Abstract
Background
While elevated body mass index (BMI) has been associated with increased risk of aggressive prostate cancer (PC), the importance of adipose tissue distribution is not well understood. We examined associations between overall and visceral obesity and aggressive PC risk. Moreover, given racial differences in adipose tissue distribution, we examined whether race modified these associations.
Methods
We conducted a cross-sectional analysis of 308 radiation-treated PC patients within the Durham VA from 2005–2011. Multivariable logistic regression examined the association between BMI categories and tertiles of waist circumference (WC), visceral fat area (VFA) and periprostatic adipose tissue area (PPAT) with high-grade PC risk (Gleason score ≥7 vs. ≤6). Models stratified by race examined whether these associations differed between black and non-black men.
Results
Both elevated BMI (p-trend=0.054) and WC (p-trend=0.040) were associated with increased high-grade PC risk, with similar results between races, although the association with BMI was not statistically significant. In contrast, elevated VFA was associated with increased aggressive PC risk in black men (p-trend=0.002) but not non-black men (p-trend=0.831), with a significant interaction between race and VFA (p-interaction=0.035). Though similar patterns were observed for PPAT, none were statistically significant.
Conclusions
Among men undergoing radiation therapy for PC, visceral obesity is associated with increased aggressive PC risk, particularly among black men. If confirmed in future studies, these results suggest adipose tissue distribution differences may contribute to PC racial disparity.
Impact
These findings highlight the need to elucidate mechanisms contributing to racial differences in the association between visceral obesity and aggressive PC.
Keywords: adipose tissue distribution, aggressive prostate cancer, biopsy Gleason score, periprostatic adipose tissue, race, visceral obesity
Introduction
In the United States, prostate cancer (PC) is the most frequently diagnosed cancer among men, and the second most common cause of cancer deaths [1]. Black men have 1.6 fold higher PC incidence and 2.5 fold higher PC mortality, relative to white men [1]. While socioeconomic factors certainly contribute to this health disparity, there is evidence that race-specific differences in tumor biology play an important role [2, 3].
Multiple meta-analyses have identified overall obesity, defined as BMI ≥30 kg/m2, to be a risk factor for aggressive PC [4]. While the prevalence of overall obesity among men in the US does not differ by race [5], there are established differences in adipose tissue distribution between black and non-black men [6, 7]. Relative to white men, black men have less visceral adipose tissue, a depot which is known to play an important role in obesity-mediated metabolic changes and inflammation [4, 8]. While visceral obesity has been associated with increased risk of aggressive PC in both white [9] and black men [10], no studies to our knowledge have examined whether the strength of the association between visceral obesity and aggressive PC differs between races. Furthermore, while one study has reported an association between increased periprostatic adipose tissue area (PPAT) and elevated risk of aggressive PC in white men [11], none have examined whether there are racial differences in PPAT quantity and whether elevated PPAT is associated with increased risk of aggressive PC in black men.
The objective of this study was to examine the association between obesity, adipose tissue distribution and risk of aggressive PC using a cross-sectional analysis of black and non-black men who received radiation therapy for biopsy-confirmed PC. We hypothesized that visceral obesity would be associated with increased risk of aggressive PC and that racial differences in adipose tissue distribution would modify this association. Specifically, we hypothesized that there would be a stronger association between visceral obesity and increased risk of aggressive PC in black men, given the higher prevalence of obesity-associated comorbidities in this racial group [12, 13].
Materials and Methods
Study Population and Design
After obtaining institutional review board approval, we identified all men (n=521) with biopsy-confirmed PC who were treated with definitive external beam radiation therapy (XRT) or brachytherapy at the Durham Veterans Affairs (VA) Medical Center between 2005 and 2011. The population used for this study was selected due to the availability of pre-treatment pelvic and abdominal CT scans for radiation planning, enabling accurate quantification of various adipose tissue depots, as described below. Thus, we excluded men with missing pelvic (n=109) or abdominal CT scans (n=80). In addition, we excluded men with missing data for BMI (n=8), PSA (n=7), clinical stage (n=7) and biopsy Gleason score (n=2), giving rise to a final population of 308 patients.
Adipose tissue measurement
Visceral fat area (VFA), subcutaneous fat area (SFA) and periprostatic adipose tissue area (PPAT) were quantified from radiation planning CT scans using Eclipse™ software (Varian Medical Systems, Palo Alto, CA) by a single investigator blinded to demographic and clinical characteristics (EHA).
