa target, Standard, Kanizsa Non-target), Hemisphere (Left, Right), and Time (Baseline, Post-treatment). The between-subject factor was Group (TMS, Wait-List). Post-hoc analyses were conducted where appropriate. Reaction time (RT), error rate (commission, omission and total error rate), were analyzed using Time and Group factor. For clinical behavioral rating scores a Treatment (pre-vs. post-TMS-NFB/or waiting period) ANOVA was completed to determine changes associated with active stimulation and wait-list conditions. Histograms with normal distribution curves along with skewness and kurtosis data were obtained for each dependent variables to determine normality of distribution and appropriateness of data for ANOVA and t-tests. For more reliable determination of normality of distribution residual plots (i.e., normal probability plot, histogram, versus fits and order) were created using Minitab statistical package to indicate that treatment with ANOVA is justified. All dependent variables in the study had normal distribution. Greenhouse-Geisser corrected p-values were employed where appropriate in all ANOVAs. A-priori hypotheses were tested with the Student's t-tests for 2 groups with equal variance. Confidence intervals (95% of mean, [95% CI]) were calculated for each ERP data sets entered for t-tests. For the estimation of the effect size and power (Murphy and Myors, 2004) we used Partial Eta Squared (η2) and observed power computed using alpha (α)=0.05. The primary statistical analyses of neurofeedback data included linear regression estimation of each EEG dependent variable over 18 sessions of post-TMS neurofeedback course. For each dependent EEG variable analyzed using t-test, normality of distribution was calculated to ensure appropriateness for the test. SPSS 19.0 and Sigma Stat 3.1 statistical packages were used for the analysis of data.
RESULTS
EEG activity measures across 18 sessions of post-TMS neurofeedback training
Relative power of gamma and theta/beta ratios
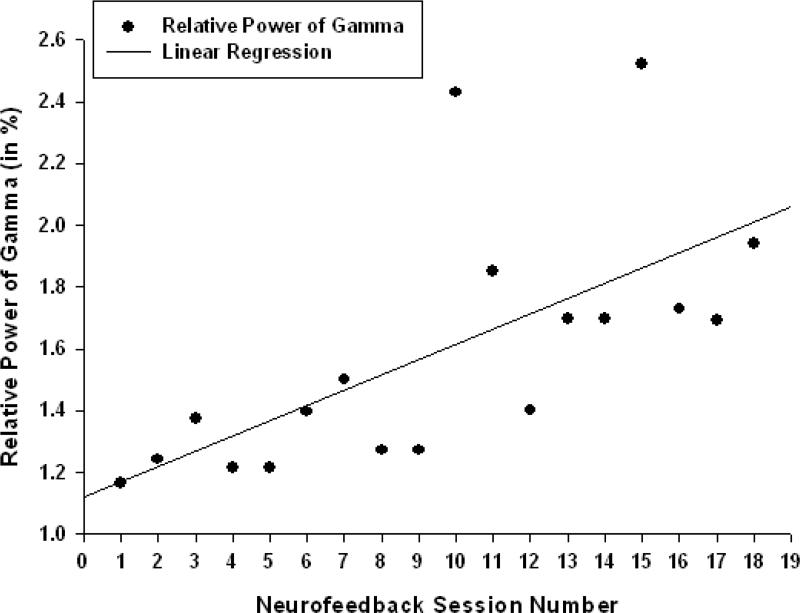
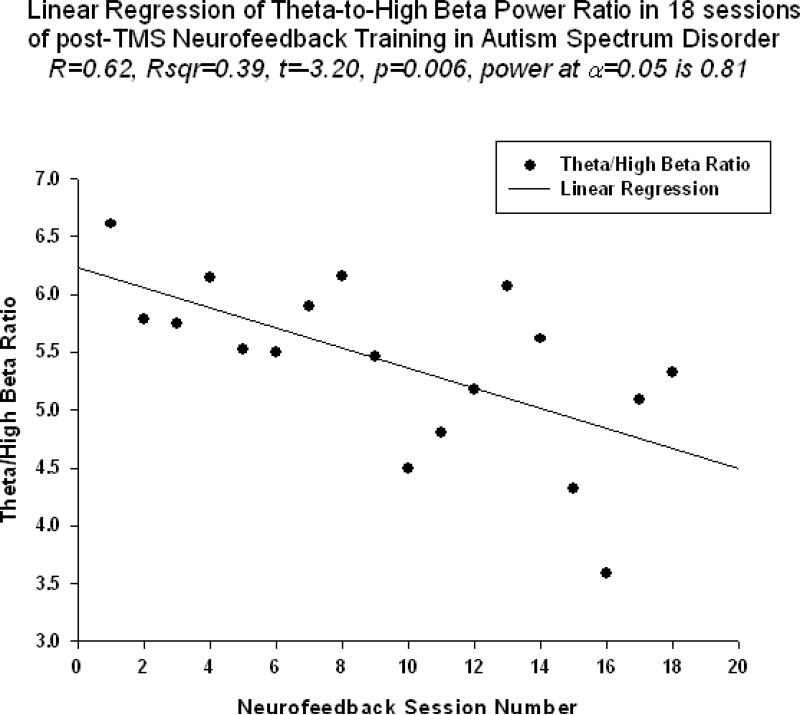
Relative power of gamma activity (power within 30-45 Hz vs. total power in 2-45 Hz, in percents) showed statistically significant linear increase over 18 sessions of neurofeedback ( linear regression: R=0.656, R2=0.431, t=3.48, p=0.003, power of test was 0.861 at alpha (α)=0.05, see Fig. 2., Table 1). Theta/low beta ratio showed statistically significant linear decrease over 18 sessions of post-TMS neurofeedback ( R=0.591, R2=0.349, t=-2.92, p=0.01, power= 0.748). Regression of the theta/high beta ratio over 18 session long post-TMS neurofeedback course was also significantly linear (R=0.625, R2=0.391, t=-3.20, p=0.006, power=0.810, see Fig. 3).
Figure 2.
Linear regression of the relative power of gamma band over 18 sessions of neurofeedback training following rTMS in children with ASD (R=0.656, y= 8.75x-4.14%, t=3.48, p=0.003).
Table 1.
Linear regression statistics of relative power of EEG bands, their ratios and neurofeedback training indices (“Focused Attention” index and “40 Hz gamma” index) over 18 sessions of neurofeedback course
| Measures | Units | t | P-value | R | R 2 | Regression Equation | Power |
|---|---|---|---|---|---|---|---|
| Gamma | % | 3.48 | .003 | .656 | .431 | y= 8.75x-4.41 | .861 |
| Theta/Low beta | N/A | −2.92 | .010 | .591 | .349 | y=−.091x+9.26 | .748 |
| Theta/High beta | N/A | −3.20 | .006 | .625 | .391 | y=−4.48x+33.72 | .810 |
| ‘Focused Attention’ | C.U. | 2.95 | .009 | .594 | .353 | y= 4.00x-288.28 | .755 |
| ‘40 Hz gamma’ | C.U. | 3.83 | .001 | .692 | .479 | y= 2.16x-85.91 | .910 |
Figure 3.
Linear regression of the theta/high beta ratio over 18 sessions of neurofeedback training in children with ASD (R=0.625, y= -4.481x+ 33.72, t=-3.20, p=0.006).
Neurofeedback training indices
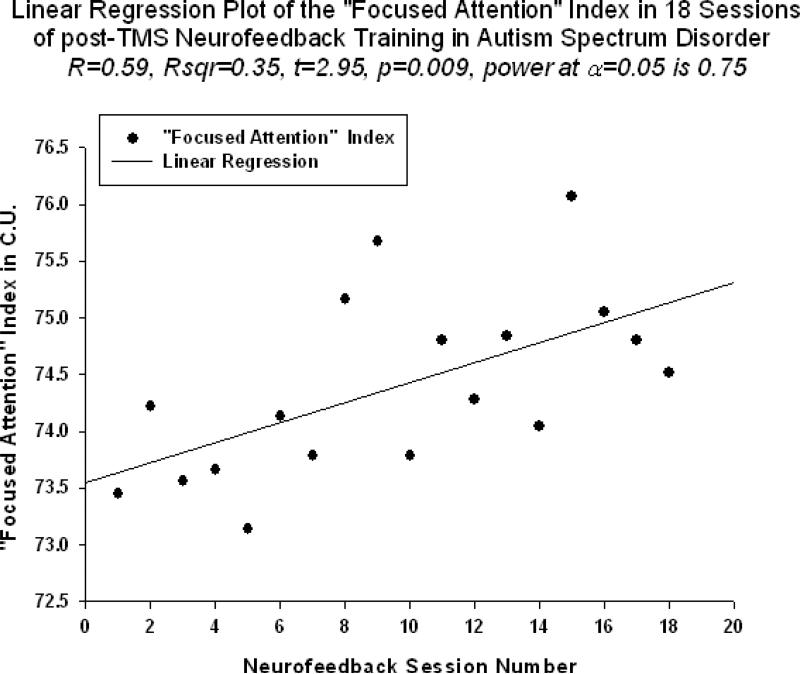
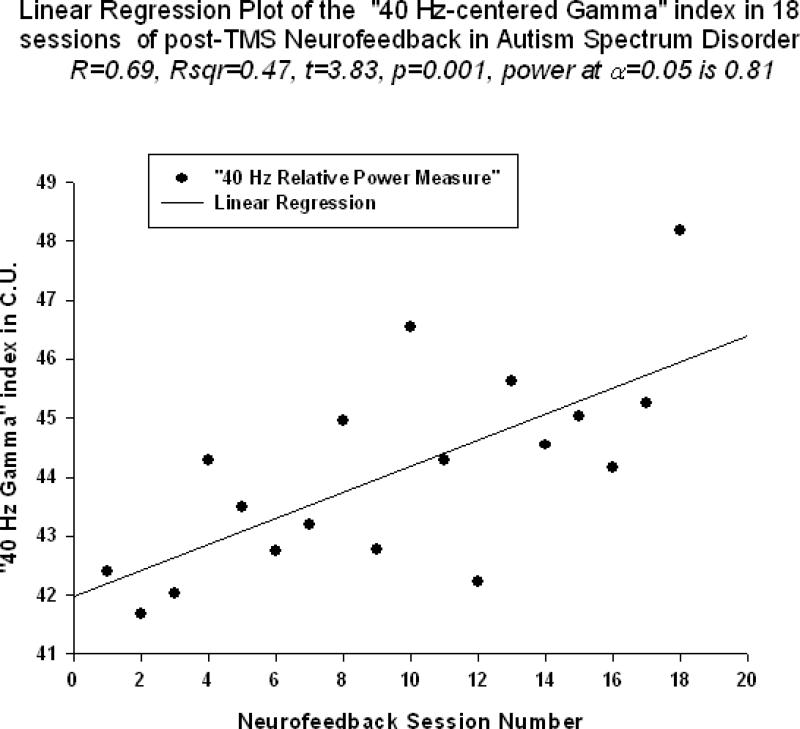
Neurofeedback measure reflecting relative power of 40-Hz centered gamma activity (i.e., “40Hz centered gamma” index) also showed significant linear increase trend over 18 sessions of training (R=0.692, R2 = 0.479, t=3.83, p=0.001, power=0.910, Fig.4). This neurofeedback training measure ( hereafter referred to as “40Hz gamma” index) showed significant positive Pearson correlation coefficient with relative gamma power across 18 session of training(r=0.659, p=0.003). “Focused Attention” index (FA index, i.e., “Inhibit All” measure in neurofeedback) also did show statistically significant linear increase over 18 sessions of post-TMS neurofeedback training (R=0.594, R2 = 0.353, t=2.95, p=0.009, Fig.5). The “Focused Attention” index showed negative correlation with the theta/low beta ratio (r=-0.629, p=0.021), but only tended to correlate with the theta/high beta ratio (r=-0.437, p=0.07, n.s.) across 18 sessions of post-TMS neurofeedback.
