Abstract
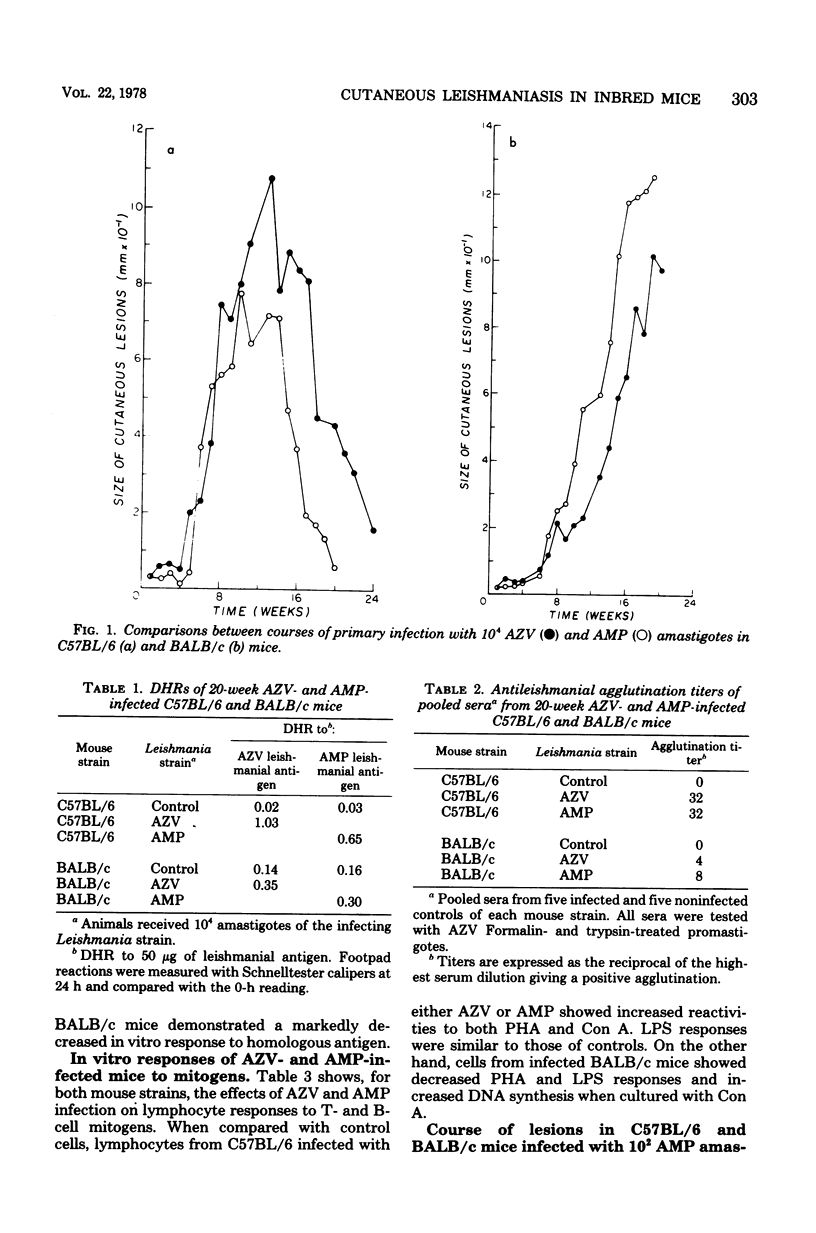
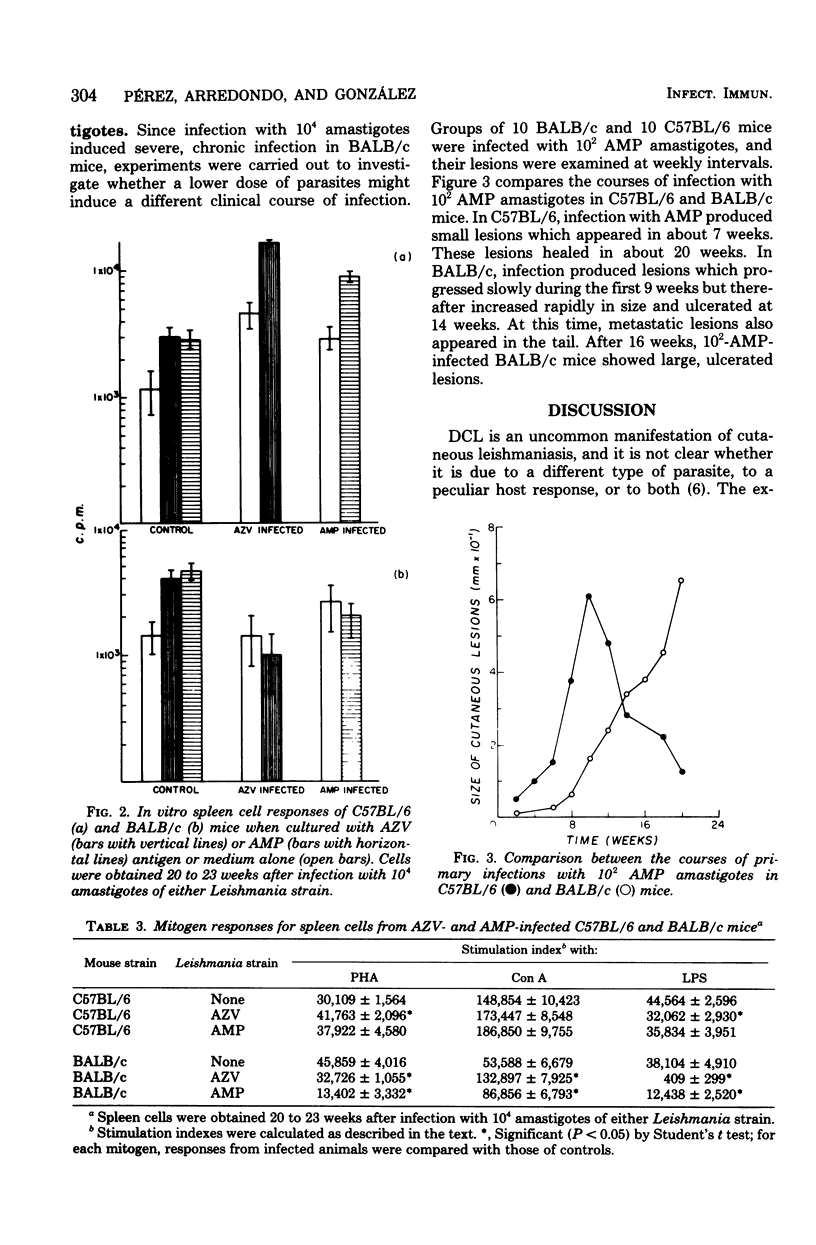
Two Leishmania strains, AZV (isolated from a typical case of American cutaneous leishmaniasis) and AMP (from a case of diffuse cutaneous leishmaniasis), were studied in C57BL/6 and BALB/c mice. After infection with 10(4) amastigotes of either strain, C57BL/6 mice developed self-resolving lesions lasting 20 to 23 weeks and showed both delayed hypersensitivity response to leishmanial antigen and specific agglutinating antibodies. On the other hand, BALB/c mice infected with 10(4) AZV or AMP amastigotes developed chronic, large, ulcerated lesions and showed impaired cellular and humoral responses to the parasite. When BALB/c and C57BL/6 mice received 10(2) AMP amastigotes, patterns of infection were similar to those observed after inoculation of 10(4) amastigotes. In vitro studies revealed that spleen cells from AZV- or AMP-infected C57BL/6 mice showed an increased DNA-synthetic response to leishmanial antigen, concanavalin A, and phytohemagglutinin. Spleen cells from AZV- or AMP-infected BALB/c mice showed an increased response to concanavalin A and diminished responses to leishmanial antigen, phytohemagglutinin, and lipopolysaccharide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S. LEISHMANIA. Adv Parasitol. 1964;2:35–96. doi: 10.1016/s0065-308x(08)60586-2. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Variation in susceptibility of mouse strains to Leishmania donovani infection. Trans R Soc Trop Med Hyg. 1972;66(4):527–528. [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. 3. Immunological studies. IV. Pathogenesis of diffuse cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1970;64(3):380–393. doi: 10.1016/0035-9203(70)90174-4. [DOI] [PubMed] [Google Scholar]