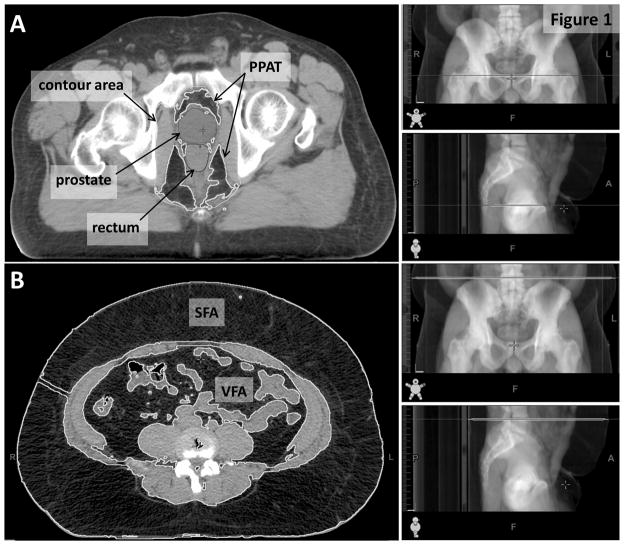
PPAT was measured using a single CT slice at the level of the first anterior point of the pubic symphysis, as previously described [14]. Briefly, the Eclipse™ freehand drawing tool was used to contour a region extending from the posterior pubic bone, along the lateral border of obturatorius internus muscle and anterior gluteus maximus muscle to the anterior coccyx bone (Figure 1). Pelvis size was defined as the total area of this contoured region, and PPAT was defined as the total area of adipose tissue within this region. We differentiated between adipose tissue and other tissues using thresholding by Hounsfield Units (HU), with adipose tissue defined as −190 to −30 HU. This method effectively excluded the prostate, rectum and mesorectum. PPAT density ratio was calculated as PPAT divided by pelvis size.
Figure 1.
Calculation of PPAT area and PPAT density measurements from a single CT slice at the level of the first point of the public symphysis (A) and calculation of VFA, SFA and WC from a single CT slice at the level of L4/L5 (B).
PPAT=periprostatic adipose tissue; SFA=subcutaneous fat area; VFA=visceral fat area
VFA and SFA were calculated using the Eclipse™ automatic area tool on a single CT slice at the level of L4/L5, as previously described [15] (Figure 1). Since waist circumference (WC) was not measured among these patients in clinic, we determined it retrospectively from the same CT slice as for VFA and SFA. Measurement of WC from a CT scan with the patient in a supine position has previously been demonstrated to correlate well with WC measured using a tape measure with the patient standing, with intraclass correlation coefficients between 0.97 and 0.98 [16–18]. Body outline was traced using the Eclipse™ freehand drawing tool and WC was calculated using ImageJ (rsbweb.nih.gov/ij/). Finally, volume of prostate was calculated automatically using the Eclipse™ volume of interest tool using the contours entered by the treating physician at the time of radiation therapy (Figure 1).
Obesity definitions
Height and weight measured at the closest time prior to radiation were abstracted from patient charts. Patients were then stratified into four BMI categories according to World Health Organization definitions; normal weight (BMI<25 kg/m2), overweight (BMI≥25 kg/m2 and <30 kg/m2), obese (BMI≥30 kg/m2 and <35 kg/m2) and severely obese (BMI≥35 kg/m2). Given that no clearly defined categories exist for VFA, SFA, WC or PPAT, these measures of obesity were divided into tertiles based on the entire cohort. In secondary analysis, race-specific VFA and PPAT tertiles were generated among black and non-black men.
Statistical analysis
Differences in demographic, clinical and anthropometric features between black (n=193) and non-black (n=115) men were examined using t-tests for normally distributed continuous variables, Wilcoxon rank-sum tests for non-normally distributed continuous variables and chi-square tests for categorical variables. Non-black men were either white (n=111) or non-black non-white (n=4). Correlation coefficients between measures of obesity (BMI, WC, SFA, VFA and PPAT), all treated as continuous variables, and between each measure of obesity and duration of pre-radiation androgen deprivation therapy (ADT) were calculated using Spearman correlation.
Multivariable logistic regression analysis was used to investigate the association between obesity (categories of BMI, WC, SFA, VFA and PPAT; each assessed individually) and risk of aggressive PC, as defined by biopsy Gleason score ≥7 vs. ≤6, using the lowest category of each obesity measure as the reference group. This standard definition of aggressive PC was selected to enable comparison of these results with prior studies using this definition [9, 10, 19–22]. When the three non-collinear adiposity measures (i.e. SFA, VFA and PPAT) were all added to the same multivariable model, the results did not substantially change relative to the multivariable model with each adiposity measure assessed individually. Thus, only models with each adiposity measure assessed individually are shown. Models were adjusted for age at radiation (continuous), race (black vs. non-black), pretreatment PSA (continuous, log-transformed), clinical stage (T1 vs. T2/T3), duration of pre-radiation ADT (none, < median (2.47 months), ≥ median) and year of radiation (continuous). We did not have access to diabetes status or family history of PC in this study. We tested for trends across increasing obesity categories using multivariable logistic regression analysis of obesity category medians. Models were stratified by race to examine whether the association of obesity categories with biopsy Gleason score differed between black and non-black men. We also tested for interaction between obesity categories and race by incorporating a product term into our models. Furthermore, we repeated our analysis using race-specific tertiles for VFA and PPAT. Finally, in exploratory analysis, we repeated our analysis among men who never received ADT (n=154) prior to radiation.
