Abstract
Purpose
To investigate the effect of VEGF-C and VEGF-D blockade via soluble VEGFR-3 (sVEGFR-3) on T cell allosensitization, corneal neovascularization, and transplant survival.
Methods
Corneal intrastromal suture placement and allogeneic transplantation were performed on BALB/c mice to evaluate the effect of sVEGFR-3 on corneal neovascularization. Soluble VEGFR-3 trap was injected intraperitoneally to block VEGF-C/D (every other day starting the day of surgery). Immunohistochemical staining of corneal whole mounts was performed using anti-CD31 (PECAM-1) and anti-LYVE-1 antibodies to quantify the levels of hem- and lymphangiogenesis, respectively. Mixed lymphocyte reaction (MLR) was performed to assess indirect and direct host T cell allosensitization and the frequencies of IFN-γ-producing T cells in the draining lymph nodes were assessed using flow cytometry. Graft opacity and survival was evaluated by slit-lamp biomicroscopy.
Results
Treatment with sVEGFR-3 resulted in a significant blockade of lymphangiogenesis 2 weeks post-transplantation and significantly prolonged corneal allograft survival compared to the control group at 8 weeks post-transplantation (87.5 % vs. 50 %), and this was associated with significant reduction in the frequencies of allosensitized T cells and decreased frequencies of IFN-γ–producing CD4 T cells.
Conclusions
Soluble VEGFR-3 suppresses corneal lymphangiogenesis and allograft rejection and may offer a viable therapeutic modality for corneal neovascularization and corneal transplantation.
Keywords: Cornea, Transplantation, Angiogenesis, Allosensitization
Introduction
Corneal transplantation (penetrating keratoplasty, PK) is by far the most commonly performed tissue transplantation worldwide [1, 2]. While PK performed under cover of topical corticosteroids on an avascular and uninflamed low-risk recipient bed enjoys a survival rate of 90 % after 2 years in the clinic, presence of corneal neovascularization (NV) at the time of transplantation, namely high-risk PK, can significantly jeopardize graft survival and increases the rejection rate to 50–90 % despite using maximal topical and systemic immune suppression [2–6]. Immunosuppressive treatments, which on the one hand may enhance graft survival, may also be complicated by several side effects [7]. Thus, development of new strategies for promoting transplant survival is of high priority.
Immune rejection is the leading cause of corneal graft failure and is composed of various interconnected mechanisms [1, 2, 8]. The central part of the cornea is normally devoid of blood and lymphatic vessels [4, 8]. However, emergence of new vessels leads to subversion of the immunologic privilege that is normally conferred to grafts [2, 4]. While both vascular components of the immune reflex arc (lymphatics-afferent; blood vessels-efferent) contribute to host allosensitization and graft rejection [2, 9], it has been suggested that corneal lymphatic vessels are more critical in mediating allosensitization [1].
Vascular endothelial growth factors (VEGF) and their receptors (VEGFR) are the principal mediators of angiogenesis [10]. VEGF-dependent hem- and lymphangiogenesis occur in concert and are closely linked to inflammatory processes [8, 11]. Moreover, depending on their location, VEGFRs can serve as both angiogenic and/or decoy (anti-angiogenic) receptors. For instance, the soluble form of VEGFR-1, which binds VEGF-A, maintains corneal avascularity [12], and lymphatic endothelial VEGFR-3 induces lymphangiogenesis by binding VEGF-C and D while corneal epithelial VEGFR-3 can act as a decoy receptor [12]. Furthermore, both innate and adaptive immune processes are modulated by VEGF signaling pathways [13–15]. VEGF-C can induce maturation of dendritic cells [15] and VEGFR-3 mediates trafficking of antigen-presenting cells (APC) to the draining lymph nodes [13–16]. However, to date, the effect of sVEGFR-3 on T cells, the primary mediators of corneal transplant rejection, remains unknown.