- CONVIT J., KERDEL-VEGAS F. DISSEMINATED CUTANEOUS LEISHMANIASIS; INNOCULATION TO LABORATORY ANIMALS, ELECTRON MICROSCOPY AND FLUORESCENT ANTIBODIES STUDIES. Arch Dermatol. 1965 May;91:439–447. doi: 10.1001/archderm.1965.01600110025007. [DOI] [PubMed] [Google Scholar]
- Clinton B. A., Stauber L. A., Palczuk N. C. Leishmania donovani: antibody response to chicken ovalbumin by infected golden hamsters. Exp Parasitol. 1969 Aug;25(1):171–180. doi: 10.1016/0014-4894(69)90063-0. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Selective triggering of B cell subpopulations by mitogens. Eur J Immunol. 1974 Nov;4(11):771–776. doi: 10.1002/eji.1830041113. [DOI] [PubMed] [Google Scholar]
- Handman E., Spira D. T. Growth of Leishmania amastigotes in macrophages from normal and immune mice. Z Parasitenkd. 1977 Aug 25;53(1):75–81. doi: 10.1007/BF00383117. [DOI] [PubMed] [Google Scholar]
- Holmes P. H., Mammo E., Thomson A., Knight P. A., Lucken R., Murray P. K., Murray M., Jennings F. W., Urquhart G. M. Immunosuppression in bovine trypanosomiasis. Vet Rec. 1974 Jul 27;95(4):86–87. doi: 10.1136/vr.95.4.86. [DOI] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Nussenzweig R. Depletion of T and B lymphocytes during malarial infections. Cell Immunol. 1974 Sep;13(3):440–446. doi: 10.1016/0008-8749(74)90263-9. [DOI] [PubMed] [Google Scholar]
- Lainson R., Shaw J. J. Leishmaniasis of the New World: taxonomic problems. Br Med Bull. 1972 Jan;28(1):44–48. doi: 10.1093/oxfordjournals.bmb.a070892. [DOI] [PubMed] [Google Scholar]
- Lamon E. W., Klein E., Andersson B., Fenyö E. M., Skurzak H. M. The humoral antibody response to a primary viral neoplasm (MSV) through its entire course in BALB-c mice. Int J Cancer. 1973 Nov 15;12(3):637–645. doi: 10.1002/ijc.2910120312. [DOI] [PubMed] [Google Scholar]
