Abstract
We describe outcomes after allogeneic hematopoietic cell transplantation (HCT) for mycosis fungoides and sezary syndrome (MF/SS). Outcomes of 129 subjects with MF/SS reported to the Center for the International Blood and Marrow Transplant (CIBMTR) from 2000–2009. Median time from diagnosis to transplant was 30 (4–206) months and most subjects were with multiply relapsed/refractory disease. Majority (64%) received non-myeloablative conditioning (NST) or reduced intensity conditioning (RIC). NST/RIC recipients were older in age compared to myeloablative recipients (median age 51 vs. 44 y p= 0.005) and transplanted in recent years. Non-relapse mortality (NRM) at 1 and 5 years was 19% (95 % CI 12–27%) and 22% (95 % CI 15–31%) respectively. Risk of disease progression was 50% (95% CI 41–60%) at 1 year and 61% (95% CI 50–71%) at 5 years. Progression free survival (PFS) at 1 and 5 years was 31% (95% CI 22–40%) and 17% (95% CI 9–26%) respectively. Overall survival at 1 and 5 years was 54% (95% CI 45–63%) and 32% (95% CI 22–44%) respectively. Allogeneic HCT in MF/SS results in 5 year survival in approximately one-third of patients and of those, half of them remain disease-free.
Keywords: Cutaneous T cell lymphoma, Allogeneic hematopoietic cell transplant, Bone marrow transplant, Mycosis fungoides, sezary syndrome
INTRODUCTION
Cutaneous T cell lymphoma (CTCL) constitutes approximately 2% of all lymphomas. Mycosis fungoides (MF) represents the most common subtype. Many patients with MF experience a prolonged clinical course. Patients with stage IVA and IVB disease have a median overall survival (OS) between 1.4 to 3.8 years from the time of diagnosis. 1,2 Newer treatment modalities have arisen over the last decade, with relapse still being common.
Historically, hematopoietic cell transplantation (HCT) was rarely used to treat MF/SS because of concern regarding the lack of skin integrity and possible increased risk of infection. However, there is renewed interest in consideration of HCT because MF/SS is incurable with conventional therapy. Autologous HCT has not resulted in long-term remissions in CTCL patients3–5 and is rarely used now. Relatively small retrospective studies of allogeneic HCT with both myeloablative (MAC) and non-myeloablative conditioning (NST)/reduced intensity conditioning (RIC) regimens have been described.5–7 Definitive conclusions are lacking because of small sample size and considerable heterogeneity in subject, disease, and transplant variables. We analyzed outcomes of allogeneic HCT for MF/SS in 129 subjects reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).8
PATIENTS AND METHODS
The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplants to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, gender, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood- derived progenitor cells), conditioning regimen, post-transplant disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR teams contribute TED data. More detailed disease and pre- and post-transplant clinical information are collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED and CRF level data are collected pre-transplant, 100 days, and six months post transplant and annually thereafter or until death.
Subject selection
We analyzed the outcomes 129 adult (≥18 years) recipients of an allogeneic HLA matched related or unrelated HCT for MF/SS reported to the CIBMTR between 2000 and 2009. A subset of these patients (N=52) were reported at a higher level with comprehensive disease data and complete case report forms (CRF). Median follow up for these 52 patients was 40 months with a range of 3–91 months. The overall cohort of 129 subjects and the comprehensive subset had similar OS outcomes.
Study endpoints and definitions
Outcomes analyzed included treatment related mortality (NRM), relapse/progression, progression-free (PFS), and overall survival (OS). Patient staging was done by Ann Arbor staging criteria at the time of treatment. Data for ISCL/EORTC for MF/SS was not captured in this data set. The intensity of conditioning was categorized based on consensus criteria.9 Neutrophil recovery was defined as the first of three subsequent days with absolute neutrophil counts ≥0.5 × 10e9/L without growth factor support. Platelet recovery was defined as the first of seven subsequent days with platelet counts ≥20 × 10e9/L without platelet transfusions. NRM was defined as death from any cause in the first 28 days after HCT or death without evidence of MF/SS progression/relapse. Progression was defined as an increase of ≥25% in the sites of lymphoma or development of new sites. Relapse was defined as recurrence of lymphoma after a complete remission (CR). For PFS, patients were considered treatment failures at the time of relapse/progression or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow up and the PFS event was summarized by a survival curve. The OS interval variable was defined as the time from date of transplant to date of death or last contact and summarized by a survival curve.
Other outcomes analyzed included the incidence of acute (aGvHD) and chronic graft versus host disease (cGvHD) graded by established criteria and cause of death.
Statistical analysis
Subject-, disease-, and transplant-related variables are described for the entire cohort along with an additional subset with higher level CRF data. Univariate probabilities of NRM, relapse/progression, aGVHD, cGVHD, PFS and survival were described and compared. Causes of death are described.
