Abstract
Increasing evidence supports the contention that many malignancies, including sporadic colorectal cancer (CRC), are driven by the self-renewing, chemotherapy-resistant cancer stem/stem-like cells (CSCs/CSLCs) underscoring the need for improved preventive and therapeutic strategies targeting CSCs/CSLCs. Omega-3 polyunsaturated fatty acids (ω-3 PUFA), have been reported to inhibit the growth of primary tumors, but their potential as a preventive agent for recurring cancers is un-explored. The primary objectives of this investigation are to examine whether eicosapentaenoic acid (EPA; one of the ω-3 PUFA) synergizes with FuOx (5-FU+Oxaliplatin), the backbone of colon cancer chemotherapy, and (b) whether EPA by itself or in combination with conventional chemotherapy prevents the recurrence of colon cancer via eliminating/suppressing CSCs/CSLCs. FuOx-resistant (chemo-resistant; CR) colon cancer cells, highly enriched in CSCs, were utilized for this study. While EPA alone was effective, combination of EPA and FuOx was more potent in (a) inhibiting cell growth, colonosphere formation and sphere-forming frequency, (b) increasing sphere disintegration, (c) suppressing the growth of SCID mice xenografts of CR colon cancer cells, and (d) decreasing pro-inflammatory metabolites in mice. Additionally, EPA + FuOx caused a reduction in CSC/CSLC population. The growth reduction by this regimen is the result of increased apoptosis as evidenced by PARP cleavage. Furthermore, increased pPTEN, decreased pAkt, normalization of β-catenin expression, localization and transcriptional activity by EPA suggests a role for PTEN/Akt axis and Wnt signaling in regulating this process. Our data suggest that EPA by itself or in combination with FuOx could be an effective preventive strategy for recurring CRC.
Introduction
Cancer stem/stem-like cells (CSCs/CSLCs), that are self-renewing undifferentiated cells, are thought to be one of the leading causes of cancer recurrence. In the colon, they are identified by specific surface epitopes such as CD44, CD166, CD133 and ESA (epithelial-specific antigen) (1, 2). Like normal stem cells, CSCs/CSLCs grow slowly and are more likely to survive chemotherapy than other tumor cells (2–5). This is exemplified by the observation that oxaliplatin treatment of colon cancer boosts the abundance of CSCs by more than 10 times (3). We have also reported that although exposure of colon cancer HCT-116 or HT-29 cells to FuOx inhibits their growth, the same treatment leads to enrichment of CSC/CSLC phenotype (4, 5). These chemo-resistant cells show an increased colonosphere formation, Wnt/β-catenin signaling, EGFR signaling, increased expression of miR21, and decreased miR145 (6, 7).
Omega 3-and 6- poly unsaturated fatty acids (ω-3 and -6 PUFAs) are substantial components of the diet, comprising about 7–10% of daily energy intake in US adults (reviewed in (8). A meta-analysis by the World Cancer Research Fund and the American Institute for Cancer Research in 2007 reported that although no definitive correlations could be drawn, there was suggestive evidence that dietary fish (main source of ω-3 PUFAs) intake protects against CRC risk in humans (9). Additional support came from clinical observations (10, 11) suggesting its significance as a chemo-preventive agent.
The current investigation examines the potential of ω-3 PUFA as an effective preventive agent for recurrent colon tumors that are reported to be enriched in CSCs/CSLCs. Two main ω-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been isolated from fish oil. Recent evidence has demonstrated that EPA and DHA reduce inflammation in humans (12, 13) and may have anti-neoplastic properties (14–16). Animal studies have revealed that EPA and to a lesser extent DHA reduced VEGF expression and micro-vessel formation (17). Recently, Fan et al demonstrated a stimulatory role of ω-6 PUFA derived PGE2 on Lgr5+ stem cell population in the colonic crypts. In contrast, ω-3 PUFA derived PGE3 had diminished ability to support stem cell expansion (18). Hawcroft et al recently showed an inhibition of liver metastasis in mice that received dietary EPA (19). However, there are no reports on the anti-neoplastic activity of this PUFA on recurrent colon cancer. The current investigation was undertaken to examine the preventive and therapeutic potential of EPA alone or when administered together with the conventional chemotherapy on chemotherapy-resistant colon cancer HT-29 and HCT-116 cells. Herein, we report that EPA alone or in combination with FuOx could be effective in prevention of recurrent colon cancer.
Materials and Methods
Cell lines and Reagents
Human colon cancer cells HT-29 and HCT-116 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). They were expanded and frozen in aliquots. Fresh aliquots were used every 6–7 months, therefore the cell lines were not authenticated again. The cells were maintained in Dulbecco’s modified Eagle’s medium as reported (5, 20). FuOx resistant (chemo-resistant; CR) cells were generated as described earlier (5, 6, 21) in our laboratory by exposing the cells to 14 consecutive cycles of exposure to increasing concentrations of 5-FU and oxaliplatin.
