Abstract
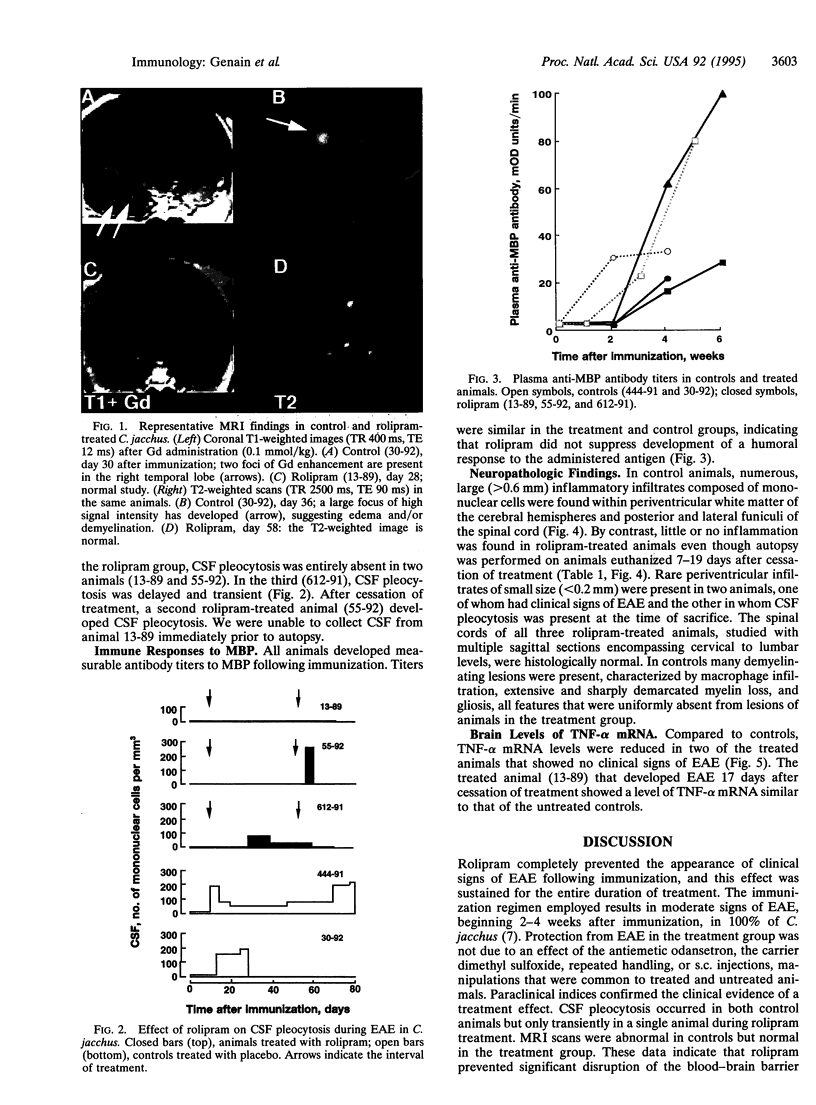
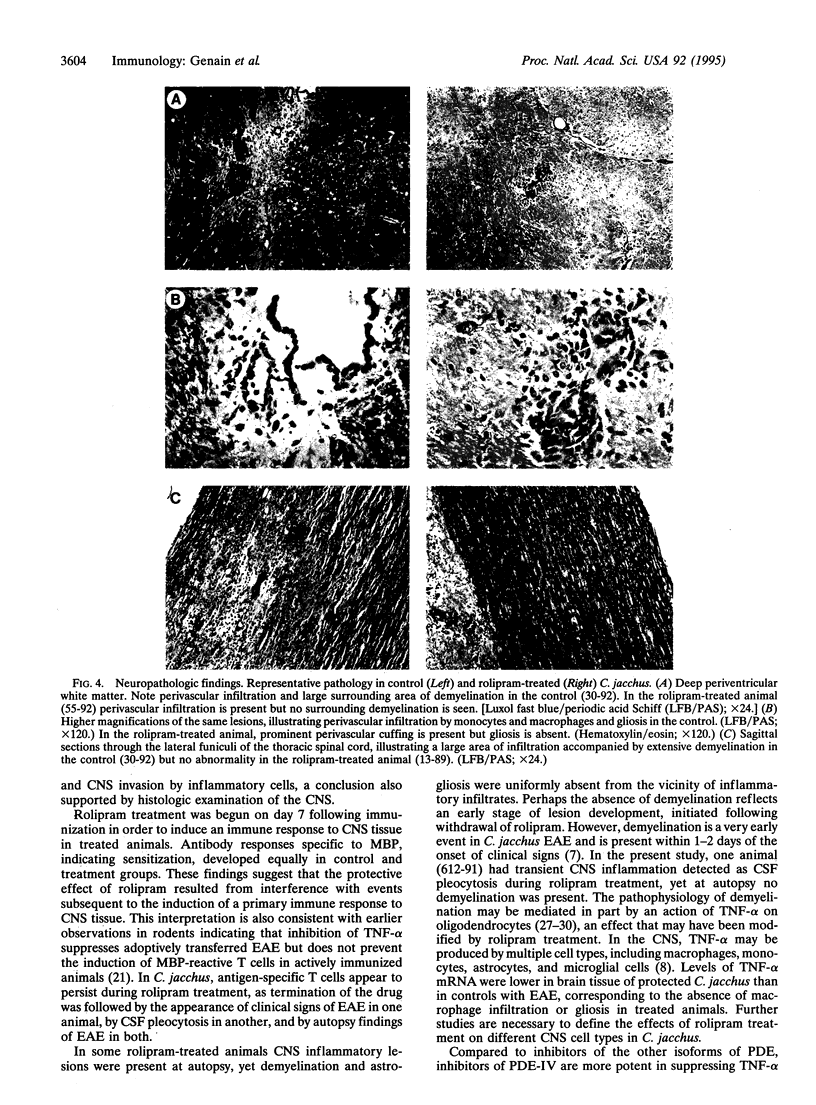
Experimental allergic encephalomyelitis (EAE) is an autoimmune disease of the central nervous system that serves as a model for the human disease multiple sclerosis. We evaluated rolipram, a type IV phosphodiesterase inhibitor, for its efficacy in preventing EAE in the common marmoset Callithrix jacchus. In a blinded experimental design, clinical signs of EAE developed within 17 days of immunization with human white matter in two placebo-treated animals but in none of three monkeys that received rolipram (10 mg/kg s.c. every other day) beginning 1 week after immunization. In controls, signs of EAE were associated with development of cerebrospinal fluid pleocytosis and cerebral MRI abnormalities. In the treatment group, there was sustained protection from clinical EAE, transient cerebrospinal fluid pleocytosis in only one of three animals, no MRI abnormality, and marked reduction in histopathologic findings. Rolipram-treated and control animals equally developed circulating antibodies to myelin basic protein. Thus, inhibition of type IV phosphodiesterase, initiated after sensitization to central nervous system antigens, protected against autoimmune demyelinating disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretta M., Nicklas J. A., Sriram S., Albertini R. J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990 Feb 9;247(4943):718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Barten D. M., Ruddle N. H. Vascular cell adhesion molecule-1 modulation by tumor necrosis factor in experimental allergic encephalomyelitis. J Neuroimmunol. 1994 May;51(2):123–133. doi: 10.1016/0165-5728(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Beck J., Rondot P., Catinot L., Falcoff E., Kirchner H., Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988 Oct;78(4):318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Selmaj K., Raine C. S. Hypothesis: a role for tumor necrosis factor in immune-mediated demyelination and its relevance to multiple sclerosis. J Neuroimmunol. 1988 Apr;18(1):87–94. doi: 10.1016/0165-5728(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Butt A. M., Jenkins H. G. Morphological changes in oligodendrocytes in the intact mouse optic nerve following intravitreal injection of tumour necrosis factor. J Neuroimmunol. 1994 Apr;51(1):27–33. doi: 10.1016/0165-5728(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Cavender D. E., Edelbaum D., Ziff M. Endothelial cell activation induced by tumor necrosis factor and lymphotoxin. Am J Pathol. 1989 Mar;134(3):551–560. [PMC free article] [PubMed] [Google Scholar]
