Abstract
Background
Evidence for an association between total cholesterol, low and high density lipoproteins (LDL and HDL, respectively), triglycerides and prostate cancer (PC) is conflicting. Given that PC and dyslipidemia affect large proportions of Western society, understanding these associations has public health importance.
Methods
We conducted a retrospective cohort analysis of 843 radical prostatectomy (RP) patients who never used statins before surgery within the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Multivariable Cox proportional hazards analysis was used to investigate the association between cholesterol, LDL, HDL and triglycerides and biochemical recurrence risk. In secondary analysis, we explored these associations in patients with dyslipidemia, defined using National Cholesterol Education Program guidelines.
Results
Elevated serum triglycerides were associated with increased risk of PC recurrence (HRper 10 mg/dl 1.03; 95%CI 1.01–1.05) but associations between total cholesterol, LDL and HDL and recurrence risk were null. However, among men with dyslipidemia, each 10 mg/dl increase in cholesterol and HDL was associated with 9% increased recurrence risk (HR 1.09; 95%CI 1.01–1.17) and 39% reduced recurrence risk (HR 0.61; 95%CI 0.41–0.91), respectively.
Conclusions
Elevated serum triglycerides were associated with increased risk of PC recurrence. Cholesterol, LDL or HDL were not associated with recurrence risk among all men. However, among men with dyslipidemia, elevated cholesterol and HDL levels were associated with increased and decreased risk of recurrence, respectively.
Impact
These findings, coupled with evidence that statin use is associated with reduced recurrence risk, suggest that lipid levels should be explored as a modifiable risk factor for PC recurrence.
Keywords: biochemical recurrence, cholesterol, dyslipidemia, high density lipoprotein, low density lipoprotein, triglyceride, prostate cancer
Introduction
Prostate cancer (PC) is the most commonly diagnosed non-cutaneous cancer in US males and the second most common cause of cancer-related deaths [1]. Approximately two thirds of the US population are overweight or obese [2], a metabolic disorder associated with increased risk of aggressive PC and PC mortality [3]. Hypercholesterolemia, a condition strongly related to obesity, currently affects approximately 20% of the US adult population [4]. Cholesterol is hypothesized to contribute to PC progression due to its established role as a signaling molecule in prostate growth and differentiation [5], in addition to evidence from laboratory studies suggesting that cholesterol may drive PC growth via multiple biologic mechanisms including Akt signaling [6] and de novo steroidogenesis [7]. Given the high prevalence of hypercholesterolemia in Western society, understanding the potential association between this modifiable risk factor and PC progression is of great public health importance.
While epidemiologic evidence does not support an association between serum cholesterol levels and risk of total PC [8, 9], there is a suggestion that elevated cholesterol may be associated with increased risk of aggressive disease [8, 10–12], although not all studies have reported this finding [9, 13–15]. There is mixed evidence for an association between serum cholesterol levels and risk of PC progression, with some studies reporting positive associations between elevated cholesterol and risk of PC recurrence [16] and mortality [17, 18], while another study reported no association with risk of PC mortality [19]. Fewer studies examined the association between cholesterol subfractions —low and high density lipoprotein (LDL and HDL, respectively) — and PC. While there is some evidence that elevated LDL [12, 20] and low HDL [10, 21] are associated with increased risk of aggressive PC, not all studies reported these findings [13, 15, 22], and the association between cholesterol subfractions and risk of PC recurrence has not been widely studied. Finally, evidence for an association between serum triglycerides and PC recurrence is mixed [14, 23]. Thus, the impact of dyslipidemia on risk of PC recurrence is not well understood.
The aim of this study was to examine the association between serum lipid levels and risk of biochemical recurrence in a retrospective cohort of radical prostatectomy (RP) patients who never used statins prior to surgery, from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. We hypothesized that elevated serum cholesterol, triglycerides and LDL would be associated with increased risk of PC recurrence, with a protective association between elevated HDL and risk of recurrence.