Unless otherwise stated, the CR cells were cultured in medium containing 2× FuOx (50µM 5-FU and 1.25µM oxaliplatin).
Determination of Cell Growth and interaction between EPA and FuOx
Cell growth was assessed by mitochondrial-dependent reduction of 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma) to formazan as described previously (22). Briefly, the cells (5×103) were seeded in quadruplicates onto 96 well culture dishes, subsequently treated with increasing concentrations of EPA and/or FuOx for 48 hr to determine the synergism between EPA and FuOx.
Colonosphere Formation and Disintegration
Formation of colonospheres and their disintegration in response to EPA and/or FuOx were carried out according to our standard protocols described previously (5, 23). Spheres of size ≥80 µm (measured by a 100 µm scale (reticule) in the eyepiece) were counted after 14 days.
Extreme limiting dilution analysis
Extreme limiting dilution analysis (ELDA) was performed y the method described by Hu and Smyth (24) with a slight modification (7, 23). Briefly, cells were pretreated with EPA and/or FuOx for 72 hr, subsequently plated at a concentration of 100, 10 and 1 cell per well and incubated for 8 days. The frequency of sphere formation was determined using ELDA webtool at http://bioinf.wehi.edu.au/software/elda.
Flow Cytometry
After 48 hr incubation in the absence (control) or presence of EPA and/or FuOx, CR-HT-29 colon cancer cells were subjected to direct immunofluorescence staining with PE-Cy7 or PerCP-Cy5 conjugated anti-human CD44 and/or CD166 antibody followed by flow cytometric analyses using a FACS DiVa (BD, San Jose, CA) at the MICR core of Karmanos Cancer Institute of Wayne State University as described previously (5). The cells stained with IgG2b (isotype-negative control) served as gating control. The proportion of CD44+/CD166+/low cells was determined on the basis of fluorescence intensity spectra.
Indirect immunoflourescence
The cells were seeded at a density of 25,000/chamber in a 8 chamber slide. After treatment with EPA+FuOx for 48hr, the cells were fixed, permeabilized and processed for indirect immunoflourescence as described (25) using appropriate antibodies. Stained cells were observed under an Olympus 1×71 microscope supporting a Hamamatsu 1394 ORCA-ERA video camera and the images were stored using Slidebook Digital Microscopy Software (Intelligent Imaging Innovations). For controls, the primary antibody was omitted.
Western blot analysis
Western blots were performed according to our standard protocol (26). Briefly, cell lysates containing 25 or 50 µg protein were separated by SDS-polyacrylamide gel electrophoresis, transferred onto a PVDF membrane (Millipore) and subjected to western blot analysis with the recommended dilution of primary and appropriate secondary antibody conjugated to IR Dye 680 or IR Dye 800 (Molecular Probes). The membranes were scanned by Odyssey Infrared Imaging System (LI-COR Biosciences) to locate the respective bands. β-actin or GAPDH was used as a loading control.
Isolation of RNA and Quantitative Polymerase Chain Reaction analysis
Total RNA was extracted from CR cells using TRIZOL reagent (Invitrogen) according to manufacturer’s instructions. RNA concentration was measured using a NanoDrop 2000C spectrophotometer.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using the GeneAmp RNA PCR Kit (Applied Biosystems, Foster City, CA). Briefly, 1 µg of purified RNA was reverse-transcribed (5, 27). For quantitative PCR amplification, 5 microliters of 1:10 diluted cDNA was amplified with SYBR Green Quantitative PCR Master Mix (Applied Biosystems) using the following PCR primers: CK20 forward: 5’TGAAGAGCTGCGAAGTCAGA-3’ and reverse: 5’-GAAGTCCTCAGCAGCCAGTT3’; β-catenin: forward 5'-ATACCACCCACTTGGCAGAC-3'; reverse 5'-GGAAGGTCTCCTTGGGACTC-3'; sequences for stem cell markers were reported earlier (27). Reactions were carried out in triplicates as described previously (5).
TCF/LEF transcriptional activity
The activation of transcription factor TCF/LEF was evaluated by using Cignal TCF/LEF reporter assay kit (SA Biosciences, Frederick, MD) as described (7, 23). The cells were grown to 70–80% confluence, co-transfected with TCF/LEF reporter constructs using Lipofectamine-2000 transfection reagent (Invitrogen) according to manufacturer’s instructions. After 16–24 hr, the cells were trypsinized, seeded into 12 wells of a 96 well plate in DMEM containing 10% FBS in the presence of EPA and/or FuOx. After 2 days of incubation they were collected and analyzed for TCF/LEF activity using a dual-luciferase assay kit (Promega-Biosciences, San Luis Obispo, CA) following manufacturer's instructions as described (7, 23). Activity of TCF/LEF was calculated in relation to positive control.
