Abstract
Objective
As international guidelines increase access to antiretroviral therapy (ART) globally, ART adherence becomes increasingly important to achieve HIV treatment as prevention (TasP) goals. In the concentrated HIV epidemic among men who have sex with men (MSM) and transgendered women (TGW) in Lima, Peru, the independent correlates of ART non-adherence were examined to inform treatment intervention priorities.
Design
Cross sectional survey of HIV-infected MSM and TGW who are engaged in clinical care in Lima, Peru.
Methods
From June to August 2012, 302 HIV-infected Peruvian MSM/TGW from three clinical care sites were recruited using convenience sampling to participate in a cross-sectional computer-assisted adherence survey. Several standardized screening measures associated with ART non-adherence were examined in order to determine the independent correlates of optimal (≥90%) and perfect (100%) adherence, which were assessed using logistic regression.
Results
Of the 302 participants recruited, 263 (87.1%) were prescribed ART. Among those prescribed ART, 229 (87.1%) reported optimal and 146 (55.5%) reported perfect adherence. The prevalence of alcohol use disorders (AUD; 43.2%), alcohol dependence (5.3%), recent drug use (6.0%) and depression (44.5%) was high and most participants had some evidence of neurocognitive impairment. Meeting criteria for having an AUD and depression were collinear (p<0.001). On multivariate analysis, having an AUD was inversely related and the only independent correlate of optimal (AOR=0.427; 95% CI=0.187–0.976) and perfect (AOR=0.552; 95% CI=0.327–0.930) ART adherence.
Conclusions
AUDs are highly prevalent among Peruvian HIV-infected MSM and contribute significantly to ART non-adherence. These findings support the need for screening and treating underlying AUDs. In order to meet HIV TasP goals, evidence-based strategies targeting AUDs are likely to directly improve ART adherence and indirectly improve overall individual health, HIV treatment engagement and reduce transmission to sexual partners among this vulnerable and disproportionally affected population.
Introduction
Increasing antiretroviral therapy (ART) access using HIV treatment as prevention (TasP) strategies is central to global HIV prevention and treatment. If successful, TasP will increasingly play a significant role in decreasing HIV incidence and mortality [1]. The World Health Organization's (WHO) recommendation for earlier ART initiation at higher CD4 lymphocyte thresholds should increase the numbers of individuals eligible for and receiving ART [2,3]. Once prescribed, maintaining high ART adherence is crucial for achieving viral suppression (VS), thereby reducing sexual transmission to heterosexuals [4,5], people who inject drugs [6,7] and men who have sex with men (MSM) [8]. Optimal ART adherence associated with VS also reduces development of drug resistance [9].
Individual and structural factors undermine optimal ART adherence. Individual factors include the presence of alcohol use disorders (AUDs) and other substance use disorders (SUDs) [10,11], HIV-associated neurocognitive impairment (NCI) [12] and psychiatric illnesses [13] – all of which are also associated with risky sexual behaviors. Social issues like HIV-related stigma, low social support, health literacy [14], and food insecurity [15] may also contribute to ART non-adherence [13]. Structural factors associated with ART non-adherence include clinic and provider characteristics, costs and availability of ART and other health care delivery considerations [2,16,17].
Globally, over half of new HIV infections in North and South America, Western Europe and Australia is concentrated among MSM [18-22]. MSM represent a particularly vulnerable population in Peru, a middle-income country where the government provides free ART. Given the high levels of stigma against same-sex relations in Peru [23], the consumption of disinhibiting substances like alcohol is common among MSM [24,25], and is associated with high-risk sexual behaviors [26-28] and being unaware of being HIV-infected [29]. In light of these concerning findings, we hypothesized that mind-altering substances like alcohol and/or drugs or underlying NCI may also negatively impact HIV treatment outcomes, even in the presence of medication reminders. We therefore sought to comprehensively explore the facilitators and barriers to optimal ART adherence among Peruvian MSM who are engaged in clinical care.
Methods
Ethics Statement
Institutional Review Boards at Impacta Peru and Yale University approved this research.
Study participants and procedures
Between June and August 2012, 302 consecutive HIV-infected Peruvian MSM were recruited to participate in a self-administered survey to examine correlates of ART adherence and access to care; only adherence was examined in this study. Participants were defined as MSM if they were born male and self-reported having sex with a man in the past year. Using convenience sampling, we recruited consecutive patients receiving HIV care from three clinics located in Lima. Eligibility included age ≥18 years, born male and diagnosed with HIV for over one year to ensure that participants had had sufficient time and opportunities to initiate ART. Recruitment included clinic-based advertisements and flyers, or clinical staff approaching patients at the end of their clinical visits; no patients refused study participation. Participants providing written informed consent then completed a 60-minute computer-assisted survey instrument (CASI) in a private setting and were paid 25 soles (∼$10 US) for their time.
Survey Content and Variable definitions
Literature review informed variable selections associated with ART non adherence [26-29]. We also collected demographic and social characteristics, education, occupation and monthly salary earned, living circumstances, year of HIV diagnosis, and self-reported sexual identity and orientation. Self-reported adherence, the dependent variable, was assessed using a validated visual analog scale (VAS) [30], and analyzed as optimal (≥90%) and perfect (100%) adherence [31].