In the present study, we used a soluble VEGFR-3 (sVEGFR-3) consisting of the extracellular, ligand-binding domain of VEGFR-3 fused to the immunoglobulin Fc domain, which traps and inactivates both VEGF-C and D [17, 18]. We evaluated the effect of this novel agent on corneal angiogenesis and transplant survival, and demonstrate that sVEGFR-3 promotes corneal transplant survival not only by inhibiting lymphangiogenesis but also by suppressing T cell allosensitization.
Methods
Animals and anesthesia
Male 6–8-week-old BALB/c mice (Taconic Farms, Germantown, NY) were used in the suture model experiments and as recipients of allogeneic PK. Age-matched C57BL/6 mice (Taconic Farms, Germantown, NY, USA) were used as donors of corneal buttons in PK. Animals were housed in a pathogen-free environment at the Schepens Eye Research Institute animal facility and were treated according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. All experiments and study protocols were approved by the Institutional Animal Care and Use Committee. Anesthesia was induced by intraperitoneal administration of a mixture of Ketamine and Xylazine at a dose of 120 mg/kg and 20 mg/kg body weight, respectively.
Induction of corneal angiogenesis
In order to induce corneal inflammation and angiogenesis, three interrupted intrastromal sutures (11–0 nylon; Sharpoint; Vanguard, Houston, TX, USA) were inserted around the paracentral cornea and fixed by figure-of-eight knots. In another model, corneal transplantation was performed on naive BALB/c recipient mice. Both of these procedures are well-established models and are known to induce robust corneal NV [8, 15, 16].
Corneal transplantation and evaluation of graft survival
Penetrating keratoplasty was performed as described previously [19, 20]. Briefly, the central cornea (2 mm in diameter) was excised from a donor C57BL/6 mouse (2 mm bore trephine) using curved Vannas scissors (Storz Instruments, San Dimas, CA, USA) and placed on ice in phosphate-buffered saline (PBS). The graft bed was prepared by excising 1.5 mm of the central cornea of a BALB/c mouse. The donor button was then placed onto the recipient site and secured with eight interrupted sutures (11–0 nylon; Sharpoint, Reading, PA, USA). The eyelids were then closed with an 8–0 tarsorrhaphy. Lid and corneal sutures were removed 3 and 7 days post-transplantation, respectively. Grafts were examined twice a week until 8 weeks after transplantation, using slit-lamp biomicroscopy. A standardized scoring scheme (0 through 5+) [20] was used to score corneal opacity and identify graft rejection. An opacity score equal to or greater than 2+ was defined as rejected.
VEGF-C/-D neutralization
VEGF-C and D were trapped and neutralized using a form of soluble VEGFR-3 (sVEGFR-3) consisting of the extracellular, ligand-binding domain of VEGFR-3 fused to the immunoglobulin Fc domain (VGX-300; Circadian Technologies Ltd/Opthea Pty Ltd, Australia). sVEGFR-3 was administered at a concentration of 35 mg/kg intraperitoneally (IP), once every other day. The agent was administered at the time of procedure (suture placement or transplantation) and continued until the mice were euthanized. The control group received PBS following the same protocol.
Mixed lymphocyte reaction (MLR)
Ipsilateral cervical and submandibular lymph nodes were harvested from allograft recipients (BALB/c) 14 days after corneal transplantation. T cells were positively isolated using CD90.2-conjugated magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instructions. For direct MLR, APCs were isolated from the spleen of naive C57BL/6 mice (n=5/group). Briefly, splenocytes were incubated in erythrocyte lysis buffer, washed, re-suspended, and APCs were negatively sorted and isolated using anti-CD90.2 magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA). APCs were then washed and re-suspended at 1×106 cells/ml in 10 % FBS supplemented RPMI 1640 (BioWhittaker, Walkersville, MD, USA) and co-cultured with purified T cells from BALB/c recipients at a 1:1 ratio in rounded-bottom 96-well plates. For indirect MLR, T cells from BALB/c recipients were isolated and co-cultured with BALB/c APCs that had been sonicated with C57BL/6 antigens. The C57BL/6 antigen was generated by re-suspension of C57BL/6 splenocytes at 3×106 cells/ml in the RPMI (containing 10 % FBS). The suspension was then sonicated with six 1-s pulsations, frozen in liquid nitrogen (for 5 min) and thawed at room temperature for 1 h. The cells were incubated in triplicates at 37 °C and 5.0 % CO2 for 5 days. Twelve hours before termination of the cultures, BrdU reagent (Sigma-Aldrich, St. Louis, MO, USA) was added and the assay was performed according to manufacturer’s protocol. T cells from naïve mice were used as a control.