Figure 4.
Linear regression of the “Focused Attention” index ( “Inhibit All” measure) over 18 sessions of training (R=0.594, y=4.00x -288.2, t=2.95, p=0.001)
Figure 5.
Linear regression of the “40-Hz centered Gamma” index over 18 sessions of training (R=0.692, y=2.165x -85.91, t=3.83, p=0.001)
Outcomes of visual oddball task with illusory figures
Behavioral Responses (Reaction Time and Accuracy, Post-error RT)
Reaction Time (RT)
Effects of TMS and NFB combination on RT to targets were not significant. Comparison of RT to targets yielded no Time X Group effects.
Accuracy
Commission and omission errors analysis yielded a significant between-group post-treatment difference only in the commission error percentage, F(1, 40) = 5.40, p = 0.024.
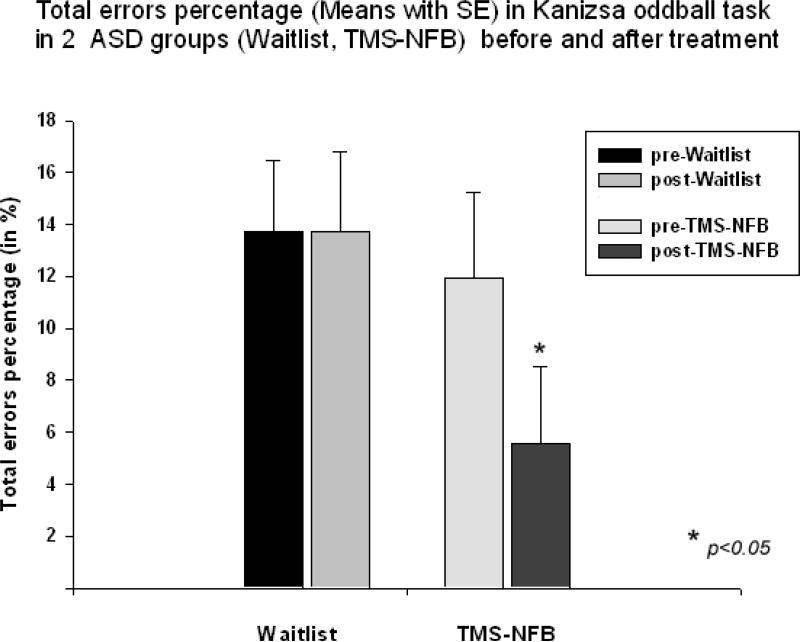
T-test showed significant decrease of commission error rate in the TMS group (mean decrease -5.29 ± 10.78 %, 95% CI from -9.95 to -0.62 %, t(19) = -2.35, p = 0.028). We could not find between group differences in omission error rate. Total error rate still did show main effect (F(1,40)=4.10, p=0.048). We found significant Time (pre, post) X Group (TMS-NFB, WTL) interaction (F(1,40)=5.89, p=0.019, η2=0.111, power=0.662). Total error rate (% errors) showed decrease only in TMS-NFB group (-6.36 ± 2.78%, 95% CI from -12.14 to -0.57 %, t(19)=2.64, p=0.013, Fig 6.).
Figure 6.
Total error percentage in visual oddball task with illusory Kanizsa figures in two groups of subjects with ASD (Waitlist, TMS-NFB) before and after treatment (wait, TMS-NFB).
Post-error RT
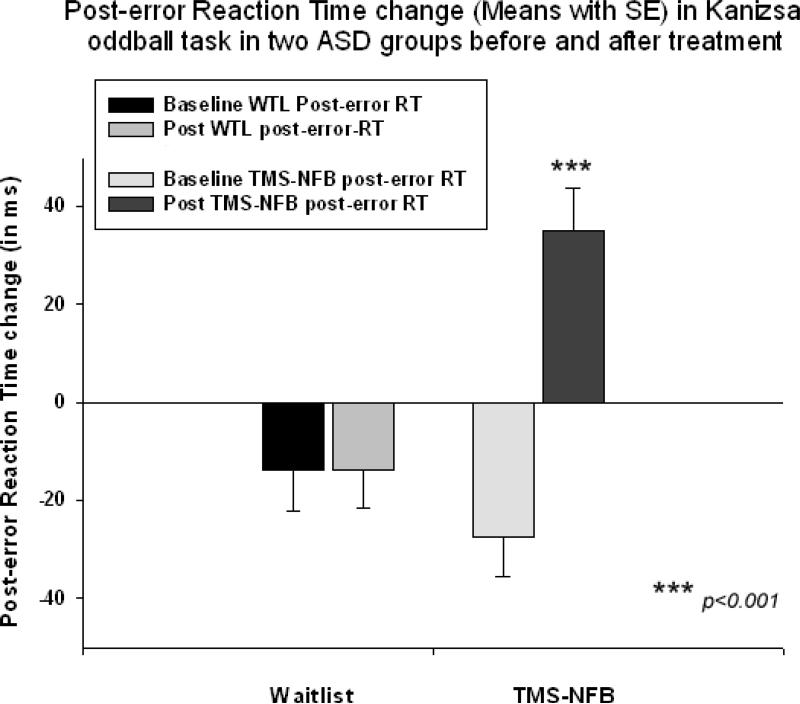
Main effect of Time (Pre, Post) on normative post-error RT slowing was highly significant (F(1,40)=16,39, p<0.001). Repeated measure ANOVA of post-error RT slowing (Time X Group interaction) also did show this strong and powerful effect (F(1,40)=27.72, p<0.001, η2=0.371, observed power =0.999). The TMS-NFB group showed post-error RT increase with significant positive change in post-error RT (Fig. 7). This change was computed as post-treatment post-error RT change minus pre-treatment post-error RT change (62.4 ± 60.5 ms, 95% CI from 36.2 to 88.5 ms, t(19) = 4.96, p< 0.001). Figure 7 shows that at the baseline both in WTL and TMS-NFB groups post-error RT was negative while in the TMS-NFB group post-error RT became positive (i.e., showed normative slowing), whereas it remained negative in the WTL group.
Figure 7.
Post-error reaction time (RT) change in visual oddball task with illusory Kanizsa figures in two groups of subjects with ASD (Waitlist, TMS-NFB) before and after treatment (wait, TMSNFB). The TMS-NFB group shows significant normative post-error RT slowing after treatment.