RESULTS
Patients and Transplant Characteristics
129 MF/SS patients received an allogeneic transplant with characteristics described in Table 1. The majority received NST/RIC (n=83), 64% vs. 36% receiving MAC. The NST/RIC cohort was older with a median age at transplant of 51 (27–72) years vs. 44 (22–63) years in the MAC cohort (P=0.005). Eighty nine percent (n=74) of patients were transplanted 12 months or longer from diagnosis, with 49% (n=41) transplanted more than 36 months after diagnosis. Only one patient received allogeneic HCT in first CR while 37% never achieved CR prior to transplant. No patient had a prior autologous HCT.
Table 1.
Characteristics of patients who underwent allogeneic transplantation for mycosis fungoides and Sezary syndrome, registered to the CIBMTR, between 2000 and 2009.
| Patient characteristicsa |
NST/RIC N% |
Myeloablative N% |
|---|---|---|
| Patient-related | ||
| Number of patients | 83 | 46 |
| Number of centers | 37 | 31 |
| Age at transplant, median (range) years | 51 (27–72) | 44 (22–63) |
| 21–30 | 1 (1) | 3 (7) |
| 31–40 | 12 (14) | 11 (24) |
| 41–50 | 24 (29) | 21 (46) |
| 51–60 | 35 (42) | 8 (17) |
| > 60 | 11 (13) | 3 (7) |
| Male sex | 52 (63) | 18 (39) |
| Karnofsky score | ||
| < 90 | 32 (39) | 28 (61) |
| ≥ 90 | 35 (42) | 11 (24) |
| Missing | 16 (19) | 7 (15) |
| Self Reported Racial group | ||
| White | 73 (88) | 30 (65) |
| Black | 7 (8) | 15 (33) |
| Asian | 3 (4) | 1 (2) |
| Disease-related | ||
| Disease status at transplant | ||
| Never in CR (PIF) | 31 (37) | 13 (28) |
| First complete remission | 1 (1) | 2 (4) |
| ≥ Second complete remission | 2 (2) | 3 (7) |
| First relapse | 10 (12) | 0 |
| ≥ Second relapse | 12 (14) | 6 (13) |
| Missing | 27 (33) | 22 (48) |
| Interval from diagnosis to transplant, median (range), months | 36 (4–206) | 20 (4–174) |
| <12 | 9 (11) | 12 (26) |
| 12–36 | 33 (40) | 17 (37) |
| > 36 | 41 (49) | 17 (37) |
| Transplant-related | ||
| Graft type | ||
| Bone marrow | 11 (13) | 7 (15) |
| Peripheral blood | 71 (86) | 36 (78) |
| Cord blood | 1 (1) | 3 (7) |
| Year of transplant | ||
| 2000–2002 | 5 (6) | 8 (17) |
| 2003–2005 | 25 (30) | 13 (28) |
| 2006–2008 | 40 (48) | 14 (30) |
| 2009 | 13 (16) | 11 (24) |
| Donor type | ||
| HLA-matched related | 45 (54) | 19 (41) |
| Unrelated donor | 34 (41) | 22 (48) |
| HLA-mismatched related | 4 (5) | 5 (11) |
| Conditioning regimen at transplant | ||
| Fludarabine + Melphalan | 36 (43) | 14 (30) |
| Busulfan + Cyclophosphamide | 0 | 4 (9) |
| Cyclophosphamide + TBI | 3 (4) | 16 (35) |
| Fludarabine + Busulfan ± Other | 30 (36) | 7 (15) |
| TBI only | 9 (11) | 5 (11) |
| BEAM | 2 (2) | 0 |
| TLI + ATG | 3 (4) | 0 |
| GVHD prophylaxis | ||
| In vivo T-cell depletion | 1 (1) | 0 |
| CSA + Alemtuzumab | 0 | 1 (2) |
| CSA ±MTX ± Other | 21 (25) | 8 (17) |
| FK506/CSA + MMF ± Other | 22 (27) | 8 (17) |
| FK506 ± MTX ± Other | 35 (42) | 25 (55) |
| Alemtuzumab alone | 3 (4) | 0 |
| Missing | 1 (1) | 4 (9) |
| Median follow-up of survivors, median (range), months | 39 (3–91) | 32 (3–97) |
Abbreviations: EVAL = evaluable; CMV= cytomegalovirus; NST = non-myeloablative; RIC = reduced intensity conditioning; Cy = cyclosphosphamide; TBI = total body irradiation; GVHD = graft versus host disease; CSA = cyclosporine; MMF= mycophenolate; MTX = methotrexate; FK506 = tacrolimus; HLA= human leukocyte antigen.