Materials and Methods
Study sample
After obtaining Institutional Review Board approval from each institution, data from patients undergoing RP (n=2,542) between 1999 and 2013 at six VA Medical Centers (West Los Angeles, CA; Palo Alto, CA; San Diego, CA; Durham, NC; Asheville, NC; and Augusta, GA) were combined into the Shared Equal Access Regional Cancer Hospital (SEARCH) database [24]. SEARCH does not include patients treated with preoperative androgen deprivation or radiation therapy. Given that preoperative serum cholesterol level was our primary exposure of interest, patients who used statins before surgery were excluded (n=1,135). We also excluded patients with missing data for serum lipid levels (n=482), preoperative PSA (n=9), body mass index (BMI; n=25), pathologic Gleason score (n=11), pathologic features (n=29) and PSA follow-up (n=8), resulting in a study sample of 843 men.
Exposure assessment and definitions
Fasting total serum cholesterol, LDL, HDL and triglyceride levels measured within the year prior to RP were abstracted from VA computerized medical records. Recommended cut-offs for normal vs. abnormal serum levels (all in mg/dl) of total cholesterol (<200 vs. ≥200), LDL (<130 vs. ≥130), HDL (≥40 vs. <40) and triglycerides (<150 vs. ≥150) were selected according to National Cholesterol Education Program (NCEP)-Adult Treatment Panel (ATP) III guidelines [25]. NCEP-ATPIII borderline and high lipid categories were combined in order to have adequate numbers of patients with abnormal lipid levels for the analysis. Based on the NCEP-ATPIII guidelines, we defined normal versus abnormal categories for each individual lipid independently of the others. For example, a patient could be included in the abnormal category for cholesterol but in the normal category for triglycerides if his cholesterol level was ≥200 mg/dl and his triglyceride level was <150 mg/dl.
Follow-up
Follow-up protocols were at the discretion of the treating physicians. Biochemical recurrence was defined as a single PSA >0.2 ng/ml, two consecutive concentrations at 0.2 ng/ml, or secondary treatment for detectable postoperative PSA. Men receiving adjuvant therapy after surgery for an undetectable PSA were considered non-recurrent at the time of adjuvant therapy, and their follow-up was censored at that point.
Statistical analysis
Given that our primary hypothesis was to test the association between total serum cholesterol levels and risk of biochemical recurrence, analysis of cholesterol was considered primary, while analysis of LDL, HDL and triglyceride levels was considered secondary. Differences in demographic, clinical and pathologic factors between patients with normal vs. abnormal total serum cholesterol (<200 vs. ≥200 mg/dl) were examined using t-tests and χ2 tests for continuous and categorical variables, respectively, and rank-sum tests for continuous variables not normally distributed.
Time from RP to biochemical recurrence was compared between normal vs. abnormal serum cholesterol categories using Kaplan-Meier plots and the log-rank test. Cox proportional hazards analysis was used to test whether serum cholesterol levels (abnormal vs. normal, as well as continuous) independently predicted time to recurrence. Continuous lipid levels were presented in 10 mg/dl increments to facilitate interpretation of the hazard ratios. The proportionality assumption was tested by examining the Schoenfeld residuals. Cox models were adjusted for age at surgery (continuous), race (black vs. non-black), pre-operative PSA (continuous; log-transformed), year of surgery (continuous), BMI (continuous; log-transformed), pathological Gleason score (2–6, 7 (3+4), 7 (4+3)–10), positive surgical margins (yes vs. no), extracapsular extension (yes vs. no), seminal vesicle invasion (yes vs. no) and surgical center. Models were also adjusted for post-RP statin use which was treated as a time-dependent variable in order to account for varying start dates and duration of post-RP statin use during follow up, based upon our previous findings that post-operative statin use impacts biochemical recurrence risk [26]. We did not have access to cardiovascular disease history. In secondary analysis using the same approach as described for serum cholesterol levels, we tested the association between LDL, HDL and triglyceride levels and risk of recurrence. While the distribution of triglyceride and HDL levels was slightly skewed, log transformation did not alter our results. Thus, in order to facilitate interpretation of hazard ratios, analysis was conducted without log transformation of triglyceride and HDL levels. We tested for interaction between abnormal lipid levels and postoperative statin use in predicting risk of recurrence by treating postoperative statin use as a categorical variable and incorporating a product term into our multivariable analysis. We examined BMI as an effect modifier of the association between lipid levels and risk of recurrence by incorporating a product term into our multivariable analysis. Given the established link between abnormal serum lipid levels and increased risk of death from causes other than PC [27], we also conducted secondary analyses using a cox proportional hazards analysis treating non-PC death as a competing risk for our primary outcome of biochemical recurrence.