- Lamon E. W., Skurzak H. M., Klein E. The lymphocyte response to a primary viral neoplasm (MSV) through its entire course in BALB-c mice. Int J Cancer. 1972 Nov;10(3):581–588. doi: 10.1002/ijc.2910100317. [DOI] [PubMed] [Google Scholar]
- MEDINA R., ROMERO J. [Clinical and parasitological study of a new strain of Leishmania]. Arch Venez Med Trop Parasitol Med. 1959 Jul;3:298–326. [PubMed] [Google Scholar]
- Matossian-Rogers A., Lumsden W. H., Dumonde D. C. Numerical immunotaxonomy of Leishmania. I. Differentiation of four strains of Leishmania by serological tests. Immunology. 1976 Jul;31(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Behin R. Cell-mediated and humoral immunity to protozoan infections. Transplant Rev. 1974;19(0):121–146. doi: 10.1111/j.1600-065x.1974.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A. Regulation of immune responses by suppressor T cells. Tohoku J Exp Med. 1976;5:91–143. [PubMed] [Google Scholar]
- Preston P. M., Carter R. L., Leuchars E., Davies A. J., Dumonde D. C. Experimental cutaneous leishmaniasis. 3. Effects of thymectomy on the course of infection of CBA mice with Leishmania tropica. Clin Exp Immunol. 1972 Feb;10(2):337–357. [PMC free article] [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976 Jan;23(1):126–138. [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Wakelin D. Genetic control of immune responses to parasites: immunity to Trichuris muris in inbred and random-bred strains of mice. Parasitology. 1975 Aug;71(1):51–60. doi: 10.1017/s0031182000053142. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- Weintraub J., Weinbaum F. I. The effect of BCG on experimental cutaneous leishmaniasis in mice. J Immunol. 1977 Jun;118(6):2288–2290. [PubMed] [Google Scholar]
- Zuckerman A. Current status of the immunology of blood and tissue Protozoa. I. Leishmania. Exp Parasitol. 1975 Dec;38(3):370–400. doi: 10.1016/0014-4894(75)90123-x. [DOI] [PubMed] [Google Scholar]