Tumor growth in SCID mice
All animal experiments were performed according to the Wayne State University's Institutional Animal Care and Use Committee (IACUC) approved protocol # A02-02-13. Animal Welfare Assurance # A3310-01.
Tumors were generated in four weeks old female SCID mice (Taconic Laboratory) by s.c. injections of 1×106 CR HCT-116 or CR HT-29 cells suspended in 100 µL Matrigel on either side. To study the chemo-preventive efficacy of EPA, animals were given EPA (250 mg/Kg in sesame oil) by oral gavage 7 days prior to inoculation of chemo-resistance colon cancer cells. The dose of EPA was selected based on previous studies (17, 28, 29). To study the therapeutic effectiveness, EPA was administered 7 days after inoculation of the cells. EPA treatment was continued for 4 weeks every day for 5 days a week (Monday to Friday). The animals in this group (control and EPA group) were also injected IP with a mixture of 25 mg/kg 5- fluorouracil and 2 mg/kg oxaliplatin (FuOx) once a week for 3 weeks. Tumor volumes were calculated as described previously (20, 23). Mice were monitored regularly. At the end of treatment period, all animals were sacrificed, blood was collected immediately from the heart in a tube containing 50 µl of 80mM EDTA, centrifuged and saved at −70°C. The tumors were harvested and tumor aliquots were frozen for RNA isolation, fixed in 10% buffered formalin or immediately digested with enzymes for single cell isolation (23, 27).
Eicosanomic analysis
Mass spectrometry based eicosanomic analysis for eicosanoids derived from both arachidonic acid and eicosapentaenoic acid was performed on the plasma extracts collected from mice as described earlier (30, 31). Briefly, plasma samples were spiked with a mixture of internal standards (5 ng each of PGE1-d4, LTB4-d4, and 15-HETE-d8), diluted with methanol to 15%, and applied to C18 solid phase extraction cartridges, washed sequentially with 15% methanol in water and hexane, followed by elution of the eicosanoids with methanol containing 1% formic acid. The eluates were evaporated to dryness and reconstituted in HPLC mobile phase for LC-MS analysis.
Eicosanomic analysis was performed by LC-MS using Luna C18 column (3µ, 2×150 mm, Phenomenex) for HPLC resolution of the eicosanoids and detected by QTRAP5500 mass analyzer (ABSCIEX) using optimized conditions for each eicosanoid by Multiple Reaction Monitoring (MRM) method as described before (31). LC-MS chromatograms were analyzed by MultiQuant (ABSCIEX) for quantitation of each eicosanoid and normalized to the internal standard signal. Under the standard conditions of the method, the detection limits for most of the eicosanoids were <2 pg on the column with a signal/ratio of 3.
Statistical Analysis
Unless otherwise stated, data were expressed as mean ± SD. Where applicable, the results were compared by using the unpaired, two-tailed Student t-test, as implemented by Excel 2007 (Microsoft, Redmond, WA). p values smaller than 0.05 were considered statistically significant.