Immunohistochemistry and morphometric evaluation of corneal neovascularization
Animals were euthanized and corneas were harvested 1 week after suture placement and 2 weeks after PK. Immunohistochemistry (IHC) was performed as described previously [2, 6]. Corneal flat mounts were stained with a FITC-conjugated anti-CD31 antibody (platelet-endothelial cell adhesion molecule [PECAM-1]; 1:100 dilution; Santa Cruz) and an unconjugated goat anti-LYVE-1 antibody (lymphatic endothelium–specific hyaluronic acid receptor; 1:300 dilution; Abcam) as a primary antibody and a rhodamine-conjugated donkey anti-goat secondary antibody (1:100 dilution; Jackson ImmunoResearch Laboratories, Inc., PA, USA). Slides were visualized by epifluorescent microscopy as described earlier [21] and micrographs were taken under 4x magnification power. The total corneal area invaded by blood (CD31hi/LYVE-1neg) and lymphatic (CD31lo/LYVE-1hi) vessels was measured morphometrically using NIH ImageJ software [6, 21].
Flow cytometry
Flow cytometry was used to evaluate T cell cytokine production. Ipsilateral draining lymph nodes of BALB/c graft recipients were harvested at 8 weeks after transplantation. T lymphocytes were positively isolated, using CD90.2 magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA) and stimulated in triplicates with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml; Sigma-Aldrich) and Ionomycin (1 μg/ml; Sigma-Aldrich) for ~12 h with the addition of GolgiPlug (0.6 μl/ml; Brefeldin, BD Pharmingen, Franklin Lakes, NJ, USA). Cells were then collected, thoroughly washed, blocked with anti-mouse CD16/CD32 (eBioscience) and stained with a FITC-conjugated anti-CD4 antibody. Cells were fixed and permeabilized with fixation/permeabilization buffers (eBioscience) and intracellularly stained with an allophycocyanin (APC)-conjugated anti-IFN-γ antibody (BD Pharmingen). All experiments were conducted with parallel staining using appropriate isotype controls (BD Pharmingen) and cells were analyzed using the PICS XL flow cytometer (Beckman Coulter).
Statistical analysis
Kaplan–Meier survival curves were used to compare corneal graft survival between treated and control mice, and the log-rank test was used to determine statistical differences between the two groups. For the MLR experiments, T cell proliferation (% BrdU incorporation) was evaluated and different groups were compared using two-tailed Student’s t test, with probability levels of less than 0.05 considered statistically significant. Error bars displayed in the figures denote ± SEM (standard error of mean).
Results
Soluble VEGFR-3 suppresses corneal lymphangiogenesis
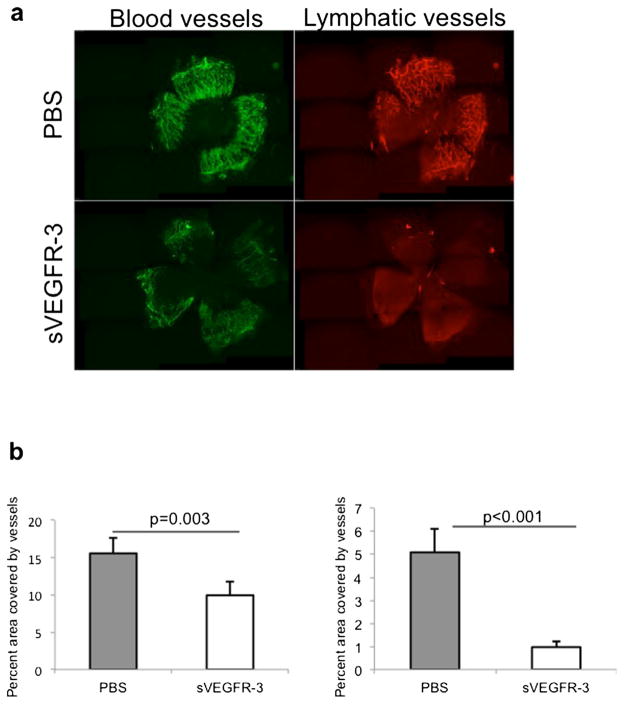
To induce neovascularization and evaluate the effect of sVEGFR-3 on corneal hem- and lymphangiogenesis [4, 8, 22], we used a corneal suture model as described previously [15]. Corneas from sVEGFR-3 and PBS (control)-treated mice were harvested 1 week after suture placement and flat mounts were double-stained for blood vessels (CD31) and lymphatic vessels (LYVE-1; Fig. 1a). Morphometric measurements demonstrated a significant reduction of blood (p= 0.003) and lymphatic vessels (p<0.001) in the sVEGFR-3-treated animals compared to the control group (PBS treated; Fig. 1b).