AUD screening deployed the WHO's validated 10-item Alcohol Use Disorders Identification Test (AUDIT) [32], with standard cut-offs to define any AUD (score ≥8) and alcohol dependence (score ≥20) which are highly correlated, have high internal and external validity [33], as well as sensitivity and specificity [34]. A drug use battery over the past 6 months was assessed using a modified Addiction Severity Index (ASI) [35]for known drugs used in Peru, while addiction severity was measured using the 10-item Drug Abuse Screening Test (DAST-10) [36], with bivariate cutoffs of >2 for moderate to severe addiction severity. Depressive symptoms were measured using the 10-item Center for Epidemiologic Studies Depression Scale (CES-D 10) with scores >7 being correlated with moderate to severe depressive symptoms [37]. HIV-related stigma and social support were analyzed continuously as previously described using the Wright Stigma Scale [38] and the Modified Social Support Survey (MSSS-5) [39], respectively. Food insecurity was measured using 6 items from the National Center for Health Statistics (NCHS) [40]. Health literacy was assessed continuously using the Short Test of Functional Health Literacy in Adults (STOHFLA) [41] and barriers to medical care deployed a validated 10-item scale [42].
NCI was assessed using the 95-item standardized Neuropsychological Impairment Scale (NIS) [43]. Though the NIS features many subscales, we focused only on the influence of general cognitive impairment, which was analyzed using a validated NCI summary score called the Global Measure of Impairment (GMI). Consistent with prior analyses involving the NIS, raw scores were transformed to t-scores and then treated as a continuous variable [44]. Facilitators of ART adherence included a battery of cues and reminders often used to assist medication-taking, like alarm clocks and calendars, association with meals and hour of sleep, reminders from friends, and putting ART in a highly visible place.
Standardized measures that have previously been translated and validated in Spanish were used. Other survey components were created in English, translated to Spanish and then back-translated into English to ensure understanding as previously described [45]. Pilot testing with HIV-infected persons in Lima was conducted to further ensure understanding.
Data Analysis
Statistical analyses were performed using SPSS (Version 20). The dependent variables were optimal (≥90%) and perfect (100%) adherence over the past 30 days. Bivariate associations of all clinically relevant variables first assessed their association with the dependent variables using chi-square and t-tests were used for categorical and continuous variables, respectively. Using a deterministic approach, bivariate associations significant at p<0.20 were then incorporated into the multivariate logistic regression model. Stepwise forward and backward elimination regression both showed the same results as our deterministic model, which was ultimately selected based on having the best goodness-of-fit using the Akaike Information Criterion (AIC). The Variance Inflation Factor (VIF) was used to test for collinearity between variables that were significant in the bivariate analysis but no longer significant in the final model.
Results
Description of the study sample
Table 1 describes the characteristics of the participants. Most were in their early thirties (mean=32.0 years), earned greater than minimal wage (58.6%) and completed high school (68.8%). Most (87.1%) participants were on ART and, of those, 87.1% reported optimal (≥90%) and 55.3% reported perfect (100%) adherence. Only 6.0% of participants reported recreational drug use and 43.2% met screening criteria for AUDs, with 5.3% meeting criteria for dependence (AUDIT≥20).
Table 1. Characteristics of the sample (N=302).
| Characteristic | N = 302 (%) |
|---|---|
| Median Age, years (S.D.) | 32.0 (± 8.1) |
| Monthly Income | |
| None | 39 (12.9) |
| Less than minimum wage | 84 (27.8) |
| Minimum wage or greater | 177 (58.6) |
| Completed High School | |
| No | 94 (31.2) |
| Yes | 207 (68.8) |
| Sexual Orientation (self-identified) | |
| Homosexual (gay) | 243 (80.7) |
| Bisexual | 45 (15.0) |
| Heterosexual | 13 (0.3) |
| Transgender | |
| No | 282 (93.4) |
| Yes | 20 (6.6) |
| Living Situation | |
| Alone | 58 (19.3) |
| With sexual partner | 49 (16.3) |
| With family or nonsexual partner | 194 (64.4) |
| Occupation | |
| Full-time | 146 (48.3) |
| Part-time | 95 (31.5) |
| Unemployed | 60 (19.9) |
| Alcohol Use Disorders | |
| No alcohol use disorder | 171 (56.8) |
| Any alcohol use disorder | 130 (43.2) |
| Hazardous drinking | 96 (31.9) |
| Harmful drinking | 18 (6.0) |
| Dependent drinking | 16 (5.3) |
| Drug Use in the past 12 months | |
| No | 281 (94.0) |
| Yes | 18 (6.0) |
| Neurocognitive Impairment | |
| Mean t-score (S.D.) | 50.8 (±10.8) |
| Depression | |
| No | 168 (55.5) |
| Yes | 134 (44.5) |
| Participants on ART | N = 263 (%) |
| Adherence ≥ 90% | (229) 87.1 |
| Adherence = 100% | (145) 55.3 |
| Mean adherence | 91.7% |
Legend: S.D.=Standard Deviation
Correlates of ART adherence
Table 2 highlights factors significantly correlated with both optimal and perfect adherence among the 263 participants prescribed ART. On bivariate analysis, only three factors were inversely correlated (p<0.05) with optimal adherence, including having an AUD, higher levels of NCI, and being transgendered women (TGW). Having moderate to severe depressive symptoms (p=0.066) and lower monthly income (p=0.084) trended towards significance. Similarly, for perfect adherence, factors that were inversely and significantly (p<0.05) correlated included having an AUD (p<0.001), higher NCI (p=0.001), increasing age (p=0.032), having moderate to severe depressive symptoms (p=0.002).