Results
EPA Synergizes with FuOx
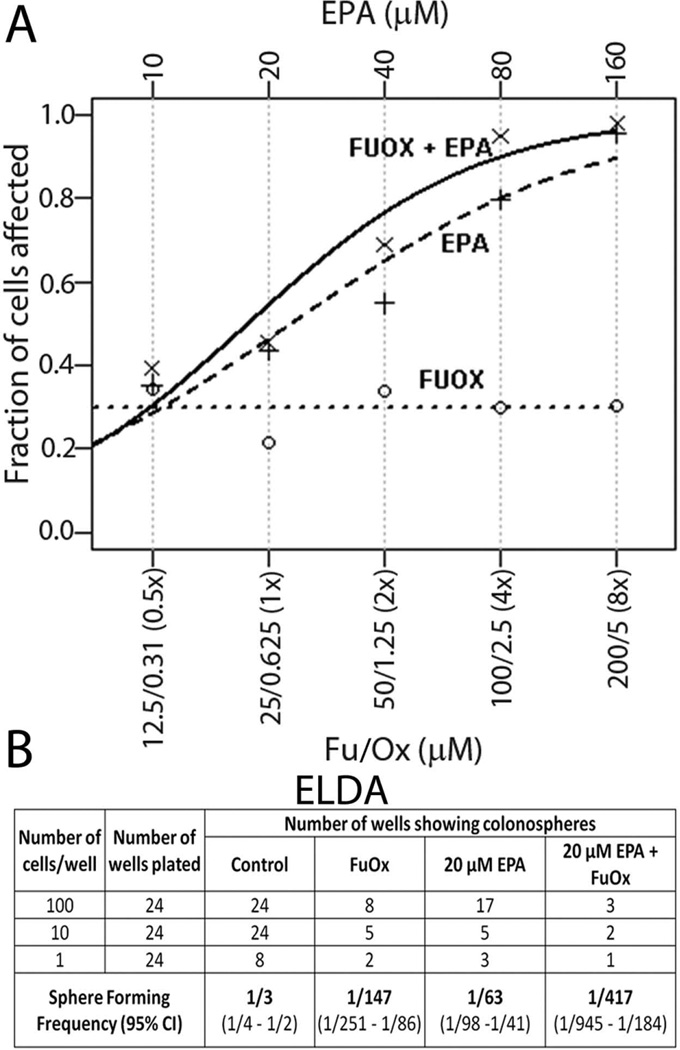
The data obtained from synergy analysis of EPA and/or FuOx treated CR HT-29 cells revealed that cells treated with the combined dosage are 6.05 times (p value=0.009) more likely to die than those treated with FuOx alone. This was calculated by the difference in intercepts of the groups in a logistic regression model with data from EPA and FuOx each alone, or in combination (Fig 1A), assuming a combined slope. The data clearly show synergism between the two.
Figure 1.
A: Dose response curves for EPA and/or FuOx in CR HT-29 cells produced by fixed ratio method demonstrating synergism between EPA and FuOx. A logistic growth curve was fitted using the R statistical software (URL http://www.R-project.org/) to the observed data points within each of the 3 measurement groups: EPA alone, FuOx alone and the combination. Concentrations of 5FU/oxaliplatin in relation to their X value are shown on the x axis. Each treatment was performed in quadruplicates.
B : Extreme Limiting Dilution Analysis (ELDA) of colonosphere forming frequency in response to EPA and /or FuOx in CR HT-29 cells
EPA or EPA+ FuOx treatment inhibits stem cell characteristics in CR cells
To analyze whether EPA and/or FuOx would affect the properties of colon CSCs/CSLCs, the ability of CR HT-29 and CR HCT-116 cells to form colonospheres was examined. Extreme limiting dilution assay (ELDA) performed on CR HT-29 cells demonstrated that the frequency to form colonospheres was 139-fold higher in control group, compared to 20 µM EPA + FuOx treated cells (Fig 1B).
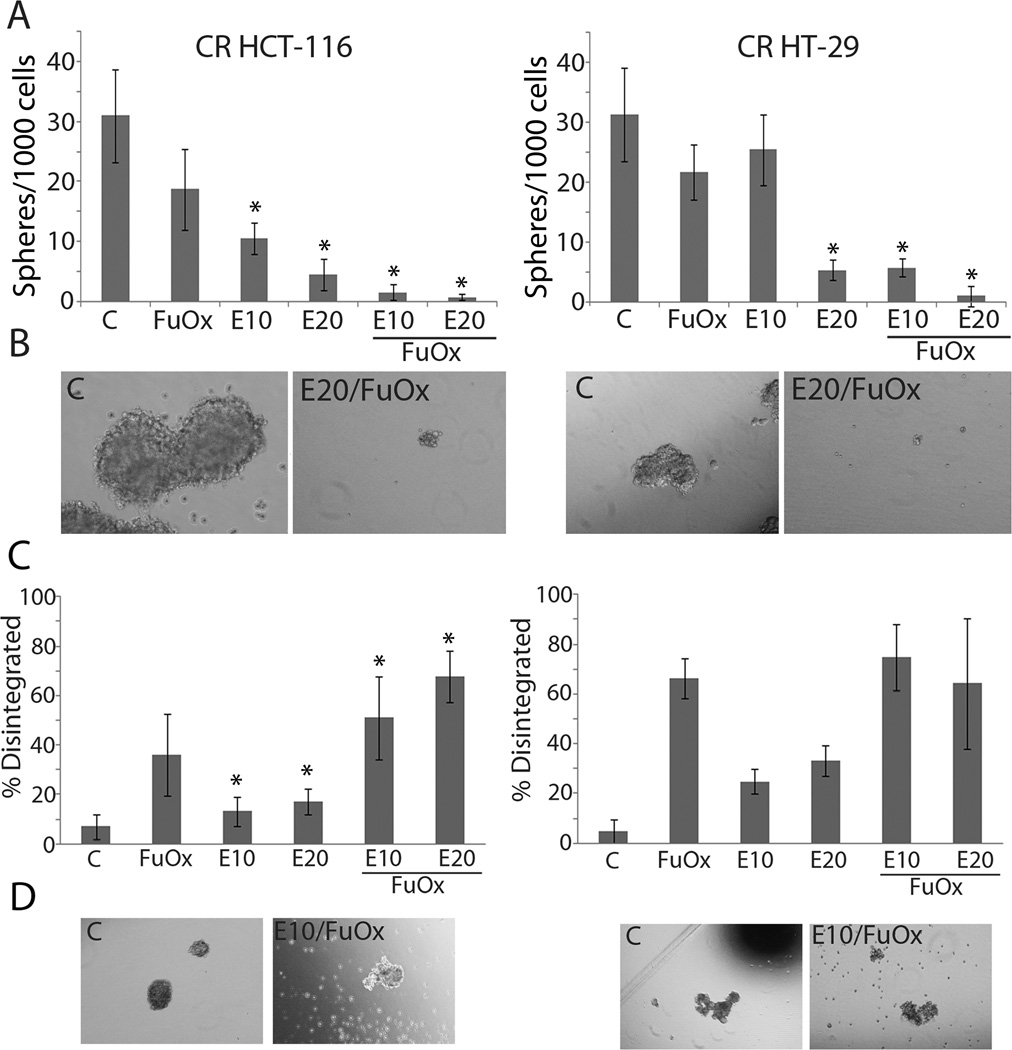