The majority of patients (64%) were transplanted after 2006. A greater utilization of NST/RIC regimens was noted for this patient population. Bone marrow grafts were used in 13%, peripheral blood grafts in 86% and cord blood in one (1%) patient. Fludarabine based combination conditioning regimens were used in 66 of the 83 (80%) patients receiving NST/RIC. Specific NST/RIC regimens are detailed in Table 1. Cyclophosphamide with total body irradiation (TBI) was the most common MAC regimen used 16 of 45 or (35%) of patients.
A subset of 52 patients had higher level reporting with more disease-related information available for analysis (CRF cohort, Table 2). In this subset, 25 of 52 patients or 48% had 4 or more lines of chemotherapy prior to transplant. The use of alemtuzumab prior to transplant was limited to (13% of patients) and total skin electron beam (TSEB) radiation was used in only one patient recorded. Radiation therapy was used before transplant in 19% of 52 patients.
Table 2.
Characteristics of patients who underwent allogeneic bone marrow or peripheral blood transplantation for mycosis fungoides and Sezary syndrome, reported to the CIBMTR, between 2001 and 2009.
| Patient characteristics | N eval | N (%) |
|---|---|---|
| Patient-related | ||
| Number of patients | 52 | |
| Number of centers | 31 | |
| Age at transplant, median (range) years | 52 | 49 (27–72) |
| 21–30 | 1 (2) | |
| 31–40 | 10 (19) | |
| 41–50 | 21 (40) | |
| 51–60 | 15 (29) | |
| > 60 | 5 (10) | |
| Male sex | 52 | 30 (58) |
| Karnofsky score at transplant < 90 | 45 | 19 (37) |
| Race | 52 | |
| White | 42 (81) | |
| Black | 8 (15) | |
| Asian | 1 (2) | |
| Missing | 1 (2) | |
| Disease-related | ||
| Disease status at transplant | 52 | |
| Primary induction failure (Never in CR) | 33 (63) | |
| First complete remission | 2 (4) | |
| First relapse | 7 (13) | |
| ≥ Second relapse | 10 (19) | |
| Interval from diagnosis to transplant, median (range), months | 52 | 38 (6 – 129) |
| <12 | 7 (13) | |
| 12–36 | 18 (35) | |
| > 36 | 27 (52) | |
| Disease stage at diagnosis | 49 | |
| I | 10 (20) | |
| II | 9 (17) | |
| III | 9 (20) | |
| IV | 19 (39) | |
| Otherb | 2 (4) | |
| LDH > upper limit at diagnosisc | 8 | 5 (71) |
| Extranodal or splenic involvement at diagnosis? | 49 | |
| Yes | 36 (74) | |
| No | 13 (26) | |
| Extranodal or splenic involvement sites prior to conditioning | 52 | |
| Yes | 34 (65) | |
| No | 11 (21) | |
| Missing | 7 (13) | |
| Alemtuzumab used | 52 | 7 (13) |
| Transplant-related | ||
| Year of transplant | 52 | |
| 2001 | 3 (6) | |
| 2002 | 3 (6) | |
| 2003 | 7 (13) | |
| 2004 | 3 (6) | |
| 2005 | 7 (13) | |
| 2006 | 11 (21) | |
| 2007 | 6 (12) | |
| 2008 | 6 (12) | |
| 2009 | 6 (12) | |
| Graft type | 52 | |
| Bone marrow | 8 (15) | |
| Peripheral blood | 43 (83) | |
| Cord blood | 1 (2) | |
| Prior radiation therapy received? | 52 | 10 (19) |
| Site for prior radiation received | 5 | |
| Locald | 4 (80) | |
| Total skin | 1 (20) | |
| Number of lines of prior chemotherapy | 52 | |
| 0 | 3 (5) | |
| 1 | 5 (10) | |
| 2 | 7 (14) | |
| 3 | 12 (23) | |
| 4 | 13 (25) | |
| ≥5 | 12(23) | |
| Donor type | 52 | |
| HLA identical sibling | 20 (38) | |
| Well matched unrelated | 15 (29) | |
| Partially matched unrelated | 10 (19) | |
| Mismatched unrelated | 5 (10) | |
| HLA –matched other relative | 1 (2) | |
| HLA-mismatched other relative | 1 (2) | |
| Subsequent DLI? | 52 | 2 (4) |
| Conditioning regimen type | 52 | |
| Myeloablative | 9 (17) | |
| NST/RIC | 43 (83) | |
| Conditioning regimen | 52 | |
| Fludarabine + Melphalan | 19 (36) | |
| Bu + Cyclophosphamide | 1 (2) | |
| Cyclophosphamide + TBI ± Other | 5 (10) | |
| Busulfan + Fludarabine ± Other | 18 (35) | |
| TBI ± Other | 7 (13) | |
| BEAM | 2 (4) | |
| GVHD prophylaxis | 52 | |
| In vivo T-cell depletion | 1 (2) | |
| CSA ± MTX ± Other | 10 (19) | |
| FK506/CSA + MMF ± Other | 13 (25) | |
| FK506 ± MTX ± Other | 23 (44) | |
| ATG + Sirolimus | 1 (2) | |
| Missing | 4 (8) | |
| Median follow up of survivors, range, months | 22 | 40 (3–91) |
Abbreviations: EVAL = evaluable; CMV= cytomegalovirus; NST = non-myeloablative; RIC = reduced intensity conditioning; Bu = busulfan; LPAM = melphalan; Cy = cyclosphosphamide; TBI = total body irradiation; GVHD = graft versus host disease; CSA = cyclosporine; MMF= mycophenolate; MTX = methotrexate; FK506 = tacrolimus; HLA= human leukocyte antigen; NMDP=national marrow donor program; DCI=donor cell infusion (DLI).