Locally-weighted regression (lowess) regression, enabling flexible modeling of complex relationships based on the local data structure, was used to model the relationship between serum lipid levels and risk of recurrence. In secondary analysis based upon lowess modelling results, we explored the association between serum lipid levels and risk of recurrence after restricting our cohort to men with abnormal lipid levels. Cox proportional hazards analysis was used to examine the association between serum lipid levels (continuous) and risk of recurrence, adjusting for aforementioned demographic, clinical and pathological characteristics among men with abnormal lipid values. This analysis was repeated among men with normal lipid levels.
Statistical analysis was performed using Stata, version 13.0 (Stata Corp, College Station, TX, USA).
Results
Baseline characteristics
Patients excluded from the analysis due to pre-operative statin use were older than those who never used statins prior to RP (Supplementary Table S1). As anticipated, pre-operative statin users also had elevated BMI and higher prevalence of diabetes, but lower levels of cholesterol and LDL, relative to men who never used statins prior to RP. However, while pre-operative statin users were less likely to have positive margins, no other pathologic features differed by pre-operative statin use. Patients excluded from the analysis due to missing lipid levels had an earlier year of surgery (2002 vs. 2005; p<0.0001) and longer median follow up (86.7 vs. 74.4 months; p=0.0076), but no other demographic or pathologic features differed by missing lipid status (data not shown).
Of the entire cohort of 843 men who never used statins prior to RP, 325 (39%) patients had abnormal preoperative cholesterol levels (≥200 mg/dl), as defined using NCEP-ATP III guidelines (Table 1) [25]. As anticipated, men with normal preoperative cholesterol levels (<200 mg/dl) were significantly less likely to use statins after RP, relative to men with abnormal preoperative cholesterol (p<0.001). However, there were no significant differences in age at surgery, race, preoperative PSA, diabetes status, BMI or any pathologic features by cholesterol status (Table 1). With the exception of LDL and triglycerides, lipid levels were modestly intercorrelated, with the strongest correlation between cholesterol and LDL (Pearson correlation coefficient=0.83). LDL and triglyceride levels were positively correlated with BMI, while HDL was negatively correlated with BMI (Supplementary Table S2).
Table 1.