Fig. 1.
Soluble VEGFR-3 inhibits heme- and lymphangiogenesis after insertion of corneal sutures. a Representative micrographs show the effect of sVEGFR-3 on corneal hem- and lymphangiogenesis. Corneal whole mounts were harvested 1 week after placement of three intrastromal sutures and immunohistochemically stained for CD31 (blood vessels, green) and LYVE-1 (lymphatic vessels, red). b Percent area invaded by blood (CD31+, p<0.001) or lymphatic (LYVE-1+, p=0.003) vessels was measured and Student’s t test was used for statistical analysis; error bars indicate ± standard error of the mean (SEM; n=5 eyes/group). Data from one of two experiments are shown
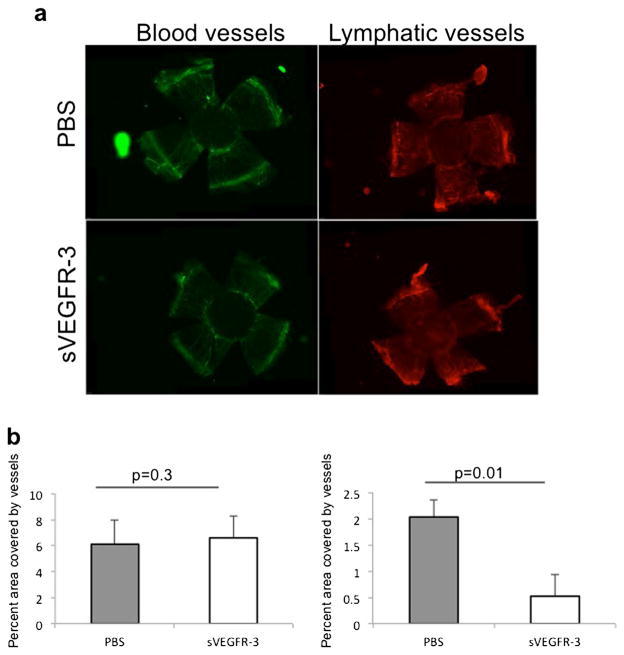
Corneal transplant rejection is associated with the extensive development of blood and lymphatic vessels post-operatively [2]. To determine whether sVEGFR-3 is effective in suppressing this post-operative neovascularization, we compared the extent of hem- and lymphangiogenesis in a corneal allotransplantation model. Grafted corneas were harvested 2 weeks after PK, stained for CD31 and LYVE-1 expression (Fig. 2a), and the percent area of the corneal graft invaded by blood or lymphatic vessels was evaluated. We observed a significant reduction in corneal lymphangiogenesis (p=0.01), but not hemangiogenesis (p=0.3), in mice receiving sVEGFR-3 compared to PBS-treated control mice (Fig. 2b).
Fig. 2.