Table 2. Correlates Associated with Optimal (≥90%) and Perfect (=100%) Antiretroviral Therapy Adherence Among HIV-infected Peruvian Men Who Have Sex with Men and Transgendered Women (N=302).
| Optimal (≥90%) Antiretroviral Therapy Adherence | Perfect (=100%) Antiretroviral Therapy Adherence | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Bivariate Associations | Multivariate Associations | Bivariate Associations | Multivariate Associations | ||||
| OR (95% CI) | p-value | AOR (95% CI) | p-value | OR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Alcohol Use Disorder | 0.357 (0.169 – 0.752) | 0.005 * | 0.427 (0.187 – 0.976) | 0.044 * | 0.438 (0.266 – 0.720) | 0.001 * | 0.552 (0.327 – 0.930) | 0.026 * |
| Neurocognitive Impairment (global score) | 0.965 (0.933 – 0.999) | 0.041 * | 0.975 (0.933 – 1.019) | 0.266 | 0.959 (0.936 – 0.983) | 0.001 * | 0.978 (0.951 – 1.007) | 0.132 |
| Age | 1.035 (0.989 – 1.083) | 0.138 | 1.026 (0.978 – 1.075) | 0.293 | 1.034 (1.003 –1.066) | 0.032 * | 1.029 (0.997 – 1.062) | 0.078 |
| Earned minimal wage or greater | 1.868 (0.913 – 3.823) | 0.084 | 1.295 (0.573 – 2.926) | 0.534 | 0.922 (0.563 – 1.512) | 0.749 | ||
| Living Alone | 0.406 (0.119 – 1.386) | 0.138 | 0.355 (0.077 – 1.627) | 0.182 | 0.846 (0.444 –1.612) | 0.611 | ||
| 0.317 (0.103 – 0.971) | 0.035 * | 0.369 (0.109 – 1.252) | 0.110 | 1.1 (0.404 – 2.994) | 0.852 | |||
| Full-time Employment (stable) | 0.948 (0.461 – 1.950) | 0.884 | 0.761 (0.465 – 1.245) | 0.276 | ||||
| Depression | 0.513 (0.250 – 1.053) | 0.066 | 1.101 (0.437 – 2.774) | 0.838 | 0.455 (0.276 – 0.748) | 0.002 * | 0.632 (0.352 – 1.138) | 0.126 |
| Any Drug Use in Previous 12 Months | 0.950 (0.310 – 2.194) | 0.928 | 1.164 (0.532 – 2.545) | 0.703 | ||||
| Domestic Violence in Past 12 Months | 2.752 (0.355 – 21.36) | 0.313 | 0.794 (0.305 – 2.074) | 0.637 | ||||
| Wright HIV Stigma Scale | 0.992 (0.938 – 1.050) | 0.789 | 0.974 (0.937 – 1.013) | 0.184 | 0.978 (0.939 – 1.019) | 0.286 | ||
| Food Insecurity | ||||||||
| Any | 1.210 (0.590 – 2.482) | 0.603 | 0.996 (0.611 – 1.622) | 0.986 | ||||
| Hunger | 1.916 (0.643 – 5.706) | 0.236 | 1.346 (0.713 – 2.538) | 0.358 | ||||
| Health Literacy | 0.610 (0.066 – 5.620) | 0.659 | 3.291 (0.363 – 29.849) | 0.263 | ||||
| Social Support | 0.992 (0.979 – 1.005) | 0.206 | 0.994 (0.986 – 1.003) | 0.220 | ||||
| Barriers to Medical Care | 0.977 (0.819 – 1.165) | 0.795 | 1.001 (0.887 – 1.130) | 0.983 | ||||
| AIC = 233.3 | AIC = 220.0 | |||||||
Legend: OR=Odds Ratio; AOR=Adjusted Odds Ratio; AIC=Akaike Information Criterion
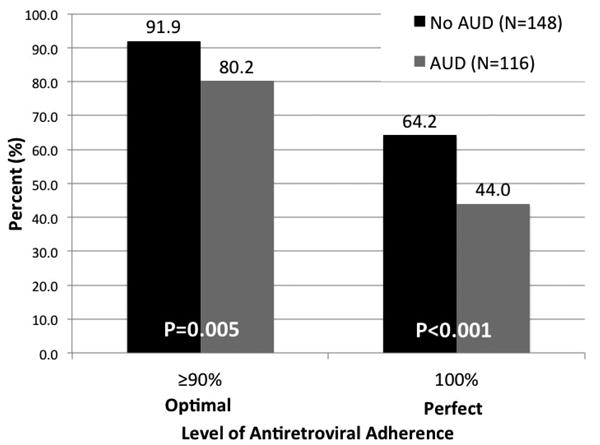
Multivariate modeling, however, confirmed that having an AUD was the only independent factor associated with ART adherence, portending greater than a 55% reduction (AOR=0.427; 95% CI=0.187–0.976) in optimal adherence and 45% reduction in perfect adherence (AOR=0.552; 95% CI=0.327–0.930). Figure 1 depicts the proportion of study participants with and without an AUD that reported optimal and perfect adherence. Compared to those without an AUD, those with an AUD were significantly less likely to report optimal (80.2% vs 91.9%; p=0.005) and perfect adherence (44.0% vs 64.2%; p<0.001). Including the use of any facilitating cuing reminder variable into the final model did not change the outcome for either of the dependent variables, suggesting that ART adherence facilitators do not overcome the influence of AUDs on non-adherence in our sample (data not shown).
Figure 1. Comparison of Adherence Between Men Who Have Sex With Men With and Without Alcohol Use Disorders (AUD).
Discussion
To our knowledge, this is the first study investigating correlates of ART adherence among HIV-infected MSM/TGW in Peru. Though alcohol and drug use have variably been associated with non-adherence in settings outside of South America and among non-MSM populations [46-49], a systematic review of the influence of alcohol and adherence showed conflicting findings, primarily because of a profound lack of using standardized measures of alcohol use, with almost none using validated screening criteria for “treatable” AUDs [50]. Findings here use standardized measures for both AUDs and ART adherence, overcoming problems identified with earlier studies. Moreover, this study was conducted among MSM, the primary drivers of HIV transmission in many regions globally [18], including South America [51].