Other disease stage at diagnosis (n=2) includes: IA: T1 Nomo (n=1); Skin (n=1)
45 patients have missing LDH values
Local site for prior radiation received include: Back, pelvis, arms (n=1), right upper ankle (n=1); scalp (n=1), local (n=1)
Engraftment
Neutrophil engraftment was achieved in 95% (95% CI 88–98) of patients at day 28. Platelet engraftment was achieved in 89% (95% CI 76–95%) of patients at day 100 (Table 3).
Table 3.
Univariate outcomes of patients who underwent allogeneic bone marrow or peripheral blood transplantation for mycosis fungoides and Sezary syndrome, registered with the CIBMTR, between 2000 – 2009a.
| Outcome of interest | N(eval) | Probability (95 % CI) |
|---|---|---|
| Total number of patients | 129 | |
| Mortality | ||
| @ 30 days | 129 | 6 (3–11) |
| @ 100 days | 16 (10–23) | |
| Neutrophil engraftment | 110 | |
| @ 28 days | 95 (88–98) | |
| @ 100 days | 95 (89–98) | |
| Platelet engraftment (20,000 × 109/L) | 53 | |
| @ 28 days | 70 (55–81) | |
| @ 100 days | 89 (76–95) | |
| Acute GVHD | 95 | |
| Grade II–IV @ 100 days | 41 (32–51) | |
| Chronic GVHD | 87 | |
| @ 180 days | 33 (23–43) | |
| @ 1 year | 42 (31–52) | |
| @ 2 years | 43 (33–54) | |
| Non relapsed mortality | 119 | |
| @ 1 year | 19 (12–27) | |
| @ 3 year | 22 (15–31) | |
| @ 5 year | 22 (15–31) | |
| Progression relapse | 119 | |
| @ 1 year | 50 (41–60) | |
| @ 3 year | 58 (48–68) | |
| @ 5 year | 61 (50–71) | |
| Progression free survival | 119 | |
| @ 1 year | 31 (22–40) | |
| @ 3 year | 19 (12–28) | |
| @ 5 year | 17 (9–26) | |
| Overall survival | 129 | |
| @ 1 year | 54 (45–63) | |
| @ 3 year | 38 (28–48) | |
| @ 5 year | 32 (22–44) | |
Abbreviations: GVHD= graft vs. host disease
Probabilities of overall survival, mortality and progression free survival were calculated using the Kaplan-Meier product limit estimate.
Probability of neutrophil & platelet engraftment, treatment related mortality, progression relapse, AGVHD and CGVHD were calculated using the cumulative incidence function.
GVHD
The incidence of grade II–IV aGVHD was 41% (95%CI 32–51%). The incidence of cGVHD was 33% (95% CI 23–43%), 42% (95%CI 31–52%), and 43% (95%CI 33–54%) at 180 days, 1 and 2 years respectively (Table 3).
Treatment and Disease Outcomes (Table 3 and 4)
Table 4.
Comparison of univariate outcomes between ablative and NST/RIC conditioning among patients who underwent allogeneic bone marrow or peripheral blood transplantation for mycosis fungoides and Sezary syndrome, reported to the CIBMTR, between 2000–2009a.