Soluble VEGFR-3 selectively inhibits inflammatory lymphangiogenesis induced by penetrating keratoplasty. a Representative micrographs show the effect of sVEGFR-3 on corneal hem- and lymphangiogenesis after allotransplantation. Corneal flat mounts were prepared 2 weeks after transplantation and immunohistochemically stained for CD31 (blood vessels, green) and LYVE-1 (lymphatic vessels, red). b Percent area invaded by blood (CD31+, p=0.3) or lymphatic (LYVE-1+, p=0.01) vessels was measured and Student’s t test was used for statistical analysis; error bars indicate ± SEM (n=5 eyes/group). Data from one of two experiments are shown
Soluble VEGFR3 suppresses T cell allosensitization
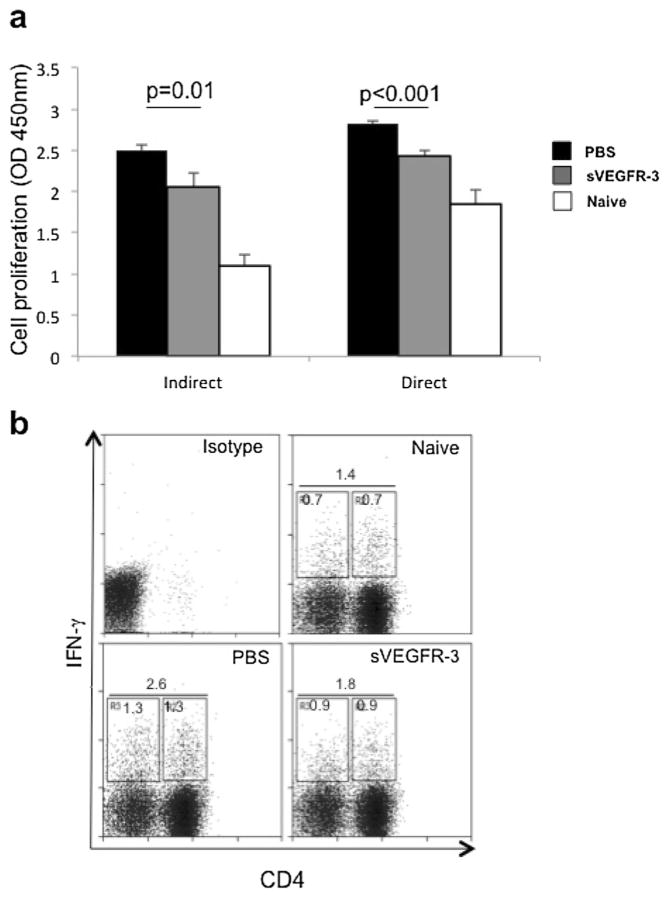
To examine whether treatment with sVEGFR-3 can suppress allosensitization of host T cells, we performed MLR assays with T cells harvested from the draining lymph nodes of allograft recipients 2 weeks after PK. T cells were co-cultured with either naïve C57BL/6 APCs (direct pathway of Tcell allosensitization) or naïve BALB/c APCs with sonicated alloantigen (indirect pathway of T cell allosensitization). The BrdU incorporation assay clearly demonstrated that administration of sVEGFR-3 significantly reduced Tcell proliferation, regardless of whether the cells were sensitized directly (p<0.001) or indirectly (p=0.01, Fig. 3a). To further confirm the effect of sVEGFR-3 on T cell proliferation, we determined whether sVEGFR-3 reduced the frequencies of IFN-γ secreting CD4 T cells in the draining lymph nodes of VEGFR-3-treated and control mice. We harvested T cells from the draining lymph nodes of allografted mice 8 weeks after transplantation and stained the cells for CD4 and IFN-γ. A 30 % reduction was seen in the frequencies of alloreactive IFN-γ+CD4+ Th1 cells in the sVEGFR-3-treated mice compared to PBS-treated mice (Fig. 3b). These data indicate that administration of sVEGFR-3 significantly inhibited the differentiation of T cells, the main mediators of allograft rejection, in the draining lymph nodes of corneal allograft recipients.
Fig. 3.