All statistical analyses were carried out in Stata version 11.0 (Stata, Corp., College Station, TX). Differences were considered to be statistically significant at p<0.05.
Results
Baseline characteristics by race
Black men were younger at the time of radiation (63.4 vs. 65.4 years old; p=0.009) and more recently treated (p=0.025) relative to non-black men (Table 1). In addition, black men had lower clinical stage compared to non-black men (p=0.001), and were significantly less likely to have received ADT prior to radiation (p=0.013). However, among men who did receive ADT prior to radiation (50% of our total cohort), there was no difference in duration of ADT by race (p=0.183). Given that ADT can promote weight gain [23], we examined whether duration of pre-radiation ADT was correlated with measures of obesity which were obtained at the time of radiation (i.e. after neo-adjuvant ADT). We found that, among men who received ADT, longer duration of ADT prior to radiation was not associated with any obesity measure (data not shown).
Table 1.
Demographic and clinical characteristics of patients by race
|
|
|||
|---|---|---|---|
| Non-black (N=115) | Black (N=193) | P-value | |
| Age, mean ± SD | 65.4 ± 6.4 | 63.4 ± 6.5 | 0.009ɷ |
| Year of radiation, median (Q1 – Q3) | 2008 (2007–2009) | 2008 (2007–2010) | 0.025† |
| Radiation type | 0.370§ | ||
| 3D, n (%) | 8 (6.9) | 14 (7.3) | |
| IMRT, n (%) | 97 (84.4) | 169 (88.0) | |
| Brachytherapy, n (%) | 10 (8.7) | 9 (4.7) | |
| ADT prior to radiation, n (%) | 68 (59.1) | 86 (44.6) | 0.013§ |
| Duration ADT (months)**, median (Q1–Q3) | 2.5 (2.0–3.2) | 2.4 (1.9–2.9) | 0.183† |
| Prostate volume (cm3), median (Q1–Q3) | 41.0 (30.9–51.4) | 40.5 (33.5–54.2) | 0.497† |
| Positive biopsy cores (%), median (Q1–Q3) | 33.3 (16.7–55.6) | 33.3 (16.7–50.0) | 0.729† |
| PSA (ng/ml), median (Q1 – Q3) | 6.7 (4.9–10.6) | 7.9 (5.3–13.6) | 0.152† |
| Biopsy Gleason score, n (%) | 0.626§ | ||
| 2 – 6 | 34 (29.6) | 58 (30.1) | |
| 7 | 57 (49.6) | 103 (53.4) | |
| 8 – 10 | 24 (20.9) | 32 (16.6) | |
| Clinical Stage, n (%) | 0.001§ | ||
| T1 | 67 (58.3) | 146 (75.7) | |
| T2/T3 | 48 (41.7) | 47 (24.4) | |
ADT=androgen deprivation therapy; IMRT=intensity-modulated radiation therapy; SD=standard deviation; Q1=25th percentile; Q3=75th percentile
Duration of ADT use prior to radiation among ADT users
p values calculated by ɷ t-test, † Wilcoxon rank-sum or § chi-square test
There were no differences in surrogate measures of obesity, BMI (29.6 vs. 28.6 kg/m2; p=0.698) or WC (104.3 vs. 104.9 cm; p=0.264), between black and non-black men (Table 2). Neither was there any significant difference in amount of subcutaneous adipose tissue between races, directly measured using SFA (296.1 vs. 289.5 cm2; p=0.349). However, black men had significantly less visceral adipose tissue (VFA; 190.3 vs. 245.8 cm2; p<0.0001) and significantly less PPAT (35.5 vs. 41.2 cm2; p=0.0001), relative to non-black men. Black men also had smaller pelvis size relative to non-black men (p=0.001), however PPAT area remained significantly lower among black men even after adjusting for pelvis size (PPAT density ratio; p=0.002; Table 2). Among all men, all measures of obesity correlated with each other, with similar correlation coefficients in black and non-black men after stratifying by race (Table 3).
Table 2.