Motor response-locked frontal and fronto-central ERN and Pe
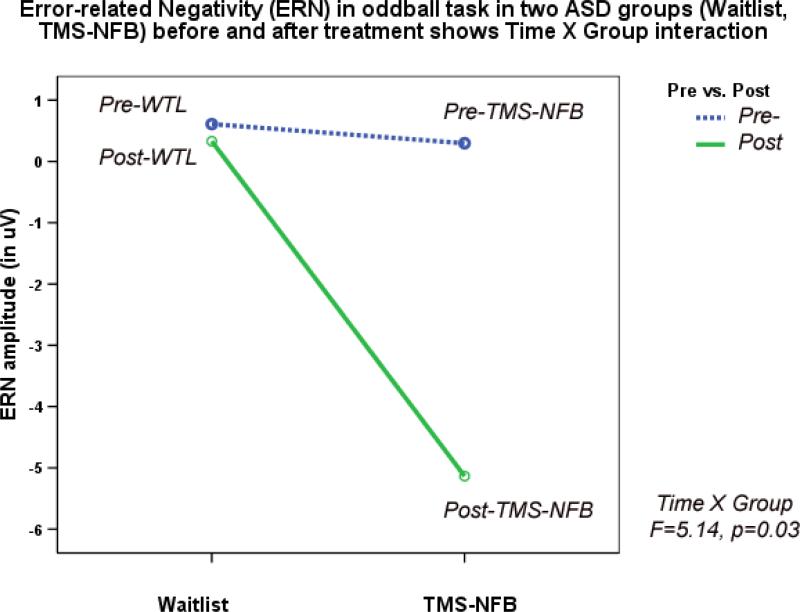
One subject did not show sufficient number of commission errors and was excluded from the analysis. TMS-NFB and WTL groups showed significant differences in ERN amplitude (F(1,39)=5.00, p=0.032) and latency (F(1,39)=8.74, p=0.006) post-treatment. Amplitude of ERN during commission errors across 5 frontal and fronto-central sites showed significant Time X Group interaction ( F(1,39)=6.32, p=0.017), and paired-sample t-test showed significant increase of ERN negativity in the TMS-NFB group (4.87± 5.33 μV, 95% CI from 2.42 to 7.29 μV, t(19)=4.17, p>0.001, see Fig. 8). Figure 8 shows ERN amplitude at the first (baseline) and at the second test, where baseline values across two groups were practically identical, whereas post-treatment TMS-NFB group shows substantial increase of negativity.
Figure 8.
Amplitude of Error-Related Negativity (ERN/Ne) in visual oddball task in two ASD groups (Waitlist, TMS-NFB) before and after treatment shows Time X Group interaction (F=5.14, p=0.03) expressed in a significant increase of the ERN negativity post-treatment in the TMS-NFB group.
The T-test of the ERN latency changes in the TMS-NFB group showed significant decrease (-25.1 ± 38.0 ms, 95% CI from -7.8 to -42.1 ms, t(18)=3.03, p=0.007). Amplitude of the Pe wave over midline frontal and fronto-central sites in the TMS-NFB group showed moderate increase (3.59 ± 6.26, t(18)=2.63, p=0.016) , but both groups were not statistically significantly different at the post-treatment test ((F(1,39)=3.20, p=0.083, n.s., see Table 2 ).
Table 2.
One way ANOVA of frontal and parietal ERPs to targets at post-treatment stage (TMS-neurofeedback [TMS-NFB, N=20] vs. Wait-list group [N=22])
| Dependent variables | TMS-NFB Mean ± SD | Wait-list Mean ± SD | F value, df F(1,40) | Significance P value |
|---|---|---|---|---|
| Frontal & fronto-central: | ||||
| N100 amplitude (μV) | -2.62±2.52 | -3.22±3.00 | 0.14 | 0.705 |
| N100 latency (ms) | 137.3±14.0 | 122.5±18.2 | 8.75 | 0.005** |
| P2d difference wave (μV) | 0.77±4.50 | -2.11±3.66 | 5.25 | 0.027* |
| P2d wave latency (ms) | 320.5±33.5 | 335.0±49.9 | 1.20 | 0.279 |
| N200 amplitude (μV) | -201±3.95 | -2.02±3.52 | 0.93 | 0.762 |
| N200 latency (ms) | 356.15±39.9 | 300.5±35.9 | 20.72 | >0.001*** |
| P300(P3a)amplitude(μV) | 5.57±4.65 | 6.54±3.99 | 1.15 | 0.289 |
| P300(P3a) latency (ms) | 434.9±27.0 | 409.6±55 | 3.45 | 0.070 |
| Parietal & centro-parietal: | ||||
| P200 amplitude (μV) | 2.58±2.69 | 3.95±2.65 | 3.03 | 0.089 |
| P200 latency (ms) | 156.5±49.2 | 124.7±16.8 | 8.11 | 0.007*** |
| N200 amplitude (μV) | -2.66±2.81 | -2.78±3.64 | 0.016 | 0.899 |
| N200 latency (ms) | 244.1±63.6 | 201.3±24.3 | 10.39 | 0.002** |
| P300(P3b) amplitude(μV) | 2.47±4.46 | 7.57±9.40 | 4.73 | 0.035** |
| P300(P3b) latency (ms) | 375.6±56.3 | 323.7±27.9 | 16.07 | >0.001** |
| Fronto-central: | ||||
| ERN/Ne amplitude (μV) | -4.31±4.79 | -0.02±6.05 | 5.00 | 0.032* |
| ERN/Ne latency (ms) | 68.44±27.7 | 108.1±49.3 | 8.74 | 0.006** |
| Pe amplitude (μV) | 8.57±5.71 | 5.53±4.14 | 3.20 | 0.083 |
| Pe latency (ms) | 176.4±41.3 | 202.2±44.8 | 3.13 | 0.086 |
| Reaction time, accuracy: | ||||
| RT (ms) | 507.9±104.39 | 455.5±123.3 | 2.53 | 0.118 |
| Commission error (%) | 2.96±3.67 | 11.50±17.24 | 5.409 | 0.024* |
| Omission error (%) | 2.65±4.35 | 2.91±4.35 | 0.178 | 0.675 |
| Total error (%) | 5.59±7.26 | 13.71±17.95 | 4.10 | 0.048* |
| Post-error RT change (ms) | 34.95±49.9 | -13.90±33.84 | 16.39 | >0.001*** |
Frontal and fronto-central ERP components
N100
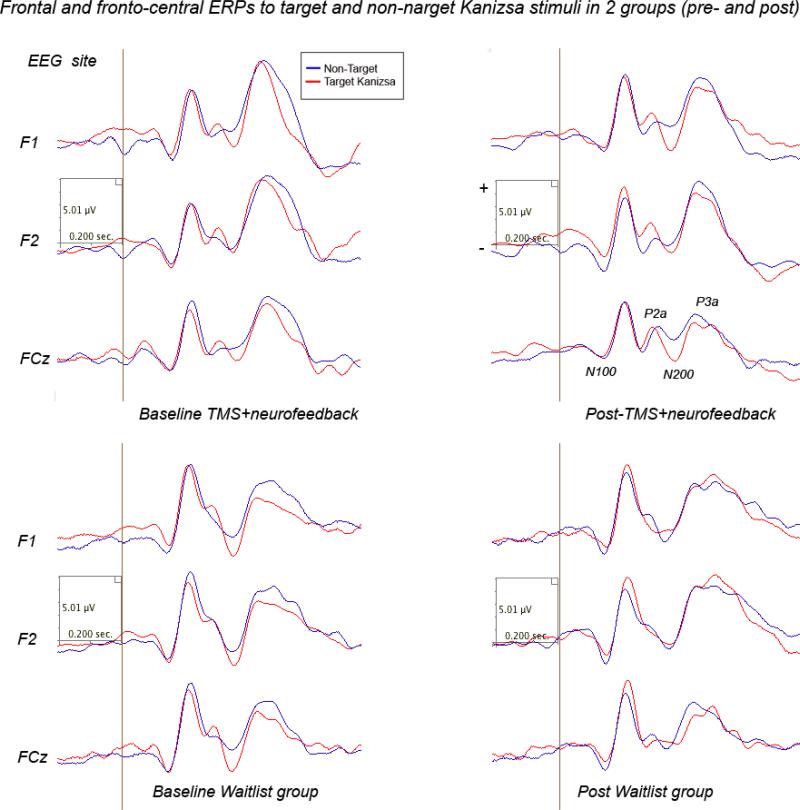
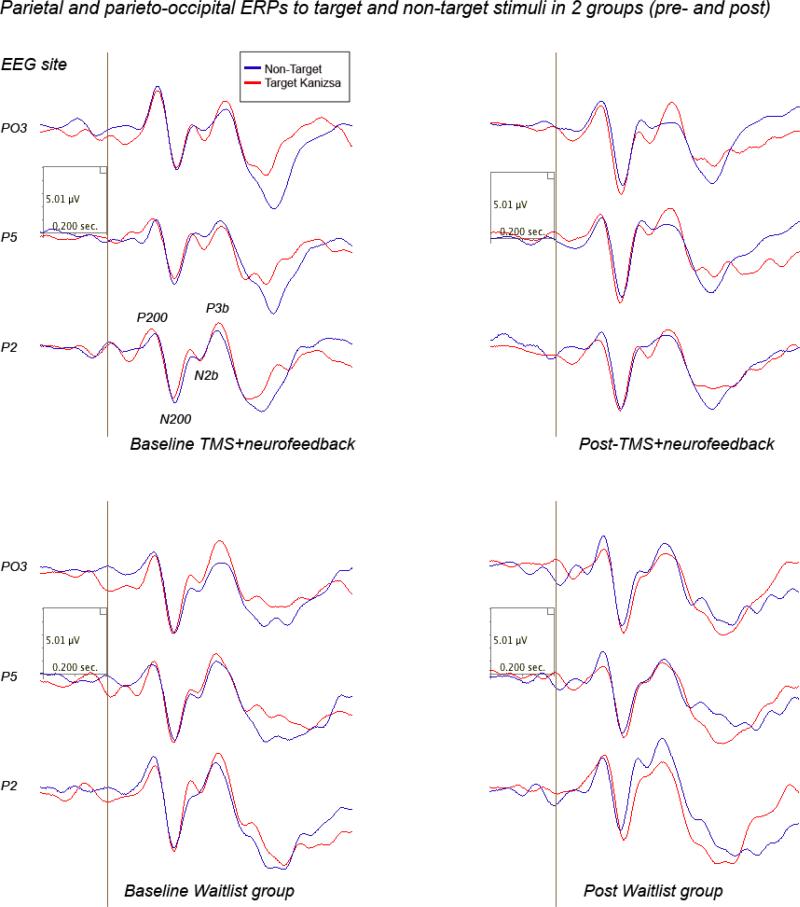
Comparison of post-treatment amplitude and latency of N100 ERP component showed prolonged latency target Kanizsa figures in the TMS-NFB group, while N100 magnitude was practically unchanged in the WTL group. Effects of Time factor on latency to targets was significant (F(1,40)=8.75, p=0.005). Effects of Time on frontal N100 to non-target Kanizsa stimuli was also statistically significant (F(1,40)=5.81, p=0.021). A Stimulus (target Kanizsa, non-target-Kanizsa) X Hemisphere (left, right) X Time (pre, post) X Group (WTL, TMS-NFB) interaction was significant, F=5.29, p=0.027, η2= 0.122, power at α=0.05 was 0.612. Interaction was expressed in a tendency to lower amplitude to non-targets and lower hemispheric differences post-treatment in the TMS-NFB group. Waveforms of frontal and fronto-central ERPs to target and non-target stimuli are depicted at Fig.9.