Treatment with sVEGFR-3 suppresses T cell allosensitization. a For a mixed lymphocyte reaction (MLR), T cells isolated from cervical and submandibular lymph nodes of allograft recipient BALB/c mice were harvested 2 weeks after transplantation and stimulated with host-derived BALB/c antigen-presenting cells sonicated with alloantigen (indirect MLR) or allogeneic, donor-derived antigen-presenting cells (direct MLR). T cell proliferation was quantified using BrdU incorporation assay and spectrometric analysis. The results showed a significant reduction in T cell allosensitization through both indirect and direct pathways (p=0.01 and p=0.001, respectively). Student’s t test was used for statistical analysis; error bars indicate ± standard error of the mean (n=5 mice/group). Naïve T cells served as controls. b Representative dot plot graph showing frequencies of IFN-γ-producing T cells in transplant recipients 8 weeks after transplantation. The cells were isolated from the draining lymph nodes of BALB/c recipients and stimulated with PMA/ionomycin, stained for CD4 and IFN-γ, and analyzed by flow cytometry. The frequencies of IFN-γ+ T cells (CD4+ and CD4−) were decreased in sVEGFR-3-treated mice compared to PBS-treated control mice (n=5 mice/group). Data from one of two experiments are shown
Treatment with sVEGFR-3 increases corneal allograft survival
We evaluated whether administration of sVEGFR-3 starting at the time of transplantation can promote corneal transplant survival (Fig. 4). We compared allograft survival in mice receiving sVEGFR-3 (n=16) versus the control group receiving PBS alone (n=10). PK was performed and the grafts were examined and scored with slit-lamp biomicroscopy twice per week for 8 weeks. A standardized opacity grading scheme (0–5+) was used to assess the transplants, and graft rejection was defined when the graft became opaque, scoring 2 or higher. In past studies, the survival rate of low-risk allogeneic murine transplants has ranged from 30–50 % [15, 23, 24], which is consistent with the present study, in which a 50 % survival rate was observed for the control-treatment group (Fig. 4). Importantly, graft survival was significantly higher in mice treated with sVEGFR-3 compared to the control group at 8 weeks post-transplantation (87.5 % vs. 50 %, p=0.04).
Fig. 4.
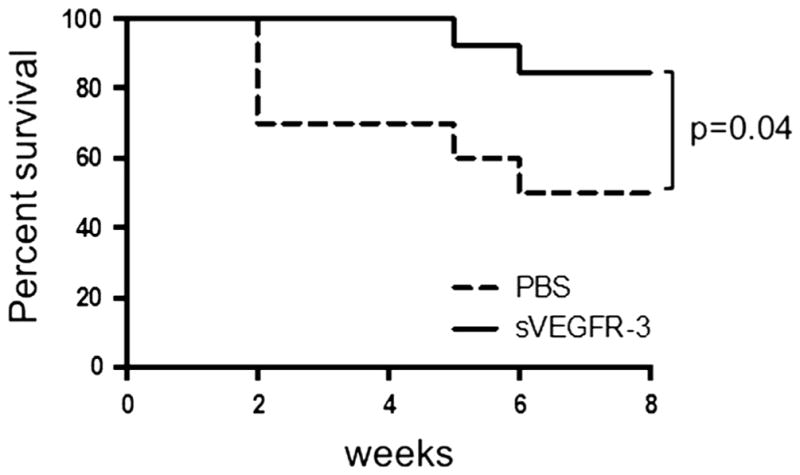
sVEGFR-3 treatment prolongs corneal allograft survival. BALB/c recipient mice received C57BL/6 corneal grafts and were treated intra-peritoneally with sVEGFR-3 or PBS starting the day of transplantation up to 8 weeks once every other day. Graft survival was followed biomicroscopically and a Kaplan–Meier curve was plotted to compare the survival in the two study groups. The log-rank test was used for statistical analysis. sVEGFR-3-treated mice showed a survival rate of 87.5 % (n=16) compared to a 50 % survival rate in PBS-treated mice (n= 10)
Discussion
The purpose of this study was to evaluate the effects of sVEGFR-3 treatment on corneal hem- and lymphangiogenesis as well as on T cell allosensitization in corneal transplantation. Neovascularization of the recipient corneal bed at the time of transplantation has long been established as a risk factor for corneal allograft rejection (high-risk PK) [1, 6]. Low-risk PK can also be associated with postoperative neovascularization of the corneal graft bed, which can appear as early as 3 days after transplantation and can abrogate the immune-privileged state of the cornea, thus triggering an alloimmune response [2]. Hence, inhibiting angiogenic responses that facilitate sensitization could be a reasonable strategy for promoting graft survival.