In South America, only one longitudinal study in Brazil showed that any alcohol use negatively influenced ART adherence [52], but did not use standardized measures to screen for AUDs or depression [53]. Our study also included a larger sample size and included only MSM. Moreover, AUDs differ from alcohol use per se in that AUDs are medically defined conditions that are amenable to intervention [54]. AUDs have previously been associated with decreased ART adherence in other settings and populations [55,56] and to other poor HIV-treatment outcomes, including decreased health care utilization [57], decreased linkage to and retention in care [58,59], reduced ART medication access [60,61] and viral non-suppression [58]. In Peru's largest biobehavioral serosurveillance study of over 5000 Peruvian MSM, AUDs were previously found to be highly prevalent (62.3%) and independently associated with high-risk sexual behaviors [26-28] and being unaware of being HIV-infected [29]. This study builds on these concerning findings and is the first to demonstrate a concerning association between AUDs and suboptimal ART adherence among HIV-infected MSM enrolled in HIV clinical care in Peru.
Overall, 31.9% met criteria for hazardous (binge) drinking (AUDIT=8-16). According to WHO recommendations, hazardous drinkers should minimally undergo brief counseling interventions to reduce heavy drinking and to seek additional assistance [62]. In HIV-infected MSM, however, the risk is augmented because several days after heavy drinking episodes are associated with continued missed ART doses [63]. Moreover, in this relatively young group of MSM, without intervention, hazardous drinkers may progress to more serious AUDs, including alcohol dependence [64].
In addition, we used standardized measures to analyze other important correlates of ART adherence. First, we examined global indicators of NCI because to our knowledge, no previous NCI assessment has been conducted among Peruvian MSM. Studies elsewhere have shown that various levels of NCI were present among HIV-infected persons [65-67], and that higher NCI levels were associated with decreased ART adherence and increased HIV risk behaviors [22,68]. The high GMI scores in our sample [50.8 (±10.8) of 80 maximum] suggest most participants had some degree of NCI. This is concerning because individuals with mild to moderate NCI may experience sub-optimal ART adherence [44] and increased HIV risk behaviors [70]. In turn, NCI symptoms will worsen in HIV-infected people not taking ART [71], because ART reverses some, but not all NCI-related symptoms [72]. Although the general measure of NCI did not remain significant in the multivariate model, it is possible that only the most severely neurocognitively impaired (e.g., worst decile) [43] or those with impairment of only one or a few of the cognitive domains (e.g., memory, learning ability, attention) [44] show suboptimal ART adherence [73]. Further exploration of NCI severity and NCI subscales on ART adherence is warranted.
For optimal, but not perfect adherence, TGW were significantly less likely to adhere to ART. This finding, however, did not remain significant in the multivariate analysis. While we speculated that TGW may have had higher levels of AUDs or HIV-related stigma [23,74], collinearity tests did not substantiate these assumptions, perhaps because TGW (N=20) comprised a small proportion (6.6%) of our sample.
Depressive symptoms were highly prevalent (44.5%) in this population. For both optimal and perfect adherence, being depressed was borderline and highly significant in the bivariate analysis, respectively, yet did not remain so in final models. Previous studies document correlations between depressive symptoms and ART non adherence [75,76], including in low- and middle-income countries [77]. Numerous studies also document a relationship between alcohol and depressive symptoms [78,79], and collinearity tests here confirm these two variables to be highly correlated (p<0.001; not shown). AUDs and depressive symptoms may both contribute to non-adherence, and from a purely clinical perspective, it is therefore essential to screen for and treat both disorders simultaneously in order to optimize treatment outcomes. This is particularly relevant since both disorders are under-diagnosed [80,81] and under-treated[82,83] in clinical settings, yet they are amenable to behavioral and/or pharmacotherapeutic interventions.
We also found that global stigma was not associated with non-adherence, yet the disclosure stigma subscale was negatively associated with perfect adherence. In our analyses, we did not explore in detail the impact of various types of stigma on ART adherence, but it is seductive to speculate that those who do not disclose their HIV status to others continually live in fear and miss doses based on concerns about someone finding out about their HIV status – as shown by previous studies conducted in Peru [84]. Further inquiry into various types of stigma is crucial for intervention development, especially with regard to HIV disclosure.
Unlike other studies examining alcohol and ART adherence, we assessed reminder interventions (e.g., alarm clocks, beepers, calendars) used clinically to improve ART adherence. Unlike a recent report conferring benefit among HIV-infected Peruvian men without assessment of alcohol or drug use [84], various reminders did not significantly influence ART adherence in our sample. Though not shown, those with AUDs were no more likely to use medication reminders than those without them. Due to our survey not examining the consistency to which participants used these reminders, we are unable to contribute further to guidelines from the International Association of Physicians in AIDS Care, recommends them as an adherence strategy [85].
Despite our analysis examining numerous validated factors that may influence ART adherence, having an AUD was the only independent correlate that remained significant in the multivariate model. This study's cross-sectional assessment does not establish causality between AUDs and suboptimal ART adherence, yet it is reasonable to expect that AUDs negatively influence adherence for numerous reasons, including intoxication impairing one's capacity to plan for or remember dosing requirements [86], living in precarious conditions with lower ART access [87], alcohol consumption used to reduce ART-related side effects [88,89] and intentional “skipping” ART when drinking, due to misconceptions about drug interactions [90-92]. Additional research is needed to clarify the behavioral, environmental, and circumstantial conditions under which alcohol use is likely to influence adherence.