Demographic, clinical, and pathological characteristics of patients by serum cholesterol status
| Cholesterol < 200 mg/dl (n=518; 61%) | Cholesterol ≥ 200 mg/dl (n=325; 39%) | p-value | |
|---|---|---|---|
|
|
|||
| Age, mean ± SD | 60.4 ± 6.3 | 60.6 ± 6.4 | 0.664‡ |
| Race, n (%) | 0.106§ | ||
| Black | 222 (43) | 121 (37) | |
| Non-black | 296 (57) | 204 (63) | |
| Year of surgery, median (Q1–Q3) | 2005 (2003, 2008) | 2004 (2002, 2007) | <0.001† |
| Follow-up, median (Q1–Q3) | 74.7 (41.3, 99.0) | 73.4 (41.5, 108.1) | 0.501† |
| PSA, median (Q1–Q3) | 6.3 (4.8, 9.4) | 7.2 (5.0, 10.5) | 0.071† |
| Statin use, n (%) | <0.001§ | ||
| Never | 308 (59) | 108 (33) | |
| Started after RP | 210 (41) | 217 (67) | |
| BMI, median (Q1–Q3) | 27.4 (24.7, 30.5) | 27.5 (24.9, 30.6) | 0.936† |
| Diabetes, n (%) | 84 (21) | 48 (20) | 0.687† |
| LDL, mean (SD) | 101.9 (21.6) | 141.9 (25.4) | <0.0001 |
| HDL, mean (SD) | 43.8 (13.7) | 51.2 (20.3) | <0.0001 |
| Triglycerides, mean (SD) | 126.0 (83.2) | 150.0 (83.9) | 0.0001 |
| Pathological Gleason score, n (%) | 0.276§ | ||
| 2–6 | 177 (34) | 125 (39) | |
| 7 (3+4) | 214 (41) | 134 (41) | |
| 7 (4+3)–10 | 127 (25) | 66 (20) | |
| Positive margins, n (%) | 228 (44) | 141 (43) | 0.857† |
| Extracapsular extension, n (%) | 87 (17) | 66 (20) | 0.198† |
| Seminal vesicle invasion, n (%) | 44 (8) | 30 (9) | 0.713† |
| Positive lymph nodes, n (%) | 12 (2) | 9 (3) | 0.919† |
SD=standard deviation; Q1=25th percentile; Q3=75th percentile; PSA=prostate specific antigen; BMI=body mass index; RP=radical prostatectomy
p values calculated by
t-test,
Wilcoxon rank-sum or
chi-square test
Total serum cholesterol and risk of recurrence
A total of 293 (35%) men experienced biochemical recurrence. Median follow-up among men who did not recur was 74.3 months (Q1–Q3: 41.5–102.8). Patients with normal cholesterol levels were more recently treated than patients with abnormal cholesterol (2004 vs. 2005; p<0.001; Table 1), but there was no significant difference in follow-up between groups (p=0.5). Kaplan Meier plots revealed no significant effect of cholesterol on risk of recurrence (log-rank p=0.334; data not shown). Total serum cholesterol was not associated with risk of recurrence, either as a continuous or categorical variable, on multivariable analysis (both p ≥0.4; Table 2). Competing risk analysis did not alter our results (Supplementary Table S3).
Table 2.
Hazard ratios for serum lipid levels predicting risk of biochemical recurrence after radical prostatectomy
| Total cholesterol | <200 mg/dL | ≥200 mg/dL | Continuous§ |
|---|---|---|---|
| Unadjusted | Ref. | 1.13 (0.90–1.43), p=0.294 | 1.02 (0.98, 1.05), p=0.365 |
| Adjusted* | Ref. | 1.09 (0.85–1.39), p=0.493 | 1.02 (0.98, 1.05), p=0.407 |
| LDL | <130 mg/dL | ≥130 mg/dL | Continuous§ |
| Unadjusted | Ref. | 1.03 (0.81–1.31), p=0.824 | 1.00 (0.96, 1.03), p=0.832 |
| Adjusted* | Ref. | 1.01 (0.78–1.30), p=0.944 | 0.99 (0.95, 1.04), p=0.768 |
| HDL | ≥40 mg/dL | <40 mg/dL | Continuous§ |
| Unadjusted | Ref. | 1.12 (0.89–1.41), p=0.339 | 0.99 (0.92, 1.07), p=0.854 |
| Adjusted* | Ref. | 1.11 (0.87–1.41), p=0.402 | 0.97 (0.89, 1.04), p=0.378 |
| Triglycerides | <150 mg/dL | ≥150 mg/dL | Continuous§ |
| Unadjusted | Ref. | 1.15 (0.91–1.46), p=0.250 | 1.02 (1.01,1.03), p=0.006 |
| Adjusted* | Ref. | 1.35 (1.05–1.74), p=0.020 | 1.02 (1.01, 1.04), p<0.001 |
Cells display hazard ratio (95% confidence interval), p-value
HDL=high density lipoprotein; LDL=low density lipoprotein
Hazard ratios are for every 10 mg/dL increase