Indeed, the inhibition of lymphangiogenesis has been shown to be effective in promoting graft survival [1, 25]. Studies have taken advantage of various pharmacologic agents to normalize or pre-condition the host bed environment prior to transplantation to suppress lymphatic vessel formation. Our data demonstrate for the first time that suppression of lymphatics starting at the time of transplantation can also be effective in enhancing graft survival.
Forms of soluble VEGFR-3, consisting of the full-length extracellular domain or the ligand binding region of human VEGFR-3 fused to the Fc domain, are capable of trapping and inactivating VEGF-C and D, two important members of the VEGF family [17, 18, 26]. We observed that treatment with sVEGFR-3 reduced both hem- and lymph-angiogenesis in a suture model of angiogenesis. This is in accord with our previous work in which a VEGF-C neutralizing antibody reduced both hem- and lymphangiogenesis in the same experimental suture model [15]. Interestingly, following sVEGFR-3 treatment in the low-risk penetrating keratoplasty model, we observed a significant decrease in lymphangiogenesis but not hemangiogenesis (Fig. 2). This difference may be due to the qualitative distinction in the suture-induced versus transplant-induced models of corneal angiogenesis. Suture-induced angiogenesis is due to non-specific inflammation induced by macrophages [27], whereas transplantation is associated with the induction of alloimmunity, which includes the recruitment of antigen-specific T cells that not only stimulate other cells to produce VEGF but can also serve as an additional source of VEGF ligands themselves [28, 29]. Our results in the transplant model are in contrast to one group’s finding that overexpression of sVEGFR-3 in transplantation can reduce the area of both corneal hem- and lymphangiogenesis, though they found the reduction in hemangiogenesis to be less robust than the reduction in lymphangiogenesis [30]. The disparity between this study and ours may be due to differences in analysis time points, as well as the mode of sVEGFR-3 treatment (local pCMV.sVEGFR-3 in their study versus intra-peritoneal sVEGFR-3 injection in our study). Nonetheless, the selective reduction of lymphangiogenesis but not hemangiogenesis with sVEGFR-3 is certainly not unprecedented, as Makinen et al. have shown that formation of lymphatic vessels is impaired in transgenic mice expressing high levels of a soluble form of VEGFR-3, whereas blood vessels develop normally [18].
We have previously shown that VEGF-C blockade not only inhibits hem- and lymphangiogenesis but also reduces trafficking of corneal APCs and promotes graft survival by inhibiting APC maturation (17). The central part of the normal cornea is populated by a phenotypically immature MHC-II-− and co-stimulatory factor (CD80, CD86)-negative population of bone marrow-derived dendritic cells [31]. In the context of allogeneic transplantation, these cells capture alloantigen and maturate, leading to upregulation of CC chemokine receptor (CCR7) and VEGF-C receptors (VEGFR-2 and 3) [15, 32], which will in turn facilitate trafficking of mature APCs toward the lymphoid tissues. This is a crucial step in sensitizing naïve T cells residing in the draining lymph nodes and is a relatively quick process, starting as early as 24 h after transplantation [15]. To investigate the effects of sVEGFR-3 on T cell immunity, we evaluated T cell proliferative ability and the induction of Tcells in the draining lymph nodes following treatment. We found that T cells isolated from sVEGFR-3-treated mice display diminished proliferative ability in both direct and indirect mixed lymphocyte reactions. This may be a downstream consequence of sVEGFR-3’s effect on APCs [15] or a result of preventing direct VEGF ligand interactions with T cells, as T cells can express VEGFR-2, a receptor for VEGF-C and D, and VEGF ligands have been shown to enhance Th1 cell polarization [28]. We also observed a 30 % reduction in the frequencies of IFN-γ secreting Th1 cells, the principal mediators of acute corneal allograft rejection [9], in the draining lymph nodes of sVEGFR-3-treated mice. These findings suggest that in addition to its known effect on APC maturation [15], one mechanism by which the neutralization of VEGF-C and D dampens alloimmunity is through inhibition of T cell allosensitization. Thus, the high survival rate of corneal transplants after treatment with sVEGFR-3 observed in our study can be attributed to the effect of VEGF-C and D blockade on both corneal lymphangiogenesis and allosensitization.