Figure 9.
Frontal and front-central ERPs to target and non-target Kanizsa stimuli in 2 ASD groups (grandaverages: Waitlist, N=22, and TMS-NFB, N=20) before and after treatment.
N200
There was observed significant between Group (TMS, WTL) difference in N200 latency at the post-treatment test (F(1,40)=20.72, p<0.001). N200 amplitude showed significant Stimulus X Hemisphere X Time X Group interaction effect (F(2,38)=5.14, p=0.03, η2=0.176, observed power =0.631) featured by lower amplitude to non-target stimuli, less pronounced hemispheric asymmetry post-treatment in the TMS-NFB group. Stimulus X Time X Group interaction was also significant (F=7.49, p=0.01).
P2d
The frontal P2a calculated as a mean difference between P2a amplitude to target Kanizsa minus P2a amplitude to non-target Kanizsa stimuli. Combined TMS-NFB treatment had significant effect at P2d amplitude (F(1,52)=5.25, p=0.027) but not on latency of P2d (p=0.279, n.s.). Difference wave (P2d) amplitude showed highly significant Time X Group interaction effect, F(1,40)=8.92, p=0.005, η2=0.182, power=0.830 expressed as higher and positive difference wave in post-treatment test in the TMS-NFB group. Paired sample t-test confirmed significance of the post TMS-NFB treatment effect on P2d amplitude (4.70 ± 8.14 μV, 95% CI from 0.77 to 8.63 μV , t(19)=2.51, p=0.021).
P300 (P3a)
The treatment had no main effect on the amplitude of the frontal P300 (P3a) component. P300 (P3a) amplitude ANOVA yielded only moderate Time X Group interaction effect, F(1,40)=4.70, p=0.036, η2=0.103, power=0.562. The effect was manifested as a tendency to lower amplitude of this component to all stimuli in the TMS-NFB group as compared to the WTL group post-treatment. The latency of P3a had a trend to be more prolonged post-treatment in the TMSNFB group, but effect did not reach significance level (p=0.07, n.s.).
Parietal and parieto-occipital ERP components
P200 and N200
TMS-NFB course had main effects on latency of posterior P200 component to targets (F(1,40)=8.11, p=0.007). In particular response of this parietal and parieto-occipital P200 component to targets showed post-treatment between group difference in latency (156.5± 49.2 ms in TMS-NFB vs. 124 7± 16.8 ms in WTL). There were no group differences in amplitude of the parietal N200 component. Latency of N200 to targets showed post-treatment between group difference (244.2± 63.5 ms in TMS-NFB vs. 201.3 ± 24.3 ms in WTL group, F(1,52)=10.39, p=0.002). ANOVA analysis of the amplitude and latency of parietal N200 to target and non-target Kanizsa stimuli showed did not show any interaction on Stimulus, Time, Hemisphere or Group factors. Posterior ERPs to target and non-target items are depicted at Fig 10.
Figure 10.
Parietal and parieto-occipital ERPs to target and non-target Kanizsa stimuli in 2 ASD groups (grandaverages: Waitlist, N=22, and TMS-NFB, N=20) before and after treatment.
P300 (P3b)
We found between group differences in P3b amplitude that were expressed as more attenuated magnitude post-treatment in TMS-NFB as compared to WTL group (lower amplitude to targets, F(1,40)=4.73, p=0.035, prolonged latency, F(1,40)=16.07, p<0.001). There were not found any ERP amplitude interaction effects. The latency of P3b showed marginally significant Stimulus x Time X Group interaction, F(2,38)=3.24, p=0.049, η2=0.14, observed power =0.585, characterized by increased latency to targets at post-treatment test in the TMS-NFB group.
Clinical Behavior Evaluations post- TMS-NFB
RBS-R
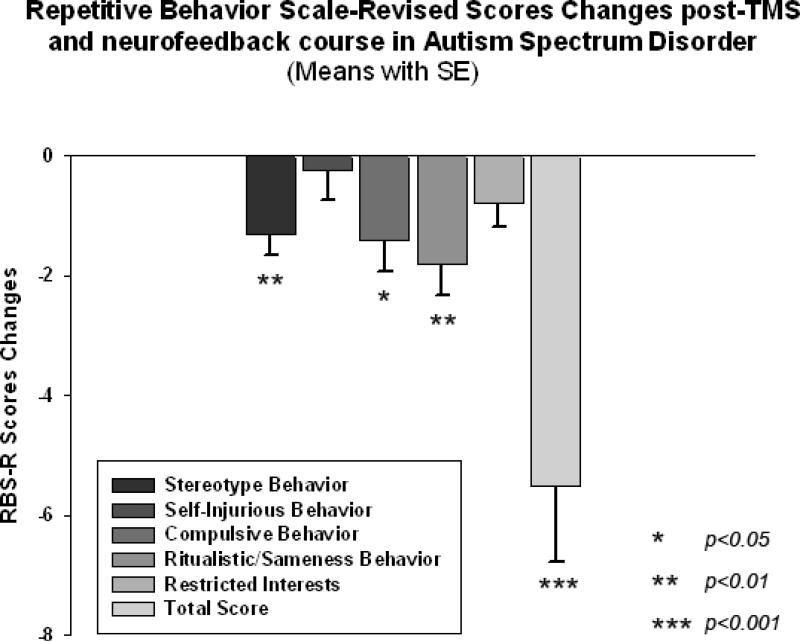
We found a significant decrease in stereotype repetitive and restricted behavior patterns and compulsive behavior following 18 sessions of combined rTMS-neurofeedback treatment as measured by the RBS-R (Bodfish et al. 1999). Time (pre, post) X Group (TMS-NFB, WTL) interaction for Total score of RBS was statistically significant (F(1,40)=7.74, p=0.008, η2=0.14, observed power was 0.99). Individual scores for the TMS-NFB group were further analyzed using a paired sample Student's t-test. Total RBS-R score decreased from 22.5 to 17.0, mean decrease being -5.5 ± 5.7, 95% CI from -2.83 to -8.16, t(19)=4.31, p<0.001. Changes in individual subscale scores is depicted at the Fig 11, where both Stereotypic Behavior subscale and Ritualistic/Sameness Behavior subscale scores show significant decrease (accordingly -1.30 ± 1.59, 95% CI from -0.55 to -2.04, t(19)=3.65, p=0.002 and -1.80 ± 2.44, 95% CI from -0.65 to -2.94 t(19)=3.29, p=0.004). None of the WTL group scores showed any statistical changes (e.g., Stereotype Behavior scores decrease was only -0.30 ± 0.60, while Ritualistic/Sameness Behavior scores did not change either, -0.05 ± 0.30, both not significant).
Figure 11.
Changes of Repetitive Behavior Scale (RBS-R) scores post-TMS-NFB treatment as compared to baseline levels in children with ASD (N=20). Stereotype Behavior, Compulsive Behavior, Ritualistic/Sameness Behavior and Total RBS scores decreased significantly in the TMS-NFB group.
ABC
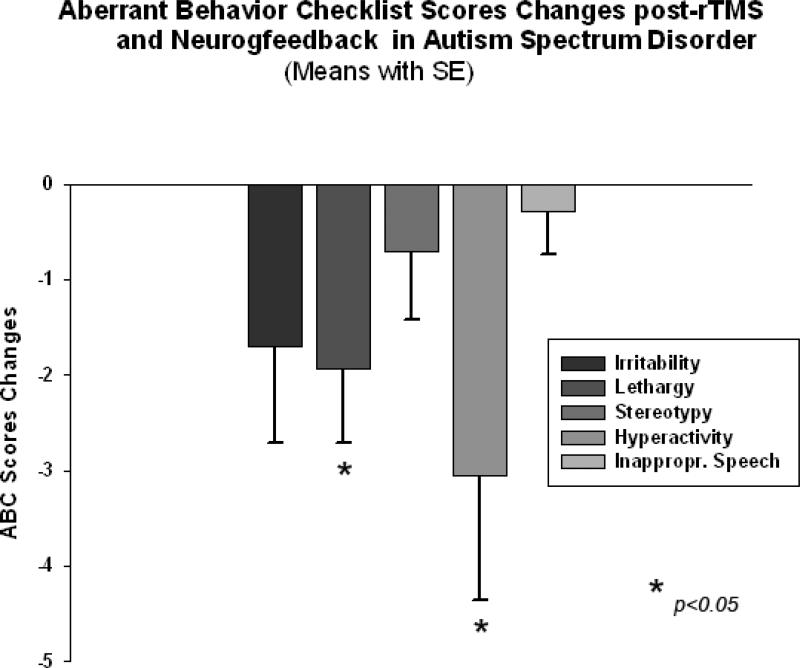
ANOVA analysis of the ABC (Aman and Singh 1994) subscale scores showed significant Time X Group interaction effect for Lethargy/Social Withdrawal scores (F(1,40)=4.45, p=0.04, η2=0.09 with observed power only 0.543) and for Hyperactivity scores (F(1,40)=5.52, p=0.023, η2=0.11, power=0.633). Paired sample t-test for the TMS-NFB group identified significant reduction in the Lethargy/Social Withdrawal subscale, i.e.,-1.94 ± 3.22, 95% CI from -0.29 to -3.59, t(19)=2.49, p=0.024. Hyperactivity subscale showed even more pronounced score reductions (-3.06 ± 5.39, 95% from -0.28 to -5.83, t(19)=2.34, p=0.033). Changes of individual subscale rating scores in TMS-NFB group are depicted at the Figure 12. The WTL group had no significant differences in any of ABC scale ratings as a result of the waiting period . For instance, the Lethargy/Social Withdrawal scores decrease in the was only -0.19 ± 0.70, while the Hyperactivity score decrease was only -1.05 ± 2.06, both not significant statistically.