With respect to formation of colonospheres, EPA alone at 10 and 20 µM caused a significant inhibition in CR HCT-116 cells, which was further exacerbated when combined with FuOx (Figs 2A & B). Interestingly, CR HT-29 cells appeared to be more resistant to the EPA at 10µM, but in the presence of combination treatment, a significant inhibition in colonosphere formation was observed. EPA at 20 µM was found to be as effective as the combinatorial treatment.
Figure 2.
Changes in formation and disintegration of colonospheres in response to EPA and/or FuOx. Chemo-resistant HT-29 or HCT-116 cells were seeded at a density of 1000/well in a 6 well dish. A&B: Formation of colonospheres after 14 days; C&D: Disintegration of colonospheres; treatments with various regimen were started 7 days after seeding B&D: photomicrographs of representative spheres using Olympus CKX41 microscope supporting Olympus DP72 camera and stored with DP2-BSW software.. ×400 Spheres ≥80µm were counted. Bars represent mean of 4–6 readings ± standard deviation. *p < 0.005.
Additionally, the combined treatment of EPA and FuOx also induced disintegration of colonospheres, especially in CR HCT-116 cells. We observed that combined treatments were significantly more effective than FuOx or EPA alone at 10 and 20 µM for differentiation and disintegration of spheres formed by CR HCT-116 cells (Fig 2C). Interestingly, EPA +FuOx treatment did not affect sphere disintegration any more than FuOx alone in CR HT-29 cells (Fig 2D) indicating a cell line specificity.
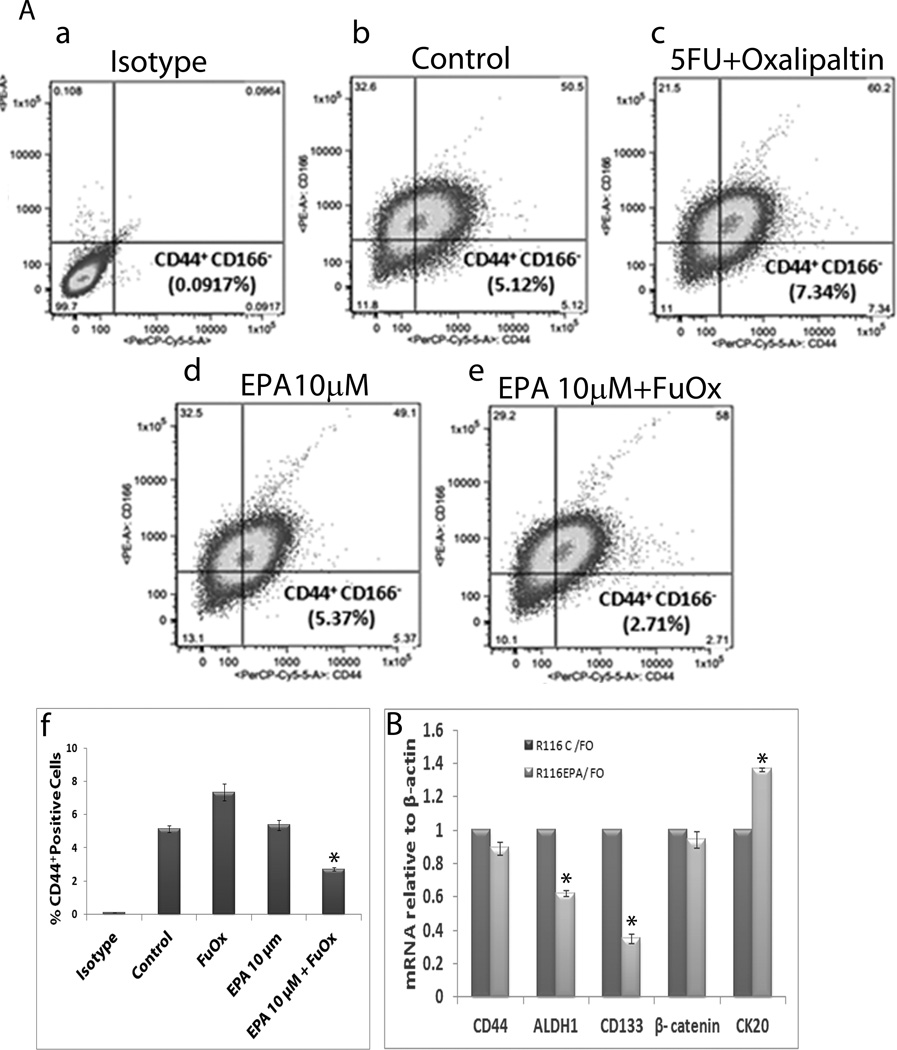