The high prevalence of AUDs among Peruvian MSM is alarming, and it can be partly explained by the cultural and sexual biases that are pervasive against same-sex relations in the wider Peruvian society [23]. As a result, MSM have found gay bars to be a sheltering environment, where the consumption of disinhibiting substances like alcohol became progressively popular, in the expectancy to overcome the taboo associated with stigmatizing behaviors [24,25]. This finding, along with the significant association between AUDs and suboptimal ART adherence, suggests the need to design and implement effective interventions targeting alcohol use among this vulnerable population. This is particularly important since Peruvian patients have been found to miss more ART doses due to alcohol use in comparison to their US and African counterparts [93]. WHO suggests that MSM with SUDs may be targeted using psychosocial interventions [62] such as cognitive-behavioral (CBT) and motivational interviewing (MI) approaches, which have been shown to be effective across a wide range of SUDs [58,85], including AUDs [94]. Counseling-based AUD strategies alone, however, have not documented HIV treatment benefits nor ART adherence. CBT and MI approaches targeting ART adherence and retention in care produce short-term, but not sustained benefits among MSM with AUDs [95]. Among the three approved pharmacological medications that treat AUDs (Naltrexone, Acamprosate and Disulfiram), only naltrexone, in its oral or extended-release formulation, is superior to either acamprosate alone [96,97] or to behavioral therapies in HIV uninfected persons [98,99]. Few studies, however, have investigated the influence of naltrexone on HIV treatment outcomes among patients prescribed ART [100], but data are beginning to emerge [101]. Since naltrexone appears to be safe in HIV-infected individuals [102-104] and in those with AUDs [105], naltrexone has the potential to improve ART adherence through reduction of alcohol use.
In addition to treating AUDs, mobile technologies that improve ART adherence are gaining traction among HIV-infected MSM in Peru who are interested in using the Internet and cellphones for HIV health promotion interventions [69,106], potentially to improve ART adherence [106-108]. Our finding of moderate levels of NCI among our sample, however, may reduce their ability to effectively engage with such technologies [109]. Future ART adherence intervention development will require tailoring and testing along the spectrum of NCI for both content and delivery.
Even though our findings have important implications for HIV prevention and treatment among MSM with AUDs, study results should be interpreted in the context of inherent methodological limitations. The cross-sectional nature of this observational study does not allow us to establish causality. Other external variables could be responsible for both AUDs and poor adherence, specifically the relationship between depressive symptoms and AUDs. Also, we did not control for other factors associated with non-adherence in other studies, such as pill burden, dosing frequency and lifestyle changes required for ART adherence. Not presented, however, is the near uniformity in twice-daily and identical ART regimens prescribed. Second, sampling bias may have occurred, since patients were recruited from three clinics that provided dedicated MSM/TGW services. Since our convenience sampling method was not designed to be fully representative of HIV-infected MSM, caution is required when generalizing these results to the MSM elsewhere without more comprehensive and culturally competent services. Additionally, we used self-report instruments to gather data about alcohol use, ART adherence, and the other independent variables. Reporting of sensitive issues such as stigma or alcohol use might have also been influenced by social desirability bias, but CASI has been documented to reduce such biases [110] and the high self-reported levels suggest this not to be a concern. Despite this being the largest sample of HIV-infected MSM assessing adherence to date, a larger sample size might help balance these limitations.
Conclusions
Our study highlights the strong and significant association of AUDs with suboptimal ART adherence among HIV-infected MSM in Peru. Given that HIV remains a concentrated epidemic among Peruvian MSM and elsewhere, our findings underscore the importance of creating, testing and implementing interventions simultaneously targeting AUDs and ART adherence. Such interventions may simultaneously integrate pharmacological and multicomponent behavioral interventions. While the influence of other factors (e.g., NCI, depression and stigma) on poor adherence was not ultimately found to be significant among this sample, further research is needed to elucidate their possible influence on this vulnerable and disproportionally affected HIV-infected population.
Acknowledgments
The authors gratefully acknowledge the subjects who gave their time to participate in this study; the medical and research personnel at Impacta, Peru and the research personnel at the Yale AIDS Program for their continued support of this project.
References
- 1.Boyd MA, Zhang X, Dorr A, Ruxrungtham K, Kolis S, et al. Lack of enzyme-inducing effect of rifampicin on the pharmacokinetics of enfuvirtide. J Clin Pharmacol. 2003;43:1382–1391. doi: 10.1177/0091270003259220. [DOI] [PubMed] [Google Scholar]
- 2.Krusi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2010;21:4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood E, Kerr T, Marshall BD, Li K, Zhang R, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7:151–156. doi: 10.1097/COH.0b013e32834f9927. [DOI] [PubMed] [Google Scholar]
- 8.Muessig KE, Smith MK, Powers KA, Lo YR, Burns DN, et al. Does ART prevent HIV transmission among MSM? AIDS. 2012;26:2267–2273. doi: 10.1097/QAD.0b013e328355713d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hicks CB, Goswami ND, Tafoya E, Ribeiro RM, et al. Evolution of drug-resistant viral populations during interruption of antiretroviral therapy. J Virol. 2011;85:6403–6415. doi: 10.1128/JVI.02389-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 12.Chander G, Himelhoch S, Fleishman JA, Hellinger J, Gaist P, et al. HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care. 2009;21:655–663. doi: 10.1080/09540120802459762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halkitis PN, Shrem MT, Zade DD, Wilton L. The physical, emotional and interpersonal impact of HAART: exploring the realities of HIV seropositive individuals on combination therapy. J Health Psychol. 2005;10:345–358. doi: 10.1177/1359105305051421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngoh LN. Health literacy: a barrier to pharmacist-patient communication and medication adherence. J Am Pharm Assoc (2003) 2009;49:e132–146. doi: 10.1331/JAPhA.2009.07075. quiz e147-139. [DOI] [PubMed] [Google Scholar]