| Outcomes | N | RIC/NST | N | Myeloablative | P-valueb |
|---|---|---|---|---|---|
| Mortality | 82 | 45 | 0.271 | ||
| @ 30 days | 5 (1–11) | 9 (2–19) | 0.394 | ||
| @ 100 days | 15 (8–23) | 18 (8–31) | 0.612 | ||
| Neutrophil engraftment | 68 | 42 | |||
| @ 28 days | 96 (86–99) | 93 (79–98) | 0.567 | ||
| @ 100 days | 97 (89–99) | 93 (79–98) | 0.353 | ||
| Platelet engraftment | 32 | 21 | |||
| @ 28 days | 75 (54–87) | 62 (38–79) | 0.335 | ||
| @ 100 days | 88 (69–95) | 90 (66–98) | 0.739 | ||
| Acute GVHD (II–IV) | 63 | 32 | |||
| Grade II–IV @ 100 days | 46 (34–58) | 32 (17–49) | 0.163 | ||
| Chronic GVHD | 56 | 31 | |||
| @ 180 days | 31 (20–44) | 37 (21–55) | 0.576 | ||
| @ 1 year | 39 (26–52) | 48 (30–66) | 0.416 | ||
| @ 2 years | 39 (26–52) | 52 (34–69) | 0.254 | ||
| NRM | 77 | 42 | |||
| @ 1 year | 16 (9–26) | 24 (12–38) | 0.372 | ||
| @ 3 years | 20 (11–30) | 27 (14–42) | 0.399 | ||
| @ 5 years | 20 (11–30) | 27 (14–42) | 0.399 | ||
| Progression relapse | 77 | 42 | |||
| @ 1 year | 50 (39–62) | 50 (35–66) | 0.982 | ||
| @ 3 years | 57 (45–69) | 60 (44–75) | 0.764 | ||
| @ 5 years | 57 (45–69) | 67 (49–82) | 0.367 | ||
| Progression free survival | 77 | 42 | |||
| @ 1 year | 33 (23–45) | 26 (13–41) | 0.412 | ||
| @ 3 years | 23 (13–35) | 13 (4–26) | 0.194 | ||
| @ 5 years | 23 (13–35) | 6 (0–21) | 0.029 | ||
| Overall survival | 83 | 46 | |||
| @ 1 year | 56 (45–67) | 51 (35–66) | 0.587 | ||
| @ 3 years | 41 (29–53) | 31 (16–49) | 0.358 | ||