Hazard ratios are adjusted for age, race, pre-operative PSA, year of surgery, BMI, surgical center, statin use, pathological Gleason score, prostate weight, positive surgical margins, extracapsular extension, and seminal vesicle invasion
Serum lipids and risk of recurrence
Similar to our null findings for total cholesterol, neither LDL nor HDL were significantly related to risk of PC recurrence, either as continuous or categorical variables, on either univariable (log-rank p=0.824 and p=0.339, respectively) or multivariable analysis (all p≥0.339; Table 2). However, elevated triglycerides were associated with 35% increased risk of recurrence on multivariable analysis (abnormal vs. normal; HR 1.35; 95% CI 1.05–1.74; Table 2). Furthermore, there was a significant 2% increased risk of recurrence for every 10 mg/dl increase in triglyceride level when treated as a continuous variable on both univariable (HRper 10 mg/dl 1.02; 95%CI 1.02–1.03) and multivariable analysis (HRper 10 mg/dl 1.02; 95%CI 1.02–1.04; Table 2). Given that abnormal serum triglycerides form part of the NCEP-ATPIII diagnostic panel for diabetes [25], we restricted our analysis to men without diabetes and found a similar association (abnormal vs. normal; HR 1.46; 95% CI 1.10–1.93). Given the strong correlation between LDL and cholesterol, we did not mutually adjust for LDL and cholesterol, but mutually adjusting for other combinations of lipids did not alter our results (data not shown). We found no significant interaction between BMI and any lipid for predicting risk of recurrence (data not shown). Treating death as a competing risk did not materially affect the associations between HDL, LDL, and triglycerides and recurrence (Supplementary Table S3). Given that preclinical disease may impact serum lipid levels, we repeated our analysis after excluding the first year of follow up and found that none of the HRs were appreciably altered (data not shown).
Risk of recurrence among men with abnormal lipid levels
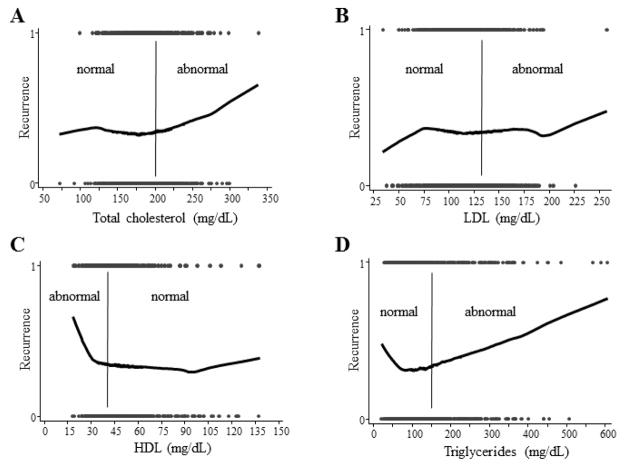
Given these null overall findings for cholesterol, LDL and HDL, we further explored the association between serum lipid levels and risk of PC recurrence by modelling these relationships using lowess plots (Figure 1). Since these plots suggested a possible relationship between lipid levels and risk of recurrence when lipid values were in the abnormal range, we performed secondary analyses restricted to patients with abnormal lipid levels. On multivariable analysis of men with abnormal lipid levels, there was a significant association between higher cholesterol levels and risk of recurrence, with each 10 mg/dl increase in cholesterol above 200 mg/dl associated with 9% increased risk of recurrence (HRper 10 mg/dl 1.09; 95%CI 1.01–1.17; Table 3). Furthermore, increasing HDL within the abnormal range was significantly protective, with each 10 mg/dL increase associated with 39% reduced risk of recurrence (HRper 10 mg/dl 0.61; 95%CI 0.41–0.91), while the association between LDL and risk of recurrence was null (HRper 10 mg/dl 1.05; 95%CI 0.94–1.17). In line with our analysis among all men, elevated triglycerides within the abnormal range remained associated with increased risk of recurrence (HRper 10 mg/dl 1.03; 95%CI 1.01–1.05). There were no significant interactions between abnormal levels of any lipid and postoperative statin use in predicting risk of recurrence (data not shown). Treating death as a competing risk did not materially affect the associations between HDL, LDL, and triglycerides and recurrence (Supplementary Table S4). Among men with normal lipid levels, total cholesterol, LDL, HDL and triglycerides were not associated with risk of recurrence (data not shown).