- 15.Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, et al. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009;24:14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 2012;15:10. doi: 10.1186/1758-2652-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21:661–665. doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djomand G, Quaye S, Sullivan PS. HIV epidemic among key populations in west Africa. Curr Opin HIV AIDS. 2014 doi: 10.1097/COH.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraguri N, Temmerman M, Geibel S. A decade of research involving men who have sex with men in sub-Saharan Africa: current knowledge and future directions. SAHARA J. 2012;9:137–147. doi: 10.1080/17290376.2012.744176. [DOI] [PubMed] [Google Scholar]
- 21.Friedman MR, Wei C, Klem ML, Silvestre AJ, Markovic N, et al. HIV infection and sexual risk among men who have sex with men and women (MSMW): a systematic review and meta-analysis. PLoS One. 2014;9:e87139. doi: 10.1371/journal.pone.0087139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolf SE, Maisto SA. Alcohol use and risk of HIV infection among men who have sex with men. AIDS Behav. 2009;13:757–782. doi: 10.1007/s10461-007-9354-0. [DOI] [PubMed] [Google Scholar]
- 23.Caceres CF, Konda K, Segura ER, Lyerla R. Epidemiology of male same-sex behaviour and associated sexual health indicators in low- and middle-income countries: 2003-2007 estimates. Sex Transm Infect 84 Suppl. 2008;1:i49–i56. doi: 10.1136/sti.2008.030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stall R, Purcell DW. Intertwining Epidemics: A Review of Research on Substance Use Among Men Who Have Sex with Men and Its Connection to the AIDS Epidemic. AIDS and Behavior. 2000;4:181–192. [Google Scholar]
- 25.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8:141–151. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- 26.Ludford KT, Vagenas P, Lama JR, Peinado J, Gonzales P, et al. Screening for drug and alcohol use disorders and their association with HIV-related sexual risk behaviors among men who have sex with men in Peru. PLoS One. 2013;8:e69966. doi: 10.1371/journal.pone.0069966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deiss RG, Clark JL, Konda KA, Leon SR, Klausner JD, et al. Problem drinking is associated with increased prevalence of sexual risk behaviors among men who have sex with men (MSM) in Lima, Peru. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.01.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blas MM, Alva IE, Cabello R, Carcamo C, Kurth AE. Risk behaviors and reasons for not getting tested for HIV among men who have sex with men: an online survey in Peru. PLoS One. 2011;6:e27334. doi: 10.1371/journal.pone.0027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vagenas P, Ludford KT, Gonzales P, Peinado J, Cabezas C, et al. Being Unaware of Being HIV-Infected is Associated with Alcohol Use Disorders and High-Risk Sexual Behaviors Among Men Who have Sex with Men in Peru. AIDS Behav. 2013 doi: 10.1007/s10461-013-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 31.Elul B, Basinga P, Nuwagaba-Biribonwoha H, Saito S, Horowitz D, et al. High levels of adherence and viral suppression in a nationally representative sample of HIV-infected adults on antiretroviral therapy for 6, 12 and 18 months in Rwanda. PLoS One. 2013;8:e53586. doi: 10.1371/journal.pone.0053586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babor T, Delafuente J, Saunders J. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. World Health Organization; Geneva: 1992. [Google Scholar]
- 33.Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21:613–619. [PubMed] [Google Scholar]
- 34.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 35.Cacciola JS, Alterman AI, Habing B, McLellan AT. Recent status scores for version 6 of the Addiction Severity Index (ASI-6) Addiction. 2011;106:1588–1602. doi: 10.1111/j.1360-0443.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.HA S. Drug Use Questionnaire (DAST-20) Addiction Research Foundation; 1982. [Google Scholar]
- 37.Lenore SR. The CES-D Scale : A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1 [Google Scholar]
- 38.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 39.Fleishman JA, Sherbourne CD, Crystal S, Collins RL, Marshall GN, et al. Coping, conflictual social interactions, social support, and mood among HIV-infected persons. HCSUS Consortium. Am J Community Psychol. 2000;28:421–453. doi: 10.1023/a:1005132430171. [DOI] [PubMed] [Google Scholar]
- 40.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security. US Department of Agriculture, Food and Nutrition Service 2000 [Google Scholar]
- 41.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 42.Kalichman SC, Catz S, Ramachandran B. Barriers to HIV/AIDS treatment and adherence among African-American adults with disadvantaged education. Journal of the National Medical Association. 1999;91:439–446. [PMC free article] [PubMed] [Google Scholar]
- 43.O'Donnell WE, De Soto CB, De Soto JL. Validity and reliability of the revised Neuropsychological Impairment Scale (NIS) J Clin Psychol. 1993;49:372–382. doi: 10.1002/1097-4679(199305)49:3<372::aid-jclp2270490311>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 44.Ezeabogu I, Copenhaver MM, Potrepka J. The influence of neurocognitive impairment on HIV treatment outcomes among drug-involved people living with HIV/AIDS. AIDS Care. 2012;24:386–393. doi: 10.1080/09540121.2011.608794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brislin RW. Back-Translation for Cross-Cultural Research. Journal of Cross-Cultural Psychology. 1970;1:185–216. [Google Scholar]
- 46.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. The American Journal of Medicine. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 47.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 48.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, et al. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 50.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vagenas P, Lama JR, Ludford KT, Gonzales P, Sanchez J, et al. A systematic review of alcohol use and sexual risk-taking in Latin America. Rev Panam Salud Publica. 2013;34:267–274. [PMC free article] [PubMed] [Google Scholar]
- 52.Teixeira C, Dourado Mde L, Santos MP, Brites C. Impact of use of alcohol and illicit drugs by AIDS patients on adherence to antiretroviral therapy in bahia, Brazil. AIDS Res Hum Retroviruses. 2013;29:799–804. doi: 10.1089/aid.2012.0296. [DOI] [PubMed] [Google Scholar]
- 53.Vagenas P, Lama JR, Ludford KT, Gonzales P, Sanchez J, et al. A systematic review of alcohol use and sexual risk-taking in Latin America. Rev Panam Salud Publica. 2013;34:267–274. [PMC free article] [PubMed] [Google Scholar]
- 54.Babor TF, Higgins-Biddle JC. Brief Intervention for Hazardous and Harmful Drinking: A Manual for Use in Primary Care. [Accessed on May 29, 2013];World Health Organization DoMHaSD. 2001 at: http://whqlibdoc.who.int/hq/2001/who_msd_msb_2001.2016b.pdf.