| @ 5 years | 36 (23–50) | 21 (5–43) | 0.208 |
Probability of treatment related mortality, progression relapse, were calculated using the cumulative incidence function.
Pointwise p-value
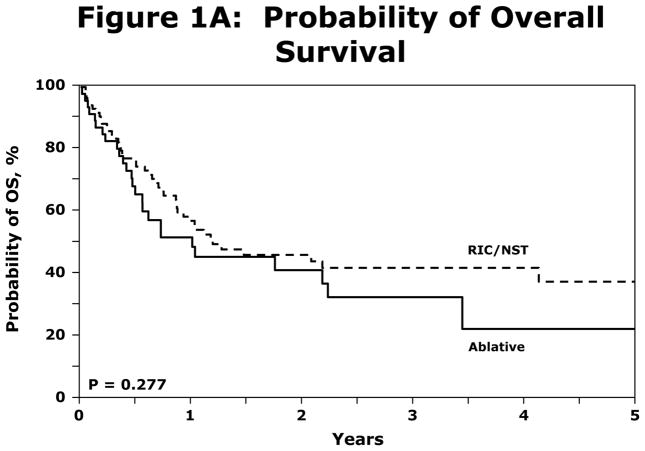
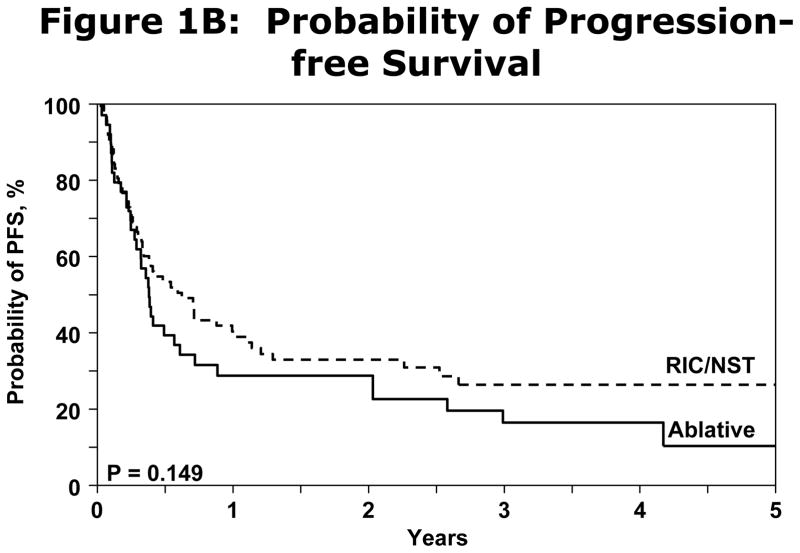
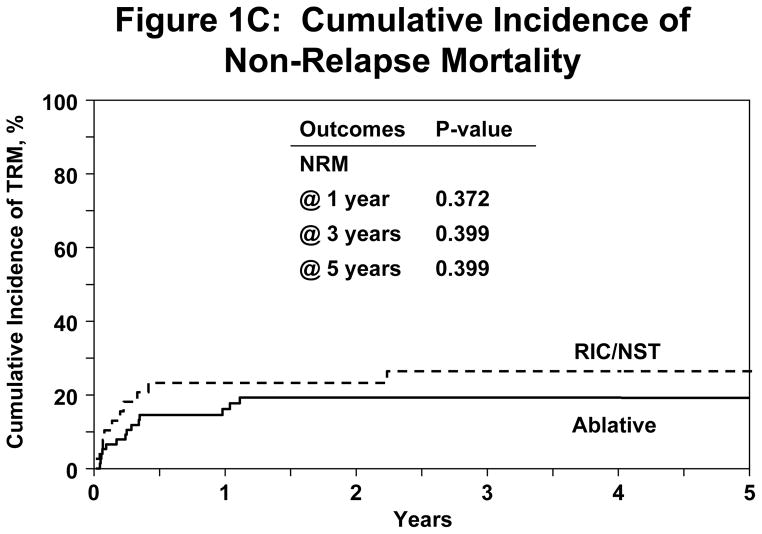
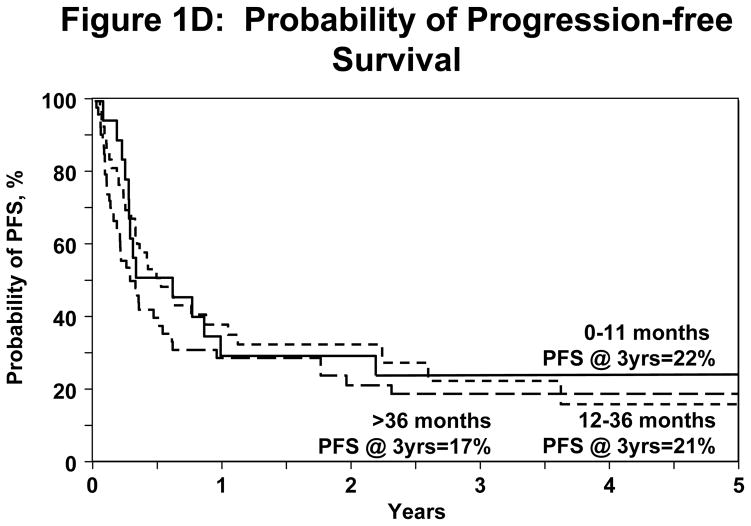
Irrespective of conditioning regimen intensity OS was similar at 56% (95% CI 45–67%) and 41% (95% CI 29–53%) at 1 and 3 years respectively for NST/RIC and 51% (95% CI 35–66%) and 31% (95% CI 16–49%) respectively for MAC (log Rank P-Value=0.277) shown in Figure 1A. NRM for registered patients at 1 y and 5y was 19% (95% CI 12–27%) and 22% (95%CI 15–31%). NRM did not differ significantly between the NST/RIC and MAC cohorts (Table 4). Progression/relapse was 50% (95% CI 41–60%) at 1 year and 61% (95% CI 50–71%) at 5 years. PFS at 1 year was 31% (95% CI 22–40%) and at 5 years 17% (95% CI 9–26%). There was no significant difference in PFS between the NST/RIC and MAC cohorts (P value=0.149; Figure 1B). There was no significant difference in the incidence of NRM with MAC vs. NST/RIC (Figure 1C). There was no significant difference in PFS based on interval of diagnosis to transplant (Figure 1D). Progressive disease was the primary cause of death and treatment failure in this cohort of patients with advanced disease. Other causes of death are summarized in Table 5.
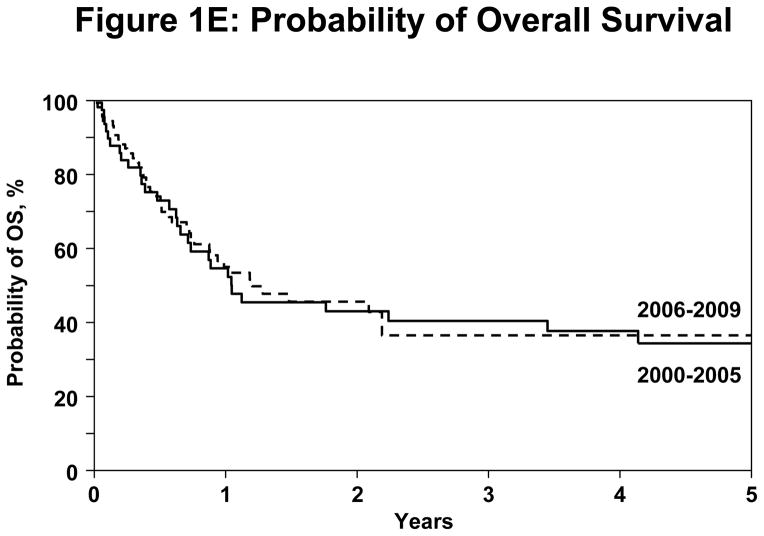
Figure 1.