Anthropometric characteristics of patients by race
|
|
|||
|---|---|---|---|
| Non-black (N=115) | Black (N=193) | P-value† | |
| BMI (kg/m2), median (Q1 – Q3) | 28.6 (26.0–34.0) | 29.6 (25.7–33.7) | 0.698 |
| SFA (cm2), median (Q1–Q3) | 289.5 (215.7–354.2) | 296.1 (215.1–412.9) | 0.349 |
| WC (cm), median (Q1 – Q3) | 104.9 (99.2–117.2) | 104.3 (95.0–115.4) | 0.264 |
| VFA (cm2), median (Q1–Q3) | 245.8 (185.0–245.8) | 190.3 (138.4–270.7) | <0.0001 |
| PPAT area (cm2), median (Q1–Q3) | 41.2 (33.4–52.4) | 35.5 (27.0–44.9) | 0.0001 |
| PPAT density ratio, median (Q1–Q3) | 0.40 (0.32–0.48) | 0.35 (0.28–0.44) | 0.002 |
| Pelvis size (cm2), median (Q1–Q3) | 104.2 (97.0–115.6) | 100.3 (93.0–108.9) | 0.001 |
BMI=body mass index; SFA=subcutaneous fat area; WC=waist circumference; VFA=visceral fat area; PPAT=periprostatic adipose tissue
PPAT density ratio=PPAT area/pelvis size
p values calculated by Wilcoxon rank-sum test
Table 3.
Correlation between adipose tissue measurements
| BMI (kg/m2) | WC (cm) | SFA (cm2) | VFA (cm2) | ||
|---|---|---|---|---|---|
| WC (cm) | All | 0.91, p<0.0001 | |||
| Non-black | 0.91, p<0.0001 | ||||
| Black | 0.91, p<0.0001 | ||||
|
| |||||
| SFA (cm2) | All | 0.88, p<0.0001 | 0.92, p<0.0001 | ||
| Non-black | 0.88, p<0.0001 | 0.90, p<0.0001 | |||
| Black | 0.88, p<0.0001 | 0.94, p<0.0001 | |||
|
| |||||
| VFA (cm2) | All | 0.65, p<0.0001 | 0.77, p<0.0001 | 0.56, p<0.0001 | |
| Non-black | 0.70, p<0.0001 | 0.82, p<0.0001 | 0.50, p<0.0001 | ||
| Black | 0.67, p<0.0001 | 0.77, p<0.0001 | 0.60, p<0.0001 | ||
|
| |||||
| PPAT (cm2) | All | 0.24, p<0.0001 | 0.31, p<0.0001 | 0.25, p<0.0001 | 0.41, p<0.0001 |
| Non-black | 0.21, p=0.024 | 0.30, p=0.001 | 0.20, p=0.036 | 0.35, p=0.0001 | |
| Black | 0.28, p=0.0001 | 0.33, p<0.0001 | 0.31, p<0.0001 | 0.39, p<0.0001 | |
Correlation coefficients calculated using Spearman’s rank test
Association between surrogate measures of obesity and risk of aggressive PC does not differ by race
In this population of radiation-treated PC patients, severely obese men (BMI ≥35 kg/m2) were nearly threefold more likely to have aggressive PC, relative to normal weight men (BMI <25 kg/m2) (OR 2.97; 95% CI 1.21–7.26; Table 4). When we restricted our analysis to black men, severe obesity remained significantly associated with approximately threefold increased risk of elevated biopsy Gleason score (OR 3.71; 95% CI 1.14–12.1). Although the direction and magnitude of this association was similar for non-black men, it did not reach statistical significance (OR 2.54; 95% CI 0.59–10.91). Similarly, larger WC, a commonly-used surrogate of visceral obesity, was associated with significantly increased risk of aggressive PC among our entire cohort of radiation-treated patients (3rd tertile vs. 1st tertile; OR 2.0; 95% CI 1.02–3.92; p-trend=0.04) and among black men only (3rd tertile vs. 1st tertile; OR 2.45; 95% CI 1.04–5.77; p-trend=0.04) with a similar direction, but lower magnitude association generating a non-significant trend among non-black men (3rd tertile vs. 1st tertile; OR 1.54; 95% CI 0.48–4.92; p-trend=0.446). Restricting our analysis to men who never received ADT did not alter our results (data not shown).
Table 4.