Figure 1. LOWESS plots of serum lipid levels and risk of biochemical recurrence.
Bars indicate the cut-off for normal vs. abnormal lipid levels, as per NCEP-ATPIII guidelines [25]
Table 3.
Hazard ratios for abnormal lipid levels predicting risk of biochemical recurrence after radical prostatectomy
| Total cholesterol | Continuous; ≥ 200 mg/dL |
|---|---|
| Unadjusted | 1.06 (0.97, 1.15), p=0.206 |
| Adjusted* | 1.09 (1.01, 1.19), p=0.027 |
| LDL | Continuous; ≥ 130 mg/dL |
| Unadjusted | 1.01 (0.90, 1.13), p=0.860 |
| Adjusted* | 1.05 (0.94, 1.17), p=0.356 |
| HDL | Continuous; < 40 mg/dL |
| Unadjusted | 0.73 (0.51, 1.04), p=0.085 |
| Adjusted* | 0.61 (0.41, 0.91), p=0.016 |
| Triglycerides | Continuous; ≥ 150 mg/dL |
| Unadjusted | 1.04 (1.02, 1.06), p<0.001 |
| Adjusted* | 1.03 (1.01, 1.05), p=0.004 |
Cells display hazard ratio (95% confidence interval), p-value
All hazard ratios are for every 10 mg/dL increase
HDL=high density lipoprotein; LDL=low density lipoprotein
Hazard ratios are adjusted for age, race, pre-operative PSA, year of surgery, BMI, surgical center, statin use, pathologic Gleason score, positive surgical margins, extracapsular extension and seminal vesicle invasion
Discussion
While obesity is an established risk factor for aggressive PC and PC mortality [3], the mechanisms contributing to this obesity-PC link are not well understood. Based upon biologic evidence supporting an important role for cholesterol in PC, we hypothesized that serum lipid levels, a potentially modifiable factor, may influence risk of PC recurrence. In contrast to this hypothesis, we found a null association between total cholesterol, LDL and HDL and risk of recurrence. However, each 10 mg/dl increase in serum triglyceride levels was associated with 2% increased risk of PC recurrence, with elevated serum triglycerides (≥150 mg/dl vs. <150 mg/dl, as defined by NCEP-ATPIII guidelines) significantly associated with 35% increased risk of recurrence. Given the scarcity of studies examining the association between triglycerides and PC progression, future studies are required to confirm these findings.
Secondary analysis, guided by modelling the relationship between serum lipid levels and risk of recurrence using lowess plots, examined these associations in men with dyslipidemia. We found that each 10 mg/dl increase in total cholesterol above the abnormal cut-off value of 200 mg/dl was significantly associated with a 9% increased risk of PC recurrence, while each 10 mg/dl increase in HDL below the abnormal cut-off value of 40 mg/dl was significantly associated with a 39% decreased risk of recurrence. These findings, which require confirmation in other studies, may highlight the importance of controlling total cholesterol and HDL levels in PC patients with dyslipidemia, not only for cardiovascular disease risk reduction but also for potentially reducing risk of PC recurrence.
In contrast to the only previous study to examine the impact of LDL on risk of recurrence in PC patients which found a positive association [16], we found a null association between LDL and risk of recurrence in our cohort. It is noteworthy that while the previous study was limited by sparse LDL data (n=169), more than half of which came from statin users [16], we had complete LDL data for our entire cohort of 843 men, none of whom were statin users at the time of LDL measurement. While future studies are required to examine the impact of elevated LDL levels on risk of recurrence, our results from this current study do not support this association.