Figure 12.
Changes of Aberrant Behavior Checklist (ABC) scores post-TMS-NFB treatment as compared to baseline levels in children with ASD (N=20). Lethargy and Hyperactivity rating scores decreased significantly post-TMS-NFB treatment course.
Discussion
The outcomes of behavioral evaluations using RBS-R (Bodfish, Symons & Lewis, 1999) and ABC (Aman and Singh, 1994) questionnaires show improvements in autism symptoms similar to those that we reported in our other study when 18 session long 1 Hz rTMS course was used in 27 children with ASD (Sokhadze et al., 2014). Psychophysiological outcomes of the study show significant changes in behavioral responses (motor response accuracy and post-error RT slowing) and both early and later-stage ERP indices of task-relevant signal processing as a result of 18 sessions of low frequency rTMS treatment over DLPFC combined with prefrontal neurofeedback training course in 20 children with ASD. Most pronounced improvements in ERP measures are observed at the frontal and fronto-central ROI (e.g., N100, P2d, N200, P3a components), as compared to posteriror ROI (i.e., parietal and parieto-occipital P200, N200 and P3b) where outcomes were mostly expressed in the latency changes. Only parietal P3b showed improvements expressed both in amplitude and latency changes post-treatment. The results of baseline analysis both in the TMSNFB and WTL groups indicate the excess of efforts needed for the differentiation of targets from non-target Kanizsa figures in individuals with ASD. Our findings demonstrate that integrated TMSNFB treatment enhanced the process of target recognition during performance on task. Especially significant and informative in this regard was positive change of the frontal P2d difference wave that indicates increase of P2a component to target Kanizsa stimuli vs. non-target Kanizsa stimuli, thus reflecting easier discrimination of target features of the stimuli (illusory square vs. illusory triangle).
At the same frontal topography N200 had longer latency resulting in globally higher magnitude of N200 to targets. The positive frontal P2d difference wave that occurs simultaneously with the posterior negative ERP N200 component (both of them peaking within 280-320 post-stimulus) in visual oddball tests tasks are associated with categorization, perceptual closure and attention focusing ultimately signaling that a perceptual representation has been formed (Potts et al. 2004). These components are enhanced if the presented stimulus contains a feature or attribute defining the target in the task according to Potts et al. (2004). It was previous reported (Baruth et al. 2010c; Sokhadze et al. 2009a,b) that individuals with ASD as compared to typical controls showed enhanced N200 to task irrelevant as compared to task relevant stimuli, and the finding that N200 became more negative to target Kanizsa figures and less negative to non-target distracters post-rTMS treatment indicates a trend to normalization of the response pattern pointing at an improved visual signal processing and a more effective discrimination of the target. We reported very comparable frontal ERP outcomes in our study on children with ASD enrolled in 18 session long TMS treatment course (Sokhadze et al. 2014).
Over-activation in the parietal cortex at the early and middle stages of processing of non-target stimuli, either standards or infrequent distracters, and at the same time under-activation of integrative frontal and fronto-central ROI at the late stages of target processing was found to occur in autism in a similar visual task that was using three-stimuli paradigm with rare novel distracters (Sokhadze et al. 2010a,b). Our results in a series of visual oddball tasks repeatedly reported enhanced and prolonged early frontal ERPs and a delayed and enhanced P3a to non-target stimuli, which would suggest low selectivity in pre-processing stage, and under-activation of integrative regions at the later stages of signal processing. Overall, this is a sign of an over-connected network where sensory inputs evoke abnormally large evoked potentials for unattended stimuli such as frequent standards and rare novel distracters at all stages of visual signal processing with signs of a reduced selectivity of the activation in response to incoming signal.
The results of the current study indicate that rTMS-NFB treatment may have facilitated attention and target discrimination by improving conflict resolutions during processing task-relevant and task-irrelevant stimuli. It should be noted, that the latency of posterior P3b was prolonged to targets but reduced to both non-target Kanzisa and non-Kanizsa stimuli following rTMS-NFB course. The P3b has been linked to task-relevance and the decision- related character of the stimulus as it indicates memory-updating and individual trial processing closure (Picton 1992). Earlier we (Sokhadze et al. 2009a,b, 2012b) noted that individuals with autism showed prolonged P300 peak to irrelevant distracters as compared to typical controls, which was similar to effects reported by other groups (Courchesne et al. 1989; Townsend et al. 2001). Majority of the ERP in studies in autism emphasize over-activation as well as an abnormal pattern of basic perceptual processes such as low selectivity regardless of modality, abnormal top-down attentional control including delayed attentional orienting to novel stimuli, and deficits in information integration processes (Belmonte and Yurgelun-Todd 2003). In typically developing children the fronto-central P3a occurs earlier in time as compared to parietal P300 (P3b), but in autistic subjects the P3a and P3b components were found to peak almost simultaneously over the frontal and parietal sites in a spatial attention test (Townsend et al. 2001). One of the most important findings of current study was replication of the increase of ERN amplitude and shortened latency post-TMS reported in previous studies using 12 and 18 sessions of rTMS (Sokhadze et al. 2012, 2014).
The results of the study may indicate facilitation of visual target discrimination processes and enhanced habituation to task-irrelevant distracters post-TMS-NFB neurotherapy. We report significant improvement in the accuracy of motor responses, lower total error rate and improved normative post-error RT slowing following 18 session long rTMS course. These result support our earlier findings that outlined improvement in attention, executive control, and irrelevant response inhibition post-TMS treatment in autism, this time using integrated treatment that combined TMS and neurofeedback.
Similar to our prior work in the treatment of ASD with neurofeedback (Wang et al. 2014) , this study indicates the utility of neurofeedback for altering the EEG characteristics associated with the disorder and suggests that the prefrontal neurofeedback could be used to alter EEG in ASD, including changes in gamma range frequencies. From the very early studies conducted in late 70s by Bird, Newton, Sheer, and Ford (Bird et al., 1978a,b, Ford et al., 1980 ) on EEG gamma frequency neurofeedback, 40 Hz activity was considered as a psychophysiological biomarker of attention in humans, and further research on association of the 40 Hz-centered gamma activity with attention, especially in neurodevelopmental disorders such as ASD definitely warrants further explorations, either as stand-alone treatment or as an adjunct arm in a combined treatment similar to one used in this study.
It was feasible to select DLPFC as a site for rTMS stimulation and prefrontal site for neurofeedback training. The DLPFC processes components of working memory, decision making process, and regulates the ability to focus attention on task-relevant goals while inhibiting responses to distracters (Enriquez-Geppert et al. 2010; Gray et al. 2003, Matzel and Kolata 2010). Suggested disruption in the ratio between cortical excitation and inhibition especially within the prefrontal cortex in individuals with autism (Casanova et al. 2002a, 2006a,b) was confirmed in individuals with Asperger syndrome (Casanova et al. 2002c). Reduced cortical inhibitory tone and an increased E/I ratio could adversely affect patterns of cortical activation (Tuchman and Rapin 1997). We proposed earlier that a course of 18 neuromodulatory sessions of low frequency rTMS may restore the cortical E/I balance by selective activation of double-bouquet cells at the periphery of cortical minicolumns (Casanova 2007, Casanova et al. 2006a,b; Sokhadze et al. 2012, 2014). It was shown that minicolumnar abnormalities in autism are most significant within the prefrontal cortex, more specifically, the DLPFC and the anterior cingulate cortex(ACC) (Casanova et al. 2006a,b, Casanova et al. 2002b; Fernandez-Duque et al. 2000; Mesulam 2000). Hence, selection of prefrontal site for neurofeedback was also driven by above listed considerations.
Rubinstein and Merzenich (2003) put forward a hypothesis that at some forms of autism could be caused by a disproportionate high level of excitation (E) or disproportionately weak inhibition (I) resulting in a high E/I ratio. Cortical circuits with such enhanced E/I level are proposed to be featured by poor functional differentiation which may lead to broad-ranging abnormalities in perception, memory and cognition, and motor control (Sokhadze et al. 2014). Among other defects, individuals with autism have well known perceptual processing abnormalities, including a hypersensitivity to sensory stimulation (Gomot et al. 2002; Plaisted et al., 2003). The E/I balance in the cortex is controlled by the relative numbers and functional activity of glutamatergic and GABA-ergic neurons. Neurodevelopmental abnormalities may lead to increased number, morphology or functional balance of excitatory vs. inhibitory neurons and can lead to a hyper-excitable state typical for autism. Excessive noise in cortical structures processing information also negatively affects development of normally differentiated representations (Casanova et al. 2012). Relatively undifferentiated representations of orienting signals or significant stimuli would result in larger and less selective response. Such over-representation by non-differentiated responses could account for the strong aversive reactions to auditory, tactile and visual stimuli that are common in autism.