Odds ratios for high-grade§ PC risk as a function of BMI, WC, SFA, VFA and PPAT area
| All (n=308)* | Non-black (n=115)** | Black (n=193)** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | n | OR | 95% CI | n | OR | 95% CI | |
| BMI (kg/m2) | |||||||||
| Normal | 64 | 1.00 | Ref | 24 | 1.00 | Ref | 40 | 1.00 | Ref |
| Overweight | 110 | 0.90 | 0.43–1.89 | 44 | 1.08 | 0.32–3.62 | 66 | 0.84 | 0.32–2.21 |
| Obese | 73 | 1.03 | 0.45–2.34 | 23 | 1.43 | 0.34–6.06 | 50 | 0.96 | 0.34–2.68 |
| Severely obese | 61 | 2.97 | 1.21–7.26 | 24 | 2.54 | 0.59–10.91 | 37 | 3.71 | 1.14–12.1 |
| p-trend | 0.054 | 0.119 | 0.170 | ||||||
|
| |||||||||
| WC (cm) | |||||||||
| T1 (≤100) | 102 | 1.00 | Ref | 33 | 1.00 | Ref | 69 | 1.00 | Ref |
| T2 (101–111) | 103 | 1.27 | 0.66–2.46 | 42 | 1.12 | 0.36–3.48 | 61 | 1.29 | 0.55–2.95 |
| T3 (≥112) | 103 | 2.00 | 1.02–3.92 | 40 | 1.54 | 0.48–4.92 | 63 | 2.45 | 1.04–5.77 |
| p-trend | 0.040 | 0.446 | 0.040 | ||||||
|
| |||||||||
| SFA (cm2) | |||||||||
| T1 (≤236) | 102 | 1.00 | Ref | 39 | 1.00 | Ref | 63 | 1.00 | Ref |
| T2 (237–353) | 103 | 1.35 | 0.70–2.60 | 47 | 1.12 | 0.39–3.24 | 56 | 1.52 | 0.64–3.62 |
| T3 (≥354) | 103 | 2.35 | 1.19–4.65 | 29 | 4.08 | 1.05–15.91 | 74 | 2.07 | 0.91–4.71 |
| p-trend | 0.025 | 0.048 | 0.093 | ||||||
|
| |||||||||
| VFA (cm2) | |||||||||
| T1 (≤168) | 102 | 1.00 | Ref | 23 | 1.00 | Ref | 79 | 1.00 | Ref |
| T2 (169–263) | 103 | 1.16 | 0.59–2.24 | 41 | 0.46 | 0.12–1.78 | 62 | 1.30 | 0.57–2.94 |
| T3 (≥264) | 103 | 2.12 | 1.07–4.22 | 51 | 0.64 | 0.18–2.24 | 52 | 4.23 | 1.67–10.69 |
| p-trend | 0.024 | 0.831 | 0.002 | ||||||
|
| |||||||||
| PPAT area (cm2) | |||||||||
| T1 (≤32) | 102 | 1.00 | Ref | 23 | 1.00 | Ref | 79 | 1.00 | Ref |
| T2 (33–43) | 103 | 1.05 | 0.55–2.00 | 42 | 0.24 | 0.06–0.98 | 62 | 1.61 | 0.72–3.59 |
| T3 (≥44) | 103 | 1.39 | 0.70–2.76 | 50 | 0.37 | 0.08–1.60 | 52 | 1.88 | 0.80–4.44 |
| p-trend | 0.342 | 0.539 | 0.135 | ||||||
BMI=body mass index; PPAT=periprostatic adipose tissue; Ref=reference group; SFA=subcutaneous fat area; T=tertile; VFA=visceral fat area; WC=waist circumference
Biopsy Gleason ≥7 versus <7
Adjusted for age, race, PSA, clinical stage, duration of ADT, year of radiation
Adjusted for age, PSA, clinical stage, duration of ADT, year of radiation
Association between visceral obesity and risk of aggressive PC is modified by race
While the associations between surrogate measures of obesity (i.e. BMI and WC), and aggressive PC risk were similar in black and non-black men, stratification by race revealed contrasting associations between VFA, a direct measure of visceral obesity, and aggressive PC risk. Specifically, while higher VFA was significantly associated with increased risk of aggressive PC among black men (3rd tertile vs. 1st tertile; OR 4.23; 95% CI 1.67–10.69; p-trend=0.002; Table 4), this association was null among non-black men (3rd tertile vs. 1st tertile; OR 0.64; 95% CI 0.18–2.24; p-trend=0.83). In contrast, SFA, a direct measure of subcutaneous adiposity, was associated with significantly increased risk of aggressive PC in non-black men (3rd tertile vs. 1st tertile; OR 4.08; 95%CI 1.05–15.91; p-trend=0.048) but this association was not significant in black men (3rd tertile vs. 1st tertile; OR 2.07; 95%CI 0.91–4.71; p-trend=0.093).
Furthermore, in this cohort of radiation-treated patients, elevated PPAT area was associated with almost twofold increased risk of higher biopsy Gleason score among black men, although this association did not reach statistical significance (3rd tertile vs. 1st tertile; OR 1.88; 95% CI 0.80–4.44; p-trend=0.135). In contrast, an opposite trend was observed among non-black men (3rd tertile vs. 1st tertile; OR 0.37; 95%CI 0.08–1.6; p-trend=0.54). Given these differing associations in black and non-black men, we tested for interaction between these visceral, subcutaneous and pelvic adipose tissue measures and race in their association with aggressive PC. While there was no significant interaction between race and SFA (p-interaction=0.586) or between race and PPAT area (p-interaction=0.444), we found a significant interaction between black race and VFA in the association with aggressive PC (p-interaction=0.035), suggesting that race may modify the association between visceral obesity and risk of aggressive PC.