Flow cytometric analysis revealed a reduction in the proportion of CD44+/CD166low CSC phenotype in CR HT-29 cells in response to 10 µM EPA and FuOx, compared to the corresponding control (Fig. 3Aa–Af). While the untreated controls contained 5.12% CD44+/CD166low phenotype, FuOx increased the proportion of CD44+/CD166low to 7.34%. This increase could be the result of enrichment of FuOx resistant phenotype. However, EPA normalized this phenotype to 5.37%, and the combined treatment further reduced it to 2.71%.
Figure 3.
Changes in CSC phenotype and expression of CSC markers CR HT-29 cells in response to EPA and /or FuOx. A: Flow cytometric analysis of CD44+ and CD166low phenotype (a–e) f: graphical representation of a–e; bars represent mean of 3 readings ± standard deviation. *p < 0.005. B: qPCR showing mRNA levels of CSC and differentiation markers normalized to β-actin. Bars represent mean of 3 readings ± standard deviation. *p<.005.
qPCR analysis of 10µM EPA and FuOx treated CR HCT-116 cells showed a down-regulation of stem cell markers CD44, ALDH1, CD133 and β-catenin, and an up-regulation of epithelial marker CK20 compared to FuOx alone (Fig. 3B), indicating that EPA+FuOx affects the colonic CSC population.
EPA + FuOx treatment retards tumor growth in SCID mice
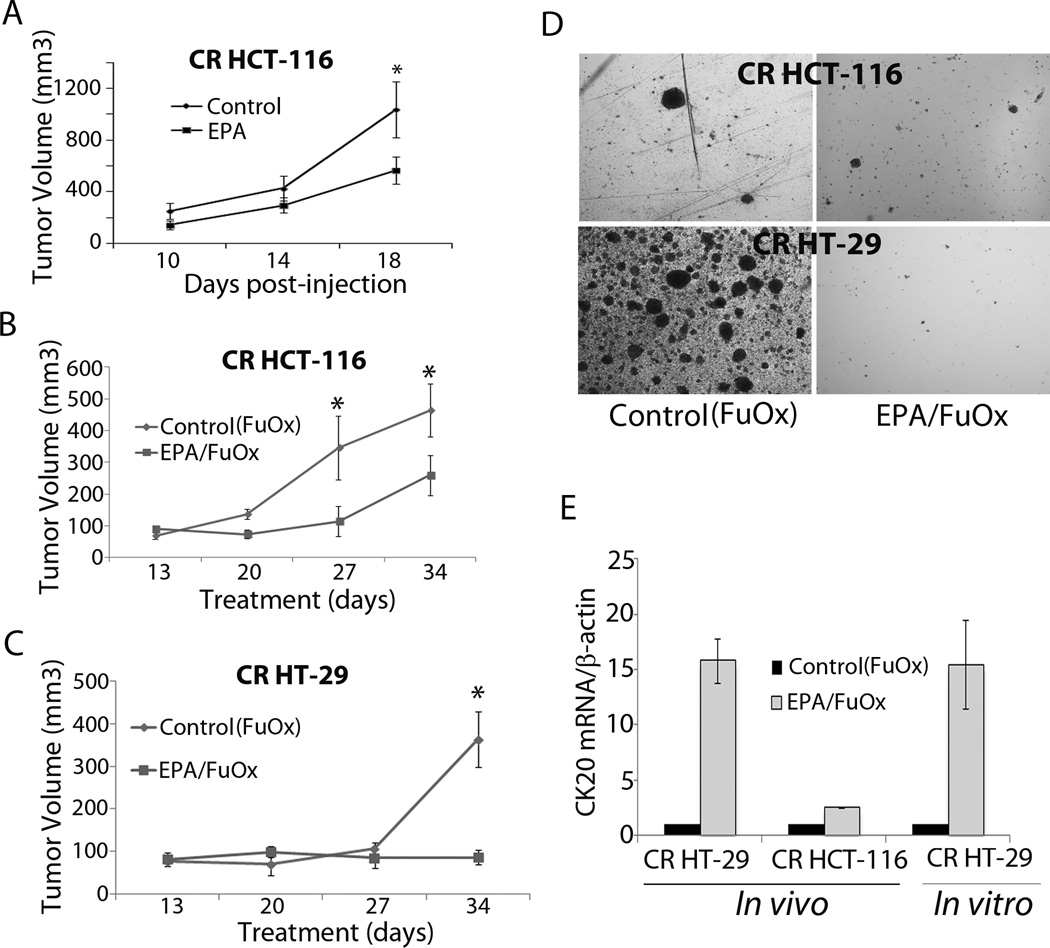
Palpable tumors were formed by 7–10 days and grew linearly in control animals. EPA pretreatment for 7 days prior to inoculation of CR cells and subsequent continuation reduced the growth, which revealed about 50% reduction at the end of 18 day treatment period (Fig 4A). The combination of EPA +FuOx was found to be more effective than EPA alone. The tumor size in EPA+FuOx