In summary, our findings demonstrate that systemic administration of sVEGFR-3 enhances corneal transplant survival by suppressing lymphangiogenesis and T cell allosensitization. Targeting lymphatic vessels is an attractive strategy for preventing graft rejection not only because of the importance of lymphatic vessels in mediating allograft rejection [1], but also because of its potential to reduce alloimmunity without compromising blood vessels, which provide delivery of nutrients and other factors necessary for wound healing [1]. Moreover, starting this treatment at the time of transplantation rather than pre-conditioning the host bed prior to grafting is of translational significance in the clinical setting.
Acknowledgments
This work was supported by the National Institutes of Health (EY012963) and Circadian Technologies Ltd. The authors thank Dr. Susanne Eiglmeier for her expert advice and assistance in manuscript preparation.
Grant support NIH EY012963, Circadian Technologies Ltd.
Footnotes
Disclosures The authors declare that they have no conflicts of interest.
References
- 1.Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–77. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich T, Onderka J, Bock F, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascu-larization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409. doi: 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 11.Nakao S, Zandi S, Hata Y, et al. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood. 2011;117:1081–1090. doi: 10.1182/blood-2010-02-267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamrah P, Chen L, Zhang Q, Dana MR. Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am J Pathol. 2003;163:57–68. doi: 10.1016/S0002-9440(10)63630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamrah P, Chen L, Cursiefen C, et al. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res. 2004;79:553–561. doi: 10.1016/j.exer.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Hajrasouliha AR, Funaki T, Sadrai Z, et al. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2012;53:1244–1250. doi: 10.1167/iovs.11-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Kim C, Kim M-J, et al. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkinen T, Jussila L, Veikkola T, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 19.Hattori T, Saban DR, Emami-Naeini P, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621–627. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice–evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Chung E-S, Chauhan SK, Jin Y, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–1992. doi: 10.2353/ajpath.2009.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streilein JW, Bradley D, Sano Y, Sonoda Y. Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas. Invest Ophthalmol Vis Sci. 1996;37:413–424. [PubMed] [Google Scholar]
- 23.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol. 2014;192:817–823. doi: 10.4049/jimmunol.1203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson W, Cheng S-F, Emami-Naeini P, et al. Gamma-irradiation reduces the allogenicity of donor corneas. Invest Ophthalmol Vis Sci. 2012;53:7151–7158. doi: 10.1167/iovs.12-9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011;52:6529–6535. doi: 10.1167/iovs.11-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thelen A, Scholz A, Benckert C, et al. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int J Cancer. 2008;122:2471–2481. doi: 10.1002/ijc.23439. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Chauhan SK, El Annan J, et al. A novel function for programmed death ligand-1 regulation of angiogenesis. Am J Pathol. 2011;178:1922–1929. doi: 10.1016/j.ajpath.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen IH, Willerslev-Olsen A, Vetter-Kauczok C, et al. Vascular endothelial growth factor receptor-3 expression in mycosis fungoides. Leuk Lymphoma. 2013;54:819–826. doi: 10.3109/10428194.2012.726720. [DOI] [PubMed] [Google Scholar]
- 30.Singh N, Tiem M, Watkins R, et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood. 2013;121:4242–4249. doi: 10.1182/blood-2012-08-453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45:722–727. 721. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y, Shen L, Chong E-M, et al. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol Vis. 2007;13:626–634. [PMC free article] [PubMed] [Google Scholar]