- 55.Amass L, Bickel WK, Higgins ST, Badger GJ. Alternate-day dosing during buprenorphine treatment of opioid dependence. Life Sci. 1994;54:1215–1228. doi: 10.1016/0024-3205(94)00848-5. [DOI] [PubMed] [Google Scholar]
- 56.Grodensky CA, Golin CE, Ochtera RD, Turner BJ. Systematic review: effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. J Stud Alcohol Drugs. 2012;73:899–910. doi: 10.15288/jsad.2012.73.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis. 1994;13:33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- 58.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 60.McNaghten AD, Hanson DL, Dworkin MS, Jones JL. Differences in prescription of antiretroviral therapy in a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:499–505. doi: 10.1097/00126334-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 61.Newman CE, Bonar M, Greville HS, Thompson SC, Bessarab D, et al. Barriers and incentives to HIV treatment uptake among Aboriginal people in Western Australia. AIDS 21 Suppl. 2007;1:S13–17. doi: 10.1097/01.aids.0000255080.46976.18. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization. Recommendations for a public health approach. Geneva: World Health Organization; 2011. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. [PubMed] [Google Scholar]
- 63.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 64.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margolin A, Avants SK, Warburton LA, Hawkins KA. Factors affecting cognitive functioning in a sample of human immunodeficiency virus-positive injection drug users. AIDS Patient Care STDS. 2002;16:255–267. doi: 10.1089/10872910260066697. [DOI] [PubMed] [Google Scholar]
- 66.Del Pesce M, Franciolini B, Censori B, Bartolini M, Ancarani F, et al. Cognitive behavior in asymptomatic (CDC stage II and III) HIV--seropositive intravenous drug users (IVDUs) Ital J Neurol Sci. 1993;14:619–625. doi: 10.1007/BF02339246. [DOI] [PubMed] [Google Scholar]
- 67.Martin EM, Nixon H, Pitrak DL, Weddington W, Rains NA, et al. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: preliminary observations. J Clin Exp Neuropsychol. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- 68.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnan A, Ferro EG, Weikum D, Vagenas P, Lama JR, et al. Communication Technology Use and mHealth Acceptance among HIV-infected Men who have Sex with Men in Peru: Implications for HIV Prevention and Treatment. AIDS Care. 2014 doi: 10.1080/09540121.2014.963014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive Impairment and HIV Risk Factors: A Reciprocal Relationship. AIDS Behav. 2010;14:1213–1226. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heaton RK, Grant I, Butters N, White DA, Kirson D, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 72.Crum-Cianflone NF, Moore DJ, Letendre S, Poehlman Roediger M, Eberly L, et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology. 2013;80:371–379. doi: 10.1212/WNL.0b013e31827f0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tozzi V, Balestra P, Galgani S, Narciso P, Ferri F, et al. Positive and sustained effects of highly active antiretroviral therapy on HIV-1-associated neurocognitive impairment. AIDS. 1999;13:1889–1897. doi: 10.1097/00002030-199910010-00011. [DOI] [PubMed] [Google Scholar]
- 74.Boyce S, Barrington C, Bolanos H, Arandi CG, Paz-Bailey G. Facilitating access to sexual health services for men who have sex with men and male-to-female transgender persons in Guatemala City. Cult Health Sex. 2012;14:313–327. doi: 10.1080/13691058.2011.639393. [DOI] [PubMed] [Google Scholar]
- 75.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16:2119–2143. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silveira MP, Guttier MC, Pinheiro CA, Pereira TV, Cruzeiro AL, et al. Depressive symptoms in HIV-infected patients treated with highly active antiretroviral therapy. Rev Bras Psiquiatr. 2012;34:162–167. doi: 10.1590/s1516-44462012000200008. [DOI] [PubMed] [Google Scholar]
- 77.Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS 26 Suppl. 2012;2:S117–135. doi: 10.1097/QAD.0b013e32835bde0f. [DOI] [PubMed] [Google Scholar]
- 78.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 79.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, et al. Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode: the BRIDGE study. Arch Gen Psychiatry. 2011;68:791–798. doi: 10.1001/archgenpsychiatry.2011.87. [DOI] [PubMed] [Google Scholar]
- 81.Chen LY, Crum RM, Martins SS, Kaufmann CN, Strain EC, et al. Service Use and Barriers to Mental Health Care Among Adults With Major Depression and Comorbid Substance Dependence. Psychiatr Serv. 2013 doi: 10.1176/appi.ps.201200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rivest J, Jutras-Aswad D, Shapiro PA. Treating the “unhealthy alcohol user” on medical wards: beyond withdrawal. J Psychiatr Pract. 2013;19:213–226. doi: 10.1097/01.pra.0000430505.52391.48. [DOI] [PubMed] [Google Scholar]
- 83.Sato S, Yeh TL. Challenges in Treating Patients with Major Depressive Disorder: The Impact of Biological and Social Factors. CNS Drugs. 2013 doi: 10.1007/s40263-012-0028-8. [DOI] [PubMed] [Google Scholar]