treated group was significantly smaller than the control at 27 and 34 days (Fig 4B). CR HT-29 cell xenografts showed a slow growth initially, but after 27 days a growth spurt was seen in control FuOx treated mice, whereas the EPA+FuOx treated mice did not show any significant tumor growth throughout the 34 days post-injection period (Fig 4C). Immunohistochemical staining of CR HT-29 xenograft showed reduced cell proliferation following EPA/FuOx treatment, as evidenced by low PCNA staining (data not shown).
Figure 4.
Inhibition of CR HCT-116 (A&B) or CR HT-29 cells (B) xenografts in EPA and/or FuOx treated SCID mice. A: EPA was administered for 7 days before inoculation of 1×106 CR HCT-116 cells and continued for the duration of the experiment. B&C: EPA+FuOx treatments were started 7 days after CR cells inoculation. Each data point represents average of 8 tumors ± SE. * p >0.05. D: Colonosphere formation in the cells isolated from CR HCT-116 and CR HT-29 xenografts ×100. E: qPCR on RNA from tumors of CR HT-29 and CR HCT-116 cells and CR HT-29 cells.
Single cell suspensions obtained from the tumors were subsequently cultured in stem cell medium to examine for colonosphere formation. The cells, isolated from the EPA/FuOx treated xenografts formed only a few spheres (Fig. 4D), strengthening our observation that EPA/FuOx combinatorial treatment inhibits the growth of CSCs/CSLCs.
Quantitative real time PCR performed on RNA isolated from tumor as well as from the CR HT-29 cells showed a significant increase in CK20 mRNA levels in EPA+FuOx treated tumors and cells indicating an increased number of differentiated cells (Fig 4E).