Casanova, Buxhoeveden & Gomez (2003) study indicated that minicolumns in the brains of individuals with autism are narrow and have altered internal organization. More specifically, their minicolumns have less peripheral neuropil space, which is the conduit for inhibitory local circuit projections. A defect in these GABAergic interneurons may correlate with the increased E/I balance and prevalence of seizures among autistic patients. The authors concluded that GABAergic interneurons are vital for sensory signal processing (e.g., filtering capacity, proper signal discrimination, etc.), thus providing a putative correlate to autistic symptomatology. As it was noted in a recent review on use of TMS in ASD (Oberman et al. 2013), TMS could be particularly informative in detecting abnormalities in E/I ratios in ASD given theoretical studies regarding role of GABAergic interneurons in autism etiology (Hussman 2001) and specifically role of high E/I balance in autism (Casanova et al. 2003; Rubenstein and Merzenich 2003). Our current study is supportive of idea that rTMS is capable to improve E/I ratio as manifested in electrocortical responses to sensory stimulus processing in visual selective attention test. Another potentially very important outcome of rTMS might be in enhancement of gamma activity. Our results indicate that post-TMS neurofeedback sessions showed gradual increase over the course of post-TMS neurofeedback training. Along with the increase of the relative power of 40 Hz centered gamma activity our results showed gradual decrease of theta/low beta and theta/high beta ratios.
This neuromodulation study was guided by the “minicolumnar” theory of autism. Topographical studies of minicolumnar morphometry in autism spectrum disorder have shown the greatest deviance from neurotypicals within the prefrontal cortex (Casanova et al. 2002d, 2006a,b, 2010). Some investigators have explained this fact as resulting from the prolonged maturation time of this structure which thus provides a larger time window of opportunity for exogenous factors to alter its development (Casanova et al. 2014). Within the rostral brain region abnormalities within the DLPFC could serve as a pathological correlate to observed executive function deficits in autism (Casanova et al., 2014). Given the vertical orientation of inhibitory elements within the periphery of the minicolumns (e.g., double bouquet cells) it has been proposed that rTMS in ASD could preferentially help build the inhibitory surround of these modular structures. Since the dorsolateral prefrontal cortex has been a source of significant minicolumnopathy in published postmortem studies it could be viewed as a target for stimulation using rTMS (Casanova et al. 2002b, 2012). Furthermore, considering the trans-synaptic effects of rTMS, the large number of DLPFC connections could provide a therapeutic cascading effect in other parts of the brain. In autism computerized image analysis suggests the presence of a minicolumnopathy characterized by an increased density of modules and a diminution in their peripheral neuropil space (Casanova et al., 2002a). The deficits previously described by our group have been corroborated using a variety of neuronomorphometric techniques(e.g. Euclidean minimum spanning tree, gray level index), in an independent sample conducted by an international study where the investigators were blind to the study variables, and in the published results of other investigators (Buxhoeveden et al., 2006; Casanova et al. 2002d, 2006). The diminished width of the minicolumnar peripheral neuropil space is seen throughout laminae II-VI, suggesting a deficit of an anatomical element in-common to all layers (Casanova et al. 2010). Since inhibitory elements populate all layers of the lateral compartment of the minicolumn pathology involving these elements could contribute to a deficit in the lateral or peripheral inhibitory surround of these modules. These findings gain credence from EEG recordings using lateral masking paradigms and threshold studies using flutter stimuli that sustain the presence of a lateral inhibitory deficit in autism (Keita et al. 2011; Puts et al. 2014). It is plausible to propose that low frequency rTMS is increasing inhibitory tone and improving lateral inhibition, and this may result in an enhancement of executive functions. Prefrontal neurofeedback can be considered as an adjunct self-regulation training that may further enhance executive function control skills if post-TMS improvements are operantly conditioned.
In general, our findings are in concordance with a recent review of rTMS applications in autism research and treatment (Oberman et al. 2010, 2013). The authors concluded that , though results of published studies are promising suggesting that specific rTMS protocols (Enticott et al. 2010, 2012, 2013; Fecteau et al. 2011) targeting selected regions of cortex may lead to improvement in behavioral deficits in some individuals with ASD, the therapeutic results have been still of preliminary character and additionally, it is necessary to conduct the large-scale, randomized, placebo-controlled trials are necessary to establish the safety and efficacy of these neuromodulation protocols (Oberman et al., 2010, 2013). There are not yet reported any studies where rTMS was followed by neurofeedback training in autism, nor in any other psychophatology, as our study design is novel and has no analog approaches reported so far to make any outcome comparisons.
Some limitations to the study should be taken into account. It should be recognized that the power (90%) and schedule (number of magnetic pulses delivered per each session, 10-20 s break between trains, once per week treatment regimen, etc.) of our rTMS is relatively lower than those used by other TMS treatment protocols. However, it must be mentioned that other known TMS protocols were targeting psychopathologies such as treatment-resistant major depression, or neurological disorders such as Parkinson disease in adults. Since our pilot study was conducted on children we preferred to select more conservative approach to monitor changes in time and avoid potential over-stimulation. One more obvious limitation of the study is the use a waiting-list group as a control group rather than using a randomized clinical trial (RCT) design with a sham rTMS followed by neurofeedback condition and post-TMS sham-neurofeedback groups. Even though our group has a custom-made sham Magstim TMS coil and interface enabling blinding of TMS delivery, and we developed several methods of delivering sham-neurofeedback using PAT system, we considered this study as a preliminary pilot with a WTL group design, and plan to consider progression to a randomized clinical trial design on the future stages. It is possible to consider as a limitation also absence of follow-up tests and observations, especially comparing effects of rTMS course alone vs. combined TMS-NFB intervention arm. Since the study is still underway and has follow-up testing on schedule, we are collecting materials to make possible comparisons of follow-up outcomes of comparable groups that underwent 18 sessions of low frequency rTMS only vs.18 sessions of combined rTMS-NFB treatment.
In conclusion, the study showed that treatment with prefrontal low frequency rTMS followed by prefrontal neurofeedback improved ERP indices of attention to targets, reduced over-reactivity to non-targets, significantly reduced motor response errors to target stimuli, and enhanced response-locked potentials reflective of error monitoring and correction (e.g., ERN, post-error RT slowing, etc). We also found significant reductions in both repetitive and stereotypic behaviors, reduced repetitive behaviors, hyperactivity and lethargy scores according to social and behavioral clinical evaluations post-TMS-NFB treatment course. We consider that it is possible to conclude that neuromodulation using low frequency, inhibitory rTMS followed by prefrontal gamma upregulation neurofeedback improved executive functioning and behavioral symptoms in autism. This study provides further support to the statement that both TMS and neurofeedback can be regarded as perspective neuromodulatory treatments targeting core symptoms of ASD such as executive function deficits.
The study targeted investigation of effects of an innovative integrated neuromodulatory intervention that combines rTMS and neurofeedback in high-functioning children with autism. It can be considered as a pilot translational clinical research study where rTMS and neurofeedback combination treatment effects are compared with a waitlist group data to explore effects on clinical, behavioral and cognitive outcomes in ASD. The study used novel approach, as to our knowledge it represents the first attempt on a combined application of rTMS and neurofeedback in children with ASD. Another novel element of the study was an application of a cognitive test with behavioral(reaction time and accuracy), dense-array based event-related potential measures to assess outcomes of integrated neuromodulatory intervention which targets impairments of behavioral, sensory, and cognitive functions in autism. The most significant aspect of the study is the investigation of neural mechanisms of neurotherapy based on rTMS and neurofeedback using behavioral and electrocortical activity measures in cognitive task, and behavioral evaluations. The study was based on a hypothesis proposing that low frequency rTMS over the dorso-lateral prefrontal cortex (DLPFC) improves excitation/inhibition ratio in autism, induces positive prefrontal EEG activity alterations that could be further enhanced by neurofeedback training of induced EEG changes.
Figure 1.
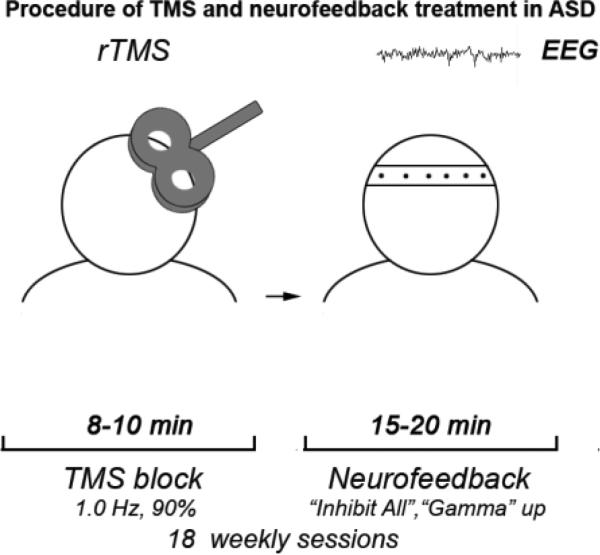
Schematic presentation of combined rTMS and neurofeedback procedure. During each TMS-NFB session participant with ASD diagnosis was administered approximately 10 min long rTMS (1 Hz, 180 pulse, 90 of MT), followed by 20 min long neurofeedback training using protocol aimed at upregulation of gamma activity and suppression of high amplitude bands.