- Chung I. Y., Norris J. G., Benveniste E. N. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991 Apr 1;173(4):801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. T., Knight M. B., Harakas N. K., Wittwer A. J., Feder J. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal Biochem. 1986 Jan;152(1):136–140. doi: 10.1016/0003-2697(86)90131-4. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Endres S., Fülle H. J., Sinha B., Stoll D., Dinarello C. A., Gerzer R., Weber P. C. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-alpha and interleukin-1 beta by human mononuclear cells. Immunology. 1991 Jan;72(1):56–60. [PMC free article] [PubMed] [Google Scholar]
- Eskandari M. K., Nguyen D. T., Kunkel S. L., Remick D. G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest. 1990 Feb;19(1):69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- Han J., Brown T., Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990 Feb 1;171(2):465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S. L., Doolittle T. H., Lincoln R., Brown R. H., Dinarello C. A. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology. 1990 Nov;40(11):1735–1739. doi: 10.1212/wnl.40.11.1735. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. C., Male D. K., Lantos P. L. Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-gamma, tumour necrosis factor and interleukin-1. Immunology. 1988 Aug;64(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Krause W., Kühne G. High-performance liquid chromatographic procedure for specificity testing of radioimmunoassays: rolipram. J Chromatogr. 1992 Jan 17;573(2):303–308. doi: 10.1016/0378-4347(92)80133-b. [DOI] [PubMed] [Google Scholar]
- Kuchroo V. K., Martin C. A., Greer J. M., Ju S. T., Sobel R. A., Dorf M. E. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol. 1993 Oct 15;151(8):4371–4382. [PubMed] [Google Scholar]
- Kuroda Y., Shimamoto Y. Human tumor necrosis factor-alpha augments experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1991 Nov;34(2-3):159–164. doi: 10.1016/0165-5728(91)90125-q. [DOI] [PubMed] [Google Scholar]
- McCarron R. M., Wang L., Racke M. K., McFarlin D. E., Spatz M. Cytokine-regulated adhesion between encephalitogenic T lymphocytes and cerebrovascular endothelial cells. J Neuroimmunol. 1993 Mar;43(1-2):23–30. doi: 10.1016/0165-5728(93)90071-6. [DOI] [PubMed] [Google Scholar]