Relative to other organs of the body, normal prostate epithelial cells have high cholesterol content and these levels increase further during progression to PC [5], suggesting that cholesterol accumulation may be advantageous to PC progression. Cholesterol promotes PC cell line growth both in vitro and in xenograft models via lipid raft-mediated Akt signaling [6]. Moreover, reduction of serum cholesterol, the precursor for sex steroid synthesis, has been demonstrated to lower tumor androgen levels and slow tumor growth in xenograft models of human PC [7]. However, epidemiologic evidence for an association between cholesterol and PC progression is mixed. One retrospective study reported a protective association between low cholesterol and risk of recurrence in radiation-treated PC patients [16] and two large studies found that elevated cholesterol levels were associated with increased risk of PC mortality [17, 18]. In contrast, another study reported a null association between cholesterol and PC mortality, although this study was conducted in a predominantly Asian population [19]. Of note, in contrast to total cholesterol, we found no association between LDL and recurrence even among men with abnormal LDL levels. While LDL levels are important for estimating cardiovascular disease risk, it is unknown which cholesterol subfractions are most important for tumor growth, and this requires further study. Although the primary mechanism by which HDL reduces cardiovascular disease risk is via reverse cholesterol transport [28], it is not known whether this same mechanism may impact PC growth. HDL has also been demonstrated to have anti-inflammatory, anti-proliferative and anti-oxidant properties [29] which may slow PC growth and progression, although epidemiologic evidence has been inconclusive [22]. Finally, high triglycerides have been associated with elevated levels of reactive oxygen species and oxidative stress, in addition to development of insulin resistance, all of which have been associated with prostate tumorigenesis [30]. Thus, the possible association between dyslipidemia and increased risk of PC recurrence is supported by these multiple biologic pathways by which serum lipids may impact PC growth and progression.
Our study has several limitations which should be considered. First, all serum lipid measurements were obtained within the year prior to RP and thus may potentially be affected by the presence of pre-clinical disease [31]. In order to address this, we explored the impact of excluding recurrences during the first year of follow up, and found that this did not alter our results. In addition, bias due to reverse causation is less likely in screened populations such as ours where PC is diagnosed early in the natural history of the disease. Second, we lacked sufficient numbers to explore the impact of abnormal levels of all four lipids simultaneously on PC recurrence. Third, we did not have access to hypertension data and therefore could not assess the association between metabolic syndrome and risk of recurrence. Neither could we assess the impact of abnormal lipid levels within the context of metabolic syndrome. However, given the use of different definitions of the metabolic syndrome across epidemiologic studies [30], in addition to recent lack of certainty regarding its pathogenesis [32], it may be equally or more informative to estimate the effect of individual components of the metabolic syndrome on risk of PC recurrence. Finally, an important limitation of all observational biomarker studies is that causality cannot be inferred from these associations. These limitations are balanced by an important strength of this study. We excluded all men who were taking statins at the time of serum lipid measurement. While exclusion of pre-operative statin users limits the generalizability of our findings to men who do not use statins, we believe that this approach strengthens our exposure assessment as the pre-operative lipid levels of statin users may not reflect the environment that their tumor developed in. Furthermore, we adjusted our multivariable models for post-RP statin use as a time-dependent variable. Thus our serum lipid level measurements were obtained in the absence of any cholesterol-lowering medications and we were able to assess the association between serum lipid levels and risk of recurrence separately from statin use. While we found no evidence for interaction between postoperative statin use and abnormal lipid levels in predicting risk of recurrence, future studies should assess whether beginning statin therapy after PC treatment could attenuate associations between abnormal lipid levels and risk of recurrence.
In summary, we found no strong evidence to support a link between serum lipid levels and risk of PC recurrence across the entire sample. However, among men with dyslipidemia, our findings suggest that normalization of serum lipid levels may be beneficial not only for cardiovascular disease prevention but also for PC recurrence risk reduction, though these results require confirmation in future studies. Although it cannot be determined from this study if these observed associations are causal, given the biologic evidence supporting an important role of cholesterol in PC growth in addition to epidemiologic data demonstrating that statin use is associated with reduced risk of recurrence, we believe that serum lipid levels should be explored further as a risk factor for PC recurrence. While the association between obesity and increased risk of PC recurrence is likely to be multifactorial [3], these findings suggest that dyslipidemia may be one of the mechanisms underlying this association. Given that 45% of deaths worldwide can be attributed to cardiovascular disease and cancer [15], with PC the second most common cause of male cancer deaths [1], understanding the role of dyslipidemia as a shared, modifiable risk factor for both of these common causes of mortality is of great importance.