Acknowledgments
The study was partially supported by National Institutes of Health Eureka R01 grant MH86784 to Manuel F. Casanova.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Forth Edition, Text Revision Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Aman MG. Management of hyperactivity and other acting out problems in patients with autism spectrum disorder. Seminars in Pediatric Neurology. 2004;11:225–228. doi: 10.1016/j.spen.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN. Aberrant Behavior Checklist—Community. Supplementary Manual. Slosson Educational Publications; East Aurora, NY: 1994. [Google Scholar]
- Arns M, de Ridder S, Strehal U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clinical EEG and Neuroscience. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The cognitive neuroscience of autism. Journal of Neurology and Neurosurgery Psychiatry. 2004;75(7):945–948. doi: 10.1136/jnnp.2003.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruth J, Casanova MF, El-Baz A, Horrell T, Mathai G, Sears L, Sokhadze E. Low-frequency repetitive transcranial magnetic stimulation modulates evoked-gamma frequency oscillations in autism spectrum disorders. Journal of Neurotherapy. 2010a;14(3):179–194. doi: 10.1080/10874208.2010.501500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruth J, Sokhadze E, El-Baz A, Mathai G, Sears L, Casanova MF. Transcaranial magentic stimulation as a treatment for autism. In: Siri K, Lyons T, editors. Cutting Edge Therapies for Autism. Skyhorse Publishing; New York: 2010b. pp. 388–397. [Google Scholar]
- Baruth J, Casanova MF, Sears L, Sokhadze E. Early-stage visual processing abnormalities in high-functioning autism spectrum disorder (ASD). Translational Neuroscience. 2010c;1(2):177–187. doi: 10.2478/v10134-010-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruth J, Williams E, Sokhadze E, El-Baz A, Sears L, Casanova MF. Repetitive transcranial stimulation (rTMS) improves electroencephalographic and behavioral outcome measures in autism spectrum disorders (ASD). Autism Science Digest. 2011;1(1):52–57. [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Research: Cognitive Brain Research. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger L, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neurosciences. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BL, Newton FA, Sheer DE, Ford M. Biofeedback training of 40-Hz EEG in humans. Biofeedback and Self-Regulation. 1978a;3(1):1–14. doi: 10.1007/BF00998559. [DOI] [PubMed] [Google Scholar]
- Bird BL, Newton FA, Sheer DE, Ford M. Behavioral and electroencephalographic correlates of 40 Hz EEG biofeedback training in humans. Biofeedback and Self-Regulation. 1978b;3:13–28. doi: 10.1007/BF00998560. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Lewis MH. Repetitive Behavior Scale. Western Carolina Center Research Reports; Morganton, NC: 1999. [Google Scholar]
- Bodfish JW, Symons FS, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Developmental Psychopathology. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Brown C. EEG in autism: is there just too much going on in there? In: Casanova MF, editor. Recent Developments in Autism Research. Nova Science Publishers; New York: 2005. pp. 109–126. [Google Scholar]
- Brown C, Gruber T, Boucher J, Rippon G, Brock J. Gamma abnormalities during perception of illusory figures in autism. Cortex. 2005;41(3):364–376. doi: 10.1016/s0010-9452(08)70273-9. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Semendeferi K, Buckwalter J, Schenker N, Switser R, Courchesne E. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathology and Applied Neurobiology. 2006;32(5):483–491. doi: 10.1111/j.1365-2990.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Casanova MF. Minicolumnar pathology in autism. In: Casanova MF, editor. Recent Developments in Autism Research. Nova Biomedical Books; New York: 2005. pp. 133–144. [Google Scholar]
- Casanova MF. Neuropathological and genetic findings in autism: the significance of a putative minicolumnopathy. Neuroscientist. 2006;12(5):435–441. doi: 10.1177/1073858406290375. [DOI] [PubMed] [Google Scholar]
- Casanova MF. The neuropathology of autism. Brain Pathology. 2007;17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002a;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002b;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Asperger's syndrome and cortical neuropathology. Journal of Child Neurology. 2002c;17:142–145. doi: 10.1177/088307380201700211. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Neuronal density and architecture (Gray Level Index) in the brains of autistic patients. Journal of Child Neurology. 2002d;17(7):515–521. doi: 10.1177/088307380201700708. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Trippe J. Regulatory mechanisms of cortical laminar development. Brain Research Reviews. 2006;51(1):72–84. doi: 10.1016/j.brainresrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten I, Switala AE, van England H, Heinsen H, Steinbuch HWM, Hof PR, Schmitz C. Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clinical Neuroscience Reseacrh. 2006a;6(3-4):127–133. [Google Scholar]
- Casanova MF, van Kooten I, van Engeland H, Heinsen H, Steinbursch HWM, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism II. Neuronal size and number. Acta Neuropathologica. 2006b;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz AS, Vanbogaert E, Narahari P, Switala A. A topographical study of minicolumnar core width by lamina comparison between autistic subjects and controls: posible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathology. 2010;20(2):451–458. doi: 10.1111/j.1750-3639.2009.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Baruth J, El-Baz A, Tasman A, Sears L, Sokhadze E. Repetitive transcranial magnetic stimulation (rTMS) modulates event-related potential (ERP) indices of attention in autism. Translational Neuroscience. 2012;3(2):170–180. doi: 10.2478/s13380-012-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz AS, Kamat SS, Dombroski BA, Khalifa F, Elnakib A, Soliman A, Allison-McNutt A, Switala AE. Focal cortical displasias in autism spectrum disorders. Acta Neuropathoogy Communications. 2014;1(1):67. doi: 10.1186/2051-5960-1-67. doi: 10.1186/2051-5960-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coben R. Autistic spectrum disorder: a controlled study of EEG coherence training focused on social skills deficits. Journal of Neurotherapy. 2008;12:57–75. [Google Scholar]
- Coben R. Neurofeedback for Autistic Disorders: Emerging empirical evidence. In: Casanova MF, El-Baz AS, Suri JS, editors. Imaging the Brain in Autism. Springer; New York: 2008. 2013. pp. 107–134. [Google Scholar]
- Coben R, Linden M, Myers TE. Neurofeedback for autistic spectrum disorder: a review of the literature. Applied Psychophysiology and Biofeedback. 2010;35(1):83–105. doi: 10.1007/s10484-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Coben R, Myers TE. The relative efficacy of connectivity guided and symptom based EEG biofeedback for autistic disorders. Applied Psychophysiology and Biofeedback. 2010;35:13–23. doi: 10.1007/s10484-009-9102-5. [DOI] [PubMed] [Google Scholar]
- Coben R, Padolsky J. Assessment-guided neurofeedback for autistic spectrum disorders. Journal of Neurotherapy. 2007;11:5–23. [Google Scholar]
- Clemans Z, Sokhadze T, El-Baz A. Custom program for extraction of event-related potential peaks in attention tasks.. Presented at Research Louisville; Louisville, KY. October 11.2011a. [Google Scholar]
- Clemans Z, Sokhadze E, El-Baz AS. A custom-made Matlab program for ERP feature detection in psychological and physiological disorders using wavelet.. Presented at the 97th Annual Meeting of Kentucky Academy of Science; Murray, KY. November 4-5.2011b. [Google Scholar]
- Courchesne E, Lincoln AJ, Yeung-Courchesne R, Elmasian R, Grillon C. Pathophysiologic findings in nonretarded autism and receptive developmental disorder. Journal of Autism and Developmental Disorders. 1989;19:1–17. doi: 10.1007/BF02212714. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Transcranial magnetic stimulation: a new investigational and treatment tool in psychiatry. Journal Neuropsychiatry Clininical Neuroscience. 2002;14:406–415. doi: 10.1176/jnp.14.4.406. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M. A framework for local cortical oscillation patterns. Trends in Cognitive Sciences. 2011;15(5):191–199. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Konrad C, Pantev C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. Neuroimage. 2010;51:877–887. doi: 10.1016/j.neuroimage.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Developmental Medicine and Child Neurology. 2010;52:e179–e183. doi: 10.1111/j.1469-8749.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. Repetitive transcranial magnetic stimulation (rTMS) improves movement-related cortical potentials in autism spectrum disorders. Brain Stimulation. 2012;5(1):30–37. doi: 10.1016/j.brs.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. GABAergic activity in autism spectrum disorders: an investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology. 2013;68:202–209. doi: 10.1016/j.neuropharm.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Oberman L, Pascual-Leone A. Brain stimulation over Broca's area differentially modulates naming skills in neurotypical adults and individuals with Asperger's syndrome. European Journal of Neuroscience. 2011;34(1):158–164. doi: 10.1111/j.1460-9568.2011.07726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Fletcher EM, Kussmaul CL, Mangun GR. Estimation of interpolation errors in scalp topographic mapping. Electroctoencephalography and Clinical Neurophysiology. 1996;98:422–434. doi: 10.1016/0013-4694(96)95135-4. [DOI] [PubMed] [Google Scholar]
- Ford M, Bird B, Newton FA, Sheer DE. Maintenance and generalization of 40-Hz EEG biofeedback effects. Biofeedback and Self-Regulation. 1980;5(2):193–205. doi: 10.1007/BF00998595. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. American Journal of Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- Gevensleben H, Holl B, Albrecht B, Vogel C, Schlamp D, et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. Journal of Child Psychology and Psychiatry. 2009;50:780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- Gomes E, Pedroso FS, Wagner MB. Auditory hypersensitivity in the autistic spectrum disorder. Pró-Fono Revista de Atualização Científica. 2008;20:279–284. doi: 10.1590/s0104-56872008000400013. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Greenberg BD. Transcranial magnetic stimulation in anxiety disorders. In: George MS, Belmaker RH, editors. Transcranial Magnetic Stimulation in Clinical Psychiatry. American Psychiatric Publishing, Inc.; Washington, DC: 2007. pp. 165–178. [Google Scholar]