- Monastra G., Cross A. H., Bruni A., Raine C. S. Phosphatidylserine, a putative inhibitor of tumor necrosis factor, prevents autoimmune demyelination. Neurology. 1993 Jan;43(1):153–163. doi: 10.1212/wnl.43.1_part_1.153. [DOI] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Powell M. B., Mitchell D., Lederman J., Buckmeier J., Zamvil S. S., Graham M., Ruddle N. H., Steinman L. Lymphotoxin and tumor necrosis factor-alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2(6):539–544. doi: 10.1093/intimm/2.6.539. [DOI] [PubMed] [Google Scholar]
- Powell M. B., Mitchell D., Lederman J., Buckmeier J., Zamvil S. S., Graham M., Ruddle N. H., Steinman L. Lymphotoxin and tumor necrosis factor-alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2(6):539–544. doi: 10.1093/intimm/2.6.539. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade F. U., Schudt C. The specific type III and IV phosphodiesterase inhibitor zardaverine suppresses formation of tumor necrosis factor by macrophages. Eur J Pharmacol. 1993 Jan 5;230(1):9–14. doi: 10.1016/0014-2999(93)90403-5. [DOI] [PubMed] [Google Scholar]
- Selmaj K. W., Farooq M., Norton W. T., Raine C. S., Brosnan C. F. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990 Jan 1;144(1):129–135. [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cross A. H. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991 Nov;30(5):694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Semmler J., Wachtel H., Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int J Immunopharmacol. 1993 Apr;15(3):409–413. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- Sharief M. K., Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991 Aug 15;325(7):467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Soliven B., Szuchet S., Nelson D. J. Tumor necrosis factor inhibits K+ current expression in cultured oligodendrocytes. J Membr Biol. 1991 Nov;124(2):127–137. doi: 10.1007/BF01870457. [DOI] [PubMed] [Google Scholar]
- Taffet S. M., Singhel K. J., Overholtzer J. F., Shurtleff S. A. Regulation of tumor necrosis factor expression in a macrophage-like cell line by lipopolysaccharide and cyclic AMP. Cell Immunol. 1989 May;120(2):291–300. doi: 10.1016/0008-8749(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman B. Mechanisms in multiple sclerosis. Nature. 1985 Nov 14;318(6042):104–105. doi: 10.1038/318104a0. [DOI] [PubMed] [Google Scholar]
- Yokota S., Geppert T. D., Lipsky P. E. Enhancement of antigen- and mitogen-induced human T lymphocyte proliferation by tumor necrosis factor-alpha. J Immunol. 1988 Jan 15;140(2):531–536. [PubMed] [Google Scholar]
- Zajicek J. P., Wing M., Scolding N. J., Compston D. A. Interactions between oligodendrocytes and microglia. A major role for complement and tumour necrosis factor in oligodendrocyte adherence and killing. Brain. 1992 Dec;115(Pt 6):1611–1631. [PubMed] [Google Scholar]