Given that the amount of VFA and PPAT differed significantly by race, we repeated our analysis using race-specific tertiles derived from black and non-black men in order to account for racial differences in adipose tissue distribution. No associations were altered; specifically, the highest tertile of VFA remained associated with increased risk of aggressive PC in black men but not in non-black men, while similar trends were observed between PPAT area and risk of aggressive PC in black and non-black men (data not shown).
Discussion
In this cross-sectional, hypothesis-generating study of radiation-treated PC patients, surrogate measures of overall and visceral obesity, BMI and WC, respectively, were associated with increased risk of high-grade PC in both black and non-black men. In contrast, the association between elevated VFA, a direct measure of visceral obesity, and increased risk of high-grade PC was modified by race with a significant positive association in black men which was absent in non-black men. These findings suggest that while surrogate measures of obesity are appropriate for studying the association between obesity and PC aggressiveness in both black and non-black men, a more precise measure of visceral adiposity reveals significantly contrasting associations by race and may provide some mechanistic insight into the racial disparity in PC.
Although it is known that obesity is associated with increased risk of aggressive, but not total PC [4], few studies have examined whether the association between obesity and risk of aggressive PC differs by race. One study reported that race modified the association between obesity and elevated risk of PC recurrence, with a significant association in black men but not in non-black men [22]. Other studies have noted a higher prevalence of obesity among black men in their cohorts, potentially contributing to the higher frequency of aggressive disease in this racial group [19, 20]. On the contrary, two studies reported obesity to be a risk factor for aggressive PC regardless of race [24, 25]. Thus, our finding adds to the literature that overall obesity is associated with increased risk of aggressive PC in both black and non-black men.
In keeping with the lack of association between overall obesity and risk of total PC [4], a large meta-analysis reported a null association between visceral obesity, predominantly assessed by WC, and risk of total PC [26]. However, several studies which examined the association between visceral obesity and risk of aggressive PC reported positive findings [21, 27, 28]. Two studies conducted exclusively among black men found a twofold increased risk of aggressive PC with elevated WC [10, 29]. Our current study is the first, to our knowledge, to suggest that visceral obesity may be more strongly associated with aggressive PC in black men, relative to non-black men. Our finding that subcutaneous adiposity, directly measured by SFA, is more strongly associated with aggressive PC among non-black men requires validation in other studies. If future studies replicate this finding, it may provide further evidence that visceral obesity is more strongly associated with aggressive PC in black men, while subcutaneous adiposity is more strongly associated in non-black men.
Consistent with other studies, we found that black men had reduced visceral adipose tissue compared to non-black men, despite similar BMI and WC [6, 30]. However, despite this reduced visceral adiposity, we found that visceral adipose tissue mass was more strongly associated with increased risk of aggressive PC in black men. While this finding may appear counterintuitive, it is noteworthy that despite lower prevalence of visceral obesity on a population level, black men actually have increased risk of visceral obesity-associated comorbidities, including coronary heart disease, hypertension, stroke and diabetes, relative to their white counterparts [12, 13]. As such, the relationship between lower prevalence of visceral obesity but yet higher rates of visceral obesity-related comorbidities in black men mirrors the relationship between lower levels of visceral obesity but yet higher rates of aggressive PC in black men. To what degree racial differences in amount or function of visceral adipose tissue contribute to racial disparity in PC is not understood and requires further study. However, it has been shown that, despite reduced visceral adipose tissue, circulating levels of obesity-associated growth factors and pro-inflammatory adipokines are elevated in black men, while adiponectin levels are reduced [31–33]. Given that these factors may contribute to the association between obesity and PC [4], future studies are warranted to explore the potential impact of race-specific differences in levels of obesity-associated growth factors and adipokines on the PC racial disparity.
Despite several in vitro studies which have reported that conditioned media from PPAT may promote an aggressive phenotype in PC cell lines [34–36]; we found no association between elevated PPAT and increased risk of aggressive PC in our cohort of black and non-black men. A previous study of 932 radiation-treated PC patients reported a significant association between high PPAT area and high-grade PC (OR 1.04; 95% CI 1.03–1.06) [11]. However, a similar analysis conducted by the same group among lower risk, brachytherapy patients found no significant association between PPAT area and risk of aggressive PC [14]. Thus, while larger epidemiologic studies are needed to examine the association between PPAT and risk of aggressive PC, these mixed results may suggest that PPAT is not the most important mediator of the link between obesity and risk of aggressive PC. In addition, as ours is the first study to report that black men have significantly less PPAT, relative to non-black men, future studies should examine whether the PPAT inflammatory profile differs by race.