- 84.Curioso WH, Kepka D, Cabello R, Segura P, Kurth AE. Understanding the facilitators and barriers of antiretroviral adherence in Peru: a qualitative study. BMC Public Health. 2010;10:13. doi: 10.1186/1471-2458-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braithwaite RS, Conigliaro J, McGinnis KA, Maisto SA, Bryant K, et al. Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcohol Clin Exp Res. 2008;32:1645–1651. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tucker JS, Orlando M, Burnam MA, Sherbourne CD, Kung FY, et al. Psychosocial mediators of antiretroviral nonadherence in HIV-positive adults with substance use and mental health problems. Health Psychol. 2004;23:363–370. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- 88.McKirnan DJ, Ostrow DG, Hope B. Sex, drugs and escape: a psychological model of HIV-risk sexual behaviours. AIDS Care. 1996;8:655–669. doi: 10.1080/09540129650125371. [DOI] [PubMed] [Google Scholar]
- 89.Nemeroff CJ, Hoyt MA, Huebner DM, Proescholdbell RJ. The Cognitive Escape Scale: measuring HIV-related thought avoidance. AIDS Behav. 2008;12:305–320. doi: 10.1007/s10461-007-9345-1. [DOI] [PubMed] [Google Scholar]
- 90.Brigido LF, Rodrigues R, Casseb J, Oliveira D, Rossetti M, et al. Impact of adherence to antiretroviral therapy in HIV-1-infected patients at a university public service in Brazil. AIDS Patient Care STDS. 2001;15:587–593. doi: 10.1089/108729101753287685. [DOI] [PubMed] [Google Scholar]
- 91.Sankar A, Wunderlich T, Neufeld S, Luborsky M. Sero-positive African Americans' beliefs about alcohol and their impact on anti-retroviral adherence. AIDS Behav. 2007;11:195–203. doi: 10.1007/s10461-006-9144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalichman SC, Amaral CM, White D, Swetsze C, Pope H, et al. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care STDS. 2009;23:449–454. doi: 10.1089/apc.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacob ST, Baeten JM, Hughes JP, Peinado J, Wang J, et al. A post-trial assessment of factors influencing study drug adherence in a randomized biomedical HIV-1 prevention trial. AIDS Behav. 2011;15:897–904. doi: 10.1007/s10461-010-9853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr HIV/AIDS Rep. 2012;9:287–312. doi: 10.1007/s11904-012-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 97.Zarkin GA, Bray JW, Aldridge A, Mitra D, Mills MJ, et al. Cost and cost-effectiveness of the COMBINE study in alcohol-dependent patients. Arch Gen Psychiatry. 2008;65:1214–1221. doi: 10.1001/archpsyc.65.10.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. see comment. [DOI] [PubMed] [Google Scholar]
- 99.Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 100.Traore AK, Thiero O, Dao S, Kounde FFC, Faye O. Impact of low dose naltrexone (LDN) on antiretroviral therapy (ART) treated HIV+ adults in Mali: A single blind randomized clinical trial. Journal of AIDS and HIV Research. 2011;3:189–198. [Google Scholar]
- 101.Springer SA, Altice FL, Herme M, Di Paola A. Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for alcohol dependent and hazardous drinking prisoners with HIV who are transitioning to the community. Contemp Clin Trials. 2013;37:209–218. doi: 10.1016/j.cct.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weinrieb RM, O'Brien CP. A case report of naltrexone for alcoholism in a liver transplant recipient: side effects and safety. Am J Addict. 2004;13:495–497. doi: 10.1080/10550490490512870. [DOI] [PubMed] [Google Scholar]
- 103.Weinrieb RM, Van Horn DH, McLellan AT, Alterman AI, Calarco JS, et al. Alcoholism treatment after liver transplantation: lessons learned from a clinical trial that failed. Psychosomatics. 2001;42:110–116. doi: 10.1176/appi.psy.42.2.110. [DOI] [PubMed] [Google Scholar]
- 104.Vagenas P, Di Paola A, Herme M, Lincoln T, Skiest DJ, et al. An evaluation of hepatitic enzyme elevations among HIV-infected released prisoners enrolled in two randomized placebo-controlled trials of extended-release naltrexone. J Subst Abuse Treat. 2014 doi: 10.1016/J.JSAT.2014.1002.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Watson B, Conigrave KM, Wallace C, Whitfield JB, Wurst F, et al. Hazardous alcohol consumption and other barriers to antiviral treatment among hepatitis C positive people receiving opioid maintenance treatment. Drug Alcohol Rev. 2007;26:231–239. doi: 10.1080/09595230701247681. [DOI] [PubMed] [Google Scholar]
- 106.Kurth AE, Curioso WH, Ngugi E, McClelland L, Segura P, et al. Personal digital assistants for HIV treatment adherence, safer sex behavior support, and provider training in resource-constrained settings. AMIA Annu Symp Proc. 2007:1018. [PubMed] [Google Scholar]
- 107.Curioso WH, Alex Quistberg D, Cabello R, Gozzer E, Garcia PJ, et al. “It's time for your life”: How should we remind patients to take medicines using short text messages? AMIA Annu Symp Proc. 2009;2009:129–133. [PMC free article] [PubMed] [Google Scholar]
- 108.Curioso WH, Kurth AE, Cabello R, Segura P, Berry DL. Usability evaluation of Personal Digital Assistants (PDAs) to support HIV treatment adherence and safer sex behavior in Peru. AMIA Annu Symp Proc. 2008:918. [PubMed] [Google Scholar]
- 109.Palepu A, Tyndall MW, Li K, Yip B, O'Shaughnessy MV, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80:667–675. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghanem KG, Hutton HE, Zenilman JM, Zimba R, Erbelding EJ. Audio computer assisted self interview and face to face interview modes in assessing response bias among STD clinic patients. Sex Transm Infect. 2005;81:421–425. doi: 10.1136/sti.2004.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]