(A) Probability of OS difference between NST/RIC and MAC patients in the registered patients.
(B) Probability of PFS difference between the NST/RIC and MAC in the registered patients
(C) Cumulative incidence of NRM between NST/RIC and MAC in the registered patients.
(D) Probability of PFS divided by time between diagnosis and transplant.
(E) Probability of OS difference between year of transplant in the registered patients.
Table 5.
Reported primary causes of death.
| Causes of Death | NST/RIC N (%) |
Myeloablative N (%) |
|---|---|---|
| Number of deaths | 32 | 37 |
| Primary disease | 14 (44) | 21 (57) |
| New malignancy | 0 | 1 (3) |
| GVHD | 3 (9) | 3 (8) |
| Interstitial Pneumonitis | 2 (6) | 0 |
| Hemorrhage | 0 | 1 (3) |
| Infection | 4 (13) | 4 (11) |
| Organ failure | 1 (3) | 2 (4) |
| Other cause-not specified | 8 (25) | 5 (14) |
DISCUSSION
To the best of our knowledge, this analysis represents the largest reported descriptive cohort of patients receiving allogeneic HCT for MF/SS. While we reviewed the data from 2000 until 2009 the majority of patients were transplanted in the latter five years. This may be due to increased availability of NST/RIC regimens, recognition of the safety of HCT in this population, and the more integrated multidisciplinary care these patients are receiving. Also surprisingly, our cohort demonstrated only 39% of the patients having stage IV MF/SS. Presumably this cohort represents a minority of the patients with MF/SS, who may have had very aggressive disease.
Molina et al. in an early report described eight patients undergoing HCT with both MAC and NST/RIC. All patients achieved complete clinical remission and resolution of molecular and cytogenetic markers of disease within 30 to 60 days after HCT. Two patients died from transplantation-related complications. A comparison of NST/RIC vs. MAC was not done due to small patient numbers.6 The European Group for Blood and Marrow Transplantation (EBMT) reviewed 60 recipients of HCT for MF and SS.7 This study confirmed the feasibility of HCT with NST/RIC or T cell depletion in MF/SS with a 10% NRM at 2 years. Our data demonstrates a NRM of 22% at 3 years which was previously thought. Duarte et al also reported the adverse impact of patient outcomes based on advanced disease phase at transplant. Although follow up is longer in our series, we did not have enough patients with limited disease to compare with late stage disease patients for survival differences. We did not find statistically significant differences in survival based on duration from diagnosis to transplant (Figure 1D). Our data also found that conditioning intensity did not have an impact on NRM, or OS. However, a prior EBMT study did show survival advantage for NST/RIC.7
In addition, our data did not find any difference in PFS with conditioning regimens. In our study the relapse was noted in 50% of patients at 1 year and 61% at 5 years. This may indicate relapses are most common in the first year post transplant and much less of a chance of progression after 1 year. Others have described evidence for a graft-versus-lymphoma (GVL) effect in CTCL patients.10 Paralkar et al. reported evidence of GVL in two CTCL patients after complete remission was achieved after post allogeneic HCT relapse with either withdrawal of immunosuppression or donor lymphocyte infusion (DLI).11,12 In our series, 4 of 55 patients received DLI, however no additional data on GVL effect was available. Further prospective study will be needed to address treatment of post transplant relapse.
One of the limitations to this analysis is the capturing of staging in this population. In 2007, the International Society for Cutaneous Lymphomas (ISCL)13 published their consensus recommendations for the staging of MF and SS and later validated independently.2 Our data did not capture all of the components of the ISCL/EORTC staging for each patient in order to be able to assign this staging approach to our patient population. Our data set relies on the diagnosis and staging at individual sites. Their data was not captured for the updated staging criteria and therefore is not consistent with this manuscript.
Duvic et al. described a prospective series of 19 patients with advanced CTCL (median age, 50 years; four prior therapies) who underwent total skin electron beam (TSEB) radiation followed by HCT with fludarabine and melphalan based conditioning. Of these patients 18 engrafted with fifteen achieving full donor chimerism. At a medium follow-up of 1.7 years, 13 of the 19 patients were still alive. Causes of death included bacterial sepsis, chronic GVHD, fungal infection and secondary malignancy. While 8 patients had relapse in their skin alone, two patients died of progressive disease. Two-year OS and PFS were 79% and 53% respectively14. Progressive disease was the primary cause of treatment failure in this cohort of patients with advanced phase disease. In the recent retrospective French cohort of 37 cases of advanced and transformed MF treated with allogeneic transplantation15, six of the 19 patients with post-transplant relapse achieved a CR with salvage therapy. While our data has a median follow up for NST/RIC patients of 39 months (3–122) and PFS at 3 years of 24%, we do not have sufficient data to be able to confirm this observation.