This study has several limitations which should be considered. All measures of obesity were obtained at the time of radiation and thus, for men who received ADT, these measures were obtained post-ADT. While ADT has been associated with significant weight gain in the first year of treatment [23, 37], the amount of weight gain after only three months of ADT has not been found to be significant [38]. In our cohort, median duration of ADT use prior to radiation was 2.48 months (IQR: 2.04–3.19) and, indeed, we did not find any association between duration of ADT and any measure of obesity, suggesting that any impact of ADT on our obesity measures was negligible. We lacked sufficient events for long-term outcomes such as PC-specific mortality, however we examined Gleason score as an intermediate endpoint for aggressive disease given that Gleason score is the strongest predictor of PC-specific mortality in men newly diagnosed with localized PC [39]. Future studies should explore alternative definitions of aggressive PC. Although central pathology review of biopsies was not available for this study, variations in Gleason grading between pathologists would likely be unrelated to obesity measures. Thus, this potential source of outcome misclassification would bias our results towards the null. Finally, given that our cohort was limited to this convenience sample of radiation-treated patients due to the availability of CT scans for adipose tissue quantification, it is important to consider that our results may not be representative of all PC patients. To what degree our results apply to all men with PC remains to be determined by future studies. Finally, an important limitation of all cross-sectional studies is that causality cannot be inferred from these associations. Despite these limitations, our study exhibited a number of important strengths. Misclassification of adipose tissue distribution is a risk for studies relying on surrogate measures of obesity and may contribute to some of the variability in epidemiologic studies exploring the association between obesity and PC [28]. Thus, an important strength of our study was accurate quantification of adipose tissue depots, enabling us to estimate more precisely the association between adipose tissue distribution and risk of aggressive PC. Finally, 63% of our cohort was black, enabling us to assess the impact of race on the association between obesity and risk of aggressive PC.
In conclusion, in this cross-sectional study of radiation-treated PC patients, while elevated BMI and WC were associated with increased risk of aggressive PC in both races, we found that the association between visceral obesity, as measured by VFA, and increased risk of aggressive PC was restricted to black men. These hypothesis-generating data suggest that racial differences in adipose tissue distribution may contribute to PC racial disparity and that visceral obesity may be particularly strongly associated with aggressive PC among black men. In addition, these findings may point to a potential mechanism which may be targeted to disrupt the obesity-aggressive PC link in black men. Together, these findings highlight the importance of considering racial differences in adipose tissue distribution when exploring the association between obesity and risk of aggressive PC.
Acknowledgments
Financial support: EHA: NCI 5R25-CA126938-03; SJF: NIH Grant 1-R01-CA131235-01A1 and NIH 1K24CA160653
Footnotes
Author contributions:
Conception and Design: EHA, SJF
Development of methodology: EHA, HS
Acquisition of data: EHA, HS, LEH, KS, JKS, SJF
Analysis and interpretation of data: EHA, LEH, SJF
Writing, review and/or revision of the manuscript: EHA, LEH, HS, KS, BFK, JKS, SJF
Administrative, technical, or material support: HS, JKS, SJF
Study supervision: SJF
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444–9. doi: 10.1016/j.juro.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Farrell J, Petrovics G, McLeod DG, Srivastava S. Genetic and Molecular Differences in Prostate Carcinogenesis between African American and Caucasian American Men. Int J Mol Sci. 2013;14:15510–31. doi: 10.3390/ijms140815510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, et al. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 2009;17:1420–7. doi: 10.1038/oby.2008.657. [DOI] [PubMed] [Google Scholar]
- 7.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 8.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–47. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Nunzio C, Albisinni S, Freedland SJ, Miano L, Cindolo L, Finazzi Agro E, et al. Abdominal obesity as risk factor for prostate cancer diagnosis and high grade disease: A prospective multicenter Italian cohort study. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Nemesure B, Wu SY, Hennis A, Leske MC. Central adiposity and Prostate Cancer in a Black Population. Cancer Epidemiol Biomarkers Prev. 2012;21:851–8. doi: 10.1158/1055-9965.EPI-12-0071. [DOI] [PubMed] [Google Scholar]