- Gruber T, Muller MM, Keil A, Elbert T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clinical Neurophysiolology. 1999;110(12):2074–2085. doi: 10.1016/s1388-2457(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Hensley MK, El-Baz AS, Sokhadze EM, Sears L, Casanova MF. Effects of 18 session therapy of gamma coherence in autism. Psychophysiology. 2014;51:S16. [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review. 2004;24:189–233. [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neurosciences. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Jarusiewicz B. Efficacy of neurofeedback for children in the autistic spectrum: A pilot study. Journal of Neurotherapy. 2002;6(4):39–49. [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosciences. 2007;30(7):317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kahana M. The cognitive correlates of human brain oscillations. Journal of Neurosciences. 2006;26(6):1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanizsa G. Subjective contours. Scientific American. 1976;235:48–52. doi: 10.1038/scientificamerican0476-48. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Ray WJ, Gruber T, Elbert T. Human gamma band activity and perception of a gestalt. Journal of Neurosciences. 1999;19(16):7152–7161. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita L, Mottron L, Dawson M, Bertone A. Atypical lateral connectivity: a neural basis for altered visuospatial processing in autism. Biological Psychiatry. 2011;70(9):806–811. doi: 10.1016/j.biopsych.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Kouijzer M, de Moor JMH, Gerrits BJ, Congedo M, van Schie H. Neurofeedback improves executive functioning in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2009a;3:145–162. [Google Scholar]
- Kouijzer M, de Moor JMH, Gerrits BJ, Congedo M, van Schie H. Long-term effects of neurofeedback treatment in autism. Research in Autism Spectrum Disorders. 2009b;3:496–501. [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: Independent validation in individuals with Autism Spectrum Disorders. Journal of Autism and developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. The Autism Diagnostic Interview—Revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Linden M, Gunkelman J. QEEG-guided neurofeedback for autism: clinical observations and outcomes. In: Casanova MF, El-Baz AS, Suri JS, editors. Imaging the Brain in Autism. Springer; New York: 2013. pp. 45–60. [Google Scholar]
- Loo C, Mitchell P. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. Journal of Affective Disorders. 2005;88:255–267. doi: 10.1016/j.jad.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Lubar JF. Neurofeedback for the management of attention deficit disorders. In: Schwartz MS, Andrasik F, editors. Biofeedback: A Practitioner's Guide. New York: Guilford Press. 3rd ed. 2003. pp. 409–437, 2003. [Google Scholar]
- Luu P, Tucker DM, Englander R, Lockfeld A, Lutsep H, Oken B. Localizing acute stroke-related EEC changes: assessing the effects of spatial undersampling. Journal of Clinical Neurophysiology. 2001;18:302–317. doi: 10.1097/00004691-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends in Neurosciences. 2007;30(7):343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neuroscience Biobehavioral Reviews. 2010;34:23–30. doi: 10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Behavioral neuroanatomy: large-networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2nd edition Oxford University Press; New York: 2000. pp. 1–120. [Google Scholar]
- Monastra VJ. Electroencephalographic biofeedback (neurotherapy) as a treatment for attention deficit hyperactivity disorder: rationale and empirical foundation. Child Adolescent Psychiatry Clinic North America. 2005;14:55–82. doi: 10.1016/j.chc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Monastra VJ. Quantitative electroencephalography and attention-deficit/hyperactivity disorder: implications for clinical practice. Current Psychiatry Reports. 2008;10:432–438. doi: 10.1007/s11920-008-0069-3. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Myors B. Statistical Power Analysis. A Simple and General Model for Traditional and Modern Hypothesis Tests. 2nd edition Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- Oberman LM, Hubbard EM, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Research Cognitive Brain Research. 2005;24(2):190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia. 2008;46(5):1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, Picker J, Rotenberg A, Pascual-Leone A. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Frontiers System Neuroscience. 2010;2:26. doi: 10.3389/fnsyn.2010.00026. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman L, Rotenberg A, Pascual-Leone A. Use of transcranial magnetic stimulation in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013 Oct 15; doi: 10.1007/s10803-013-1960-2. [Epub ahead of print], doi: 10.1007/s10803-013-960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S. Casual mechanisms of autism: unifying perspectives from an information-processing framework. In: Cohen DJ, Volkmar FR, editors. Handbook of autism and pervasive developmental disorders. John Wiley; New York: 1997. pp. 868–879. [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Current Opinions in Neurobiololgy. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Perrin E, Pernier J, Bertrand O, Giard M, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology. 1987;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. Journal o Clinical Neurophysiology. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Plaisted K, Saksida L, Alcantara J, Weisblatt E. Towards an understanding of the mechanisms of weak central coherence effects: experiments in visual configural learning and auditory perception. Proceedings Royal Society (London) 2003;358:375–386. doi: 10.1098/rstb.2002.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Journal of Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF, Patel SH, Azzam PN. Impact of instructed relevance on the visual ERP. International Journal of Psychophysiology. 2004;52:197–209. doi: 10.1016/j.ijpsycho.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Puts NA, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RA. Impaired tactile processing in children with autism spectrum disorder. Journal of Neurophysiology. 2014;111(9):1803–1811. doi: 10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. International Journal of Psychophysiology. 2007;63(2):164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genetics Brain Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: A meta-analysis. Psychology and Medicine. 2009;39(1):65–75. doi: 10.1017/S0033291708003462. [DOI] [PubMed] [Google Scholar]
- Sherlin L, Arns M, Lubar J, Sokhadze E. A position paper on neurofeedback for the treatment of ADHD. Journal of Neurotherapy. 2010;14:66–78. [Google Scholar]
- Sichel A, Fehmi LG, Goldstein D. Positive outcome with neurofedbacktreatmetnt in a case of mild autism. Journal of Neurotherapy. 1995;1(1):60–64. [Google Scholar]
- Szentagothai J, Arbib MA. Conceptual Models of Neural Organization. MIT Press; Cambridge, Mass.: 1975. [PubMed] [Google Scholar]
- Sohal VS. Insights into cortical oscillations arising from optogenetic studies. Biological Psychiatry. 2012;71(12):1039–1045. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Stewart C, Hollifield M. Integrating cognitive neuroscience, psychiatry and behavioral treatment with neurofeedback in addictive disorders. Journal of Neurotherapy. 2007;11(2):13–44. [Google Scholar]
- Sokhadze EM, Cannon R, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy and recommendations for future research. Applied Psychophysiology and Biofeedback. 2008;33(1):1–28. doi: 10.1007/s10484-007-9047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, Tasman A, Sears L, Mathai G, El-Baz A, Casanova MF. Event-related potential study of novelty processing abnormalities in autism. Applied Psychophysiology and Biofeedback. 2009a;34:37–51. doi: 10.1007/s10484-009-9074-5. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, El-Baz A, Baruth J, Mathai G, Sears L, Casanova MF. Effect of a low-frequency repetitive transcranial magnetic stimulation (rTMS) on induced gamma frequency oscillations and event-related potentials during processing of illusory figures in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009b;39:619–634. doi: 10.1007/s10803-008-0662-7. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, Tasman A, Mansoor M, Ramaswamy R, Sears L, Mathai G, El-Baz A, Casanova MF. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Applied Psychophysiology and Biofeedback. 2010a;35:147–161. doi: 10.1007/s10484-009-9121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, El-Baz A, Horrell T, Sokhadze G, Carroll T, Tasman A, Sears L, Casanova MF. Impaired error monitoring and correction function in autism. Journal of Neurotherapy. 2010b;14:79–95. doi: 10.1080/10874201003771561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Casanova MF. Prefrontal neuromodulation using rTMS improves error monitoring and correction functions in autism. Applied Psychophysiology and Biofeedback. 2012a;37(2):91–102. doi: 10.1007/s10484-012-9182-5. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Williams E.l., Klapheke R, Casanova MF. Event-related potential study of attention regulation during illusory figure categorization task in ADHD, Autism Spectrum Disorder, and typical children. Journal of Neurotherapy. 2012b;16(1):12–31. doi: 10.1080/10874208.2012.650119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, Baruth J, Tasman A, Casanova MF. Event-related potential studies of cognitive processing abnormalities in autism. In: Casanova MF, El-Baz A, Suri JS, editors. Imaging Methods in Autism. Springer; New York: 2013a. pp. 61–86. [Google Scholar]
- Sokhadze EM, Casanova MF, Baruth J. Transcranial magnetic stimulation in autism spectrum disorders. In: Alba-Ferrara L, editor. Transcranial Magnetic Stimulation: Methods, Clinical Uses and Effect on the Brain. NOVA Publishers; New York: 2013b. pp. 219–231. [Google Scholar]
- Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Frontiers in Systems Neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00134. Article 134, doi: 10. 3389/fnsys.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Tucker DM, Murias M. Estimating the spatial Nyquist of the human EEG. Behavioral Research Methods, Instrumentation and Computers. 1998;30:8–19. [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cerebral Cortex. 2005;15(5):654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Townsend J, Westerfield M, Leaver E, Makeig S, Jung T, Pierce K, Courchesne E. Event-related brain response abnormalities in autism: evidence for impaired cerebello-frontal spatial attention networks. Brain Research Cognitive Brain Research. 2001;11:127–145. doi: 10.1016/s0926-6410(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Thompson L, Thompson M, Reid A. Neurofeedback outcomes in clients with Asperger's syndrome. Applied Psychophysiology and Biofeedback. 2010;35:63–81. doi: 10.1007/s10484-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Tuchman RF, Rapin I. Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics. 1997;99(4):560–566. doi: 10.1542/peds.99.4.560. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller R-A. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. NeuroImage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, El-Baz A, Sears L, Casanova MF, Tasman A, Sokhadze E. Prefrontal neurofeedback approaches in autism.. Presented at the ISNR annual conference; San Diego, CA. October 16.2014. [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical Neurophysiology. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. Harcourt Assessment, Inc.; San Antonio, TX: 2003. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment, Inc.; San Antonio, TX: 1999. [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biological Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]