Duvic et al. postulate the use of TSEB may have improved allogeneic HCT outcomes by reducing antigen presenting cells in the skin. Only one of our patients, by report, had TSEB therapy prior to transplant. It is of interest whether TSEB conditioning is superior in inducing a remission prior to HCT or if there will be a prolongation of the disease-free interval with longer follow-up from Duvic’s study. Newer approaches to MF/SS such as TSEB, new anti-neoplastic and immunosuppressive agents may improve these patients’ outcomes.
In conclusion, this large series of allogeneic HCT in MF/SS confirms feasibility, acceptable NRM (19–28%) and evidence of benefit in an advanced cohort of MF/SS patients. Allogeneic HCT in MF/SS appears to be superior to autologous transplantation based on previous reports but relapse remains the major cause of mortality3–5. Prospective studies will be necessary to determine the role of new modalities of therapy as well as the optimal timing of allogeneic HCT.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
CONFLICT OF INTEREST: There is no conflict of interest relevant to the content of this manuscript.
References
- 1.Sausville EA, Eddy JL, Makuch RW, Fischmann AB, Schechter GP, Matthews M, et al. Histopathological staging at initial diagnosis of mycosis fungoides and the Sezary syndrome. Definition of three distinctive prognostic groups. Ann Intern Med. 1988;109:372–382. doi: 10.7326/0003-4819-109-5-372. [DOI] [PubMed] [Google Scholar]
- 2.Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 3.Bigler RD, Crilley P, Micaily B, Brady LW, Topolsky D, Bulova S, et al. Autologous bone marrow transplantation for advanced stage mycosis fungoides. Bone Marrow Transplant. 1991;7:133–137. [PubMed] [Google Scholar]
- 4.Olavarria E, Child F, Woolford A, Whittaker SJ, Davis JG, McDonald C, et al. T-cell depletion and autologous stem cell transplantation in the management of tumour stage mycosis fungoides with peripheral blood involvement. Br J Haematol. 2001;114:624–631. doi: 10.1046/j.1365-2141.2001.02919.x. [DOI] [PubMed] [Google Scholar]
- 5.Duarte RF, Schmitz N, Servitje O, Sureda A. Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplant. 2008;41:597–604. doi: 10.1038/sj.bmt.1705968. [DOI] [PubMed] [Google Scholar]
- 6.Molina A, Zain J, Arber DA, Angelopolou M, O’Donnell M, Murata-Collins J, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol. 2005;23:6163–6171. doi: 10.1200/JCO.2005.02.774. [DOI] [PubMed] [Google Scholar]
- 7.Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W, et al. Allogeneic Hematopoietic Cell Transplantation for Patients With Mycosis Fungoides and Sezary Syndrome: A Retrospective Analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:4492–4499. doi: 10.1200/JCO.2010.29.3241. [DOI] [PubMed] [Google Scholar]
- 8.Lechowicz M, Agovi M, Carreras J, Lazarus HM, Laport GG, Montoto S, et al. Allogeneic Hematopoietic Cell Transplantation (AHCT) for Primary Cutaneous T Cell Lymphoma (CTCL): a Center for International Blood and Marrow Transplant Research (CIBMTR) Review. Blood (ASH Annual Meeting Abstracts) 2010 Nov;116:2246. (Abstract 364) [Google Scholar]
- 9.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbert KE, Spencer A, Grigg A, Ryan G, McCormack C, Prince HM. Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma after reduced-intensity HLA-matched sibling allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:521–525. doi: 10.1038/sj.bmt.1704641. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen ED, Kim HT, Ho VT, Cutler CS, Koreth J, Fisher DC, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;22:1608–1613. doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu PA, Kim YH, Lavori PW, Hoppe RT, Stockerl-Goldstein KE. A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sézary syndrome. Biol Blood Marrow Transplant. 2009;15:982–990. doi: 10.1016/j.bbmt.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007 Sep 15;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 14.Duvic M, Donato M, Dabaja B, Richmond H, Singh L, Wei W, et al. Total Skin Electron Beam and Non-Myeloablative Allogeneic Hematopoietic Stem-Cell Transplantation in Advanced Mycosis Fungoides and Sézary Syndrome. J Clin Oncol. 2010;28:2365–2372. doi: 10.1200/JCO.2009.25.8301. [DOI] [PubMed] [Google Scholar]
- 15.De Masson A, Beylot-Barry M, Bouaziz J-D, Peffault de Latour R, Aubin F, Garciaz S, et al. Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica. 2014 Mar;99(3):527–34. doi: 10.3324/haematol.2013.098145. Epub 2013 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]