- 11.van Roermund JG, Hinnen KA, Tolman CJ, Bol GH, Witjes JA, Bosch JL, et al. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011;107:1775–9. doi: 10.1111/j.1464-410X.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang SH, Pollack LM, Colditz GA. Life Years Lost Associated with Obesity-Related Diseases for U.S. Non-Smoking Adults. PLoS One. 2013;8:e66550. doi: 10.1371/journal.pone.0066550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Roermund JG, Bol GH, Witjes JA, Ruud Bosch JL, Kiemeney LA, van Vulpen M. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World J Urol. 2010;28:699–704. doi: 10.1007/s00345-009-0497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zilli T, Nguyen TV, Bahary JP, Chagnon M, Dufresne A, Taussky D. Prognostic impact of abdominal adiposity, waist circumference and body mass index in patients with intermediate-risk prostate cancer treated with radiotherapy. Int J Obes (Lond) 2011;35:1421–6. doi: 10.1038/ijo.2010.279. [DOI] [PubMed] [Google Scholar]
- 16.Ciudin A, Salvador R, Budoy A, Ciudin A, Spinu C, Diaconu MG, et al. Measurement of waist circumference for retrospective studies - prospective validation of use of CT images to assess abdominal circumference. Endocrinol Nutr. 2014;61:147–52. doi: 10.1016/j.endonu.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Sousa AS, de Sousa OL, Amaral TF. The effect of posture on body circumferences in older adults. J Hum Nutr Diet. 2014;27:80–7. doi: 10.1111/jhn.12093. [DOI] [PubMed] [Google Scholar]
- 18.Waninge A, Ligthart KA, Kramer J, Hoeve S, van der Schans CP, Haisma HH. Measuring waist circumference in disabled adults. Res Dev Disabil. 2010;31:839–47. doi: 10.1016/j.ridd.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–45. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 20.Caire AA, Sun L, Polascik TJ, Albala DM, Moul JW. Obese African-Americans with prostate cancer (T1c and a prostate-specific antigen, PSA, level of <10 ng/mL) have higher-risk pathological features and a greater risk of PSA recurrence than non-African-Americans. BJU Int. 2010;106:1157–60. doi: 10.1111/j.1464-410X.2010.09340.x. [DOI] [PubMed] [Google Scholar]
- 21.Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3252–61. doi: 10.1158/1055-9965.EPI-08-0609. [DOI] [PubMed] [Google Scholar]
- 22.Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol. 2007;178:1939–44. doi: 10.1016/j.juro.2007.07.021. discussion 1945. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Moreira DM, Smith MR, Presti JC, Jr, Aronson WJ, Terris MK, et al. A natural history of weight change in men with prostate cancer on androgen-deprivation therapy (ADT): results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2011;107:924–8. doi: 10.1111/j.1464-410X.2010.09679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayachandran J, Banez LL, Aronson WJ, Terris MK, Presti JC, Jr, Amling CL, et al. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115:5263–71. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su LJ, Arab L, Steck SE, Fontham ET, Schroeder JC, Bensen JT, et al. Obesity and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Cancer Epidemiol Biomarkers Prev. 2011;20:844–53. doi: 10.1158/1055-9965.EPI-10-0684. [DOI] [PubMed] [Google Scholar]
- 26.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 27.Qu YY, Dai B, Kong YY, Chang K, Ye DW, Yao XD, et al. Influence of obesity on localized prostate cancer patients treated with radical prostatectomy. Asian J Androl. 2013;15:747–52. doi: 10.1038/aja.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zilli T, Chagnon M, Van Nguyen T, Bahary JP, Guay JP, Dufresne A, et al. Influence of abdominal adiposity, waist circumference, and body mass index on clinical and pathologic findings in patients treated with radiotherapy for localized prostate cancer. Cancer. 2010;116:5650–8. doi: 10.1002/cncr.25539. [DOI] [PubMed] [Google Scholar]
- 29.Beebe-Dimmer JL, Dunn RL, Sarma AV, Montie JE, Cooney KA. Features of the metabolic syndrome and prostate cancer in African-American men. Cancer. 2007;109:875–81. doi: 10.1002/cncr.22461. [DOI] [PubMed] [Google Scholar]
- 30.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 31.Demerath EW. Causes and consequences of human variation in visceral adiposity. Am J Clin Nutr. 2010;91:1–2. doi: 10.3945/ajcn.2009.28948. [DOI] [PubMed] [Google Scholar]
- 32.Gardener H, Crisby M, Sjoberg C, Hudson B, Goldberg R, Mendez AJ, et al. Serum adiponectin in relation to race-ethnicity and vascular risk factors in the Northern Manhattan Study. Metab Syndr Relat Disord. 2013;11:46–55. doi: 10.1089/met.2012.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim CX, Bailey KR, Klee GG, Ellington AA, Liu G, Mosley TH, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One. 2010;5:e9065. doi: 10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, et al. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol. 2009;182:1621–7. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A, et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res. 2012;31:32. doi: 10.1186/1756-9966-31-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toren P, Venkateswaran V. Periprostatic adipose tissue and prostate cancer progression: new insights into the tumor microenvironment. Clin Genitourin Cancer. 2014;12:21–6. doi: 10.1016/j.clgc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–7. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 38.Timilshina N, Breunis H, Alibhai SM. Impact of androgen deprivation therapy on weight gain differs by age in men with nonmetastatic prostate cancer. J Urol. 2012;188:2183–8. doi: 10.1016/j.juro.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]