Supplementary Material
Acknowledgments
Financial support: EHA: NCI 5R25-CA126938-03; SJF: NIH Grant 1-R01-CA131235-01A1 and NIH 1K24CA160653
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 5.Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim Biophys Acta. 2013;1835:219–29. doi: 10.1016/j.bbcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–31. [PubMed] [Google Scholar]
- 7.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7:e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–8. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22:1545–52. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer. 2012;12:25. doi: 10.1186/1471-2407-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103:885–92. doi: 10.1093/jnci/djr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs EJ, Stevens VL, Newton CC, Gapstur SM. Plasma total, LDL, and HDL cholesterol and risk of aggressive prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control. 2012;23:1289–96. doi: 10.1007/s10552-012-0006-y. [DOI] [PubMed] [Google Scholar]
- 14.Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009;20:1181–92. doi: 10.1007/s10552-009-9319-x. [DOI] [PubMed] [Google Scholar]
- 15.His M, Zelek L, Deschasaux M, Pouchieu C, Kesse-Guyot E, Hercberg S, et al. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur J Epidemiol. 2014 doi: 10.1007/s10654-014-9884-5. [DOI] [PubMed] [Google Scholar]
- 16.Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28:2653–9. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]
- 17.Colli JL, Amling CL. High cholesterol levels are associated with reduced prostate cancer mortality rates during periods of high but not low statin use in the United States. Urol Oncol. 2009;27:170–3. doi: 10.1016/j.urolonc.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22:311–8. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxley R. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8:199–205. [PubMed] [Google Scholar]
- 20.Kok DE, van Roermund JG, Aben KK, den Heijer M, Swinkels DW, Kampman E, et al. Blood lipid levels and prostate cancer risk; a cohort study. Prostate Cancer Prostatic Dis. 2011;14:340–5. doi: 10.1038/pcan.2011.30. [DOI] [PubMed] [Google Scholar]
- 21.Magura L, Blanchard R, Hope B, Beal JR, Schwartz GG, Sahmoun AE. Hypercholesterolemia and prostate cancer: a hospital-based case-control study. Cancer Causes Control. 2008;19:1259–66. doi: 10.1007/s10552-008-9197-7. [DOI] [PubMed] [Google Scholar]
- 22.Kotani K, Sekine Y, Ishikawa S, Ikpot IZ, Suzuki K, Remaley AT. High-density lipoprotein and prostate cancer: an overview. J Epidemiol. 2013;23:313–9. doi: 10.2188/jea.JE20130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post JM, Beebe-Dimmer JL, Morgenstern H, Neslund-Dudas C, Bock CH, Nock N, et al. The Metabolic Syndrome and Biochemical Recurrence following Radical Prostatectomy. Prostate Cancer. 2011;2011:245642. doi: 10.1155/2011/245642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allott EH, Abern MR, Gerber L, Keto CJ, Aronson WJ, Terris MK, et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2013 doi: 10.1038/pcan.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 26.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: Results from the SEARCH database. BJU Int. 2014 doi: 10.1111/bju.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGrowder D, Riley C, Morrison EY, Gordon L. The role of high-density lipoproteins in reducing the risk of vascular diseases, neurogenerative disorders, and cancer. Cholesterol. 2011;2011:496925. doi: 10.1155/2011/496925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toth PP. High-density lipoprotein as a therapeutic target: clinical evidence and treatment strategies. Am J Cardiol. 2005;96:50K–58K. doi: 10.1016/j.amjcard.2005.08.008. discussion 34K–35K. [DOI] [PubMed] [Google Scholar]
- 30.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006;169:1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2814–21. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.