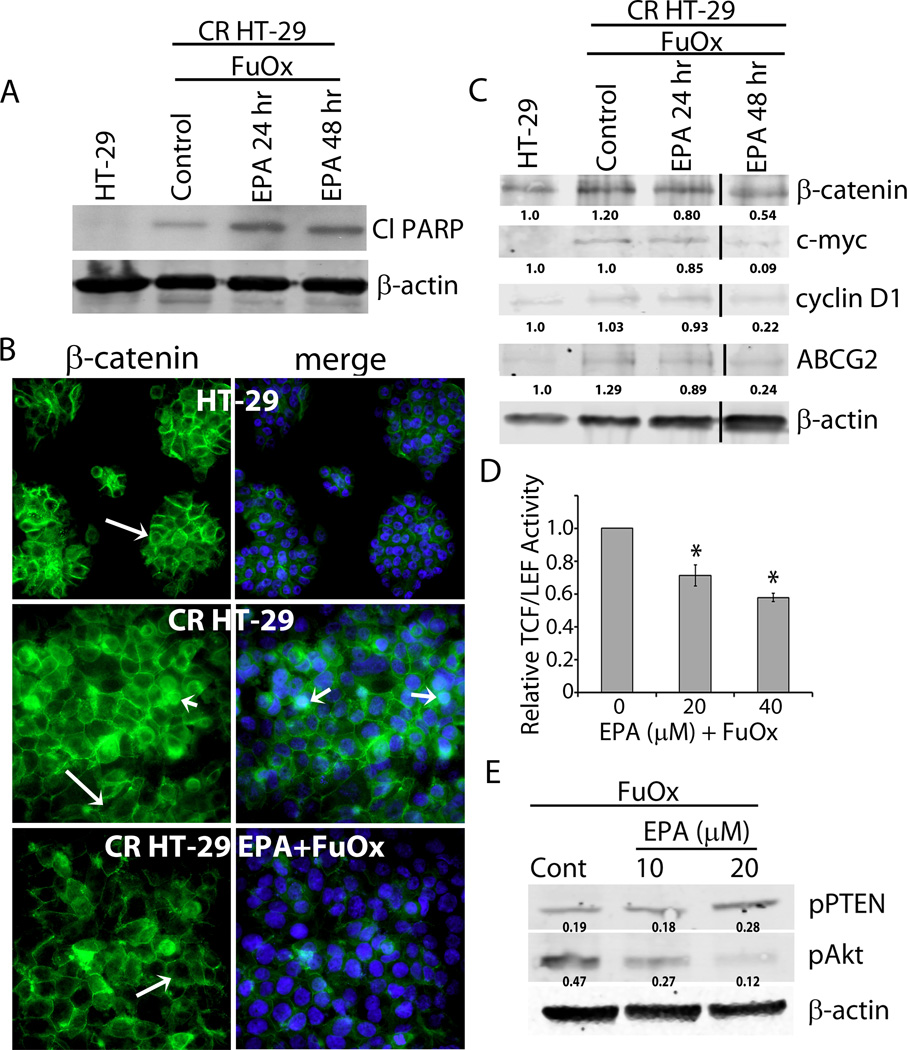
The EPA+FuOx-mediated growth inhibition could be due to induction of apoptosis as western blot analysis of the EPA+FuOx treated CR HT-29 cells showed an increased PARP cleavage (Fig 5A).
Figure 5.
Inhibition of Wnt/β-catenin signaling and PTEN/Akt axis in CR HT-29 cells in response to EPA + FuOx. A: Western blot analysis of cleaved PARP in CR HT-29 cells treated with 10µM EPA/ 2× FuOx for 24 and 48 hr. B: Indirect immunofluorescence staining showing membrane (long arrow) and nuclear (short arrow) localization of β-catenin. Counter staining was done with DAPI (blue) ×100. C: Western blot analysis of CR HT-29 cells treated with 10µM EPA/ 2× FuOx for 24 and 48 hr. 50 µg total protein was loaded in each lane. β-actin was used as loading control. Lines indicate removal of a band. D: Transcriptional activity of TCF/LEF in EPA/FuOx treated cells. Bars represent mean of 3 readings ± standard deviation. *p<.005. E: Western blot analysis of pPTEN and pAkt in EPA+FuOx treated CR HT-29 cells. β-actin was used as loading control.
EPA + FuOx treatment regulates β-catenin activity via PTEN/Akt axis
To elucidate the regulatory mechanism(s) for EPA and FuOx mediated inhibition of growth and development of CSCs in chemo-resistant colon cancer cells in vivo and in vitro, we examined the localization and transcriptional activity of β-catenin, which is known to be activated in CRC (32) and plays a critical role in maintaining the growth and functional properties of colon CSCs (33). Indirect immunofluorescence staining showed β-catenin to be primarily localized on the cell membrane in normal HT-29 cells (Fig 5B top panel, long arrows). In contrast, CR HT-29 cells showed nuclear localization of β-catenin in a number of cells (Fig 5B middle panel, short arrow). However, in EPA/FuOx treated CR HT-29 cells, β-catenin was found to be mainly localized on the cell membrane (Fig 5B bottom panel). Western blot analysis showed a decreased expression of β-catenin as well as its target proteins c-myc and cyclin D1 in EPA+FuOx treated cells after 24 and 48 hr (Fig 5C). EPA+FuOx treatment also caused approximately 40% reduction in the transcriptional activity of TCF/LEF in CR cells (Fig 5D). These data indicate that EPA+FuOx treatment inhibits the nuclear localization of β-catenin, thus preventing its transcriptional activity. In addition, decreased levels of ATP-binding cassette (ABC) transporter ABCG-2 protein, which is involved with drug efflux (3, 34), indicate reduced drug resistance in EPA/FuOx treated cells (Fig 5C).
Although the precise mechanism(s) for EPA+FuOx-mediated changes in β-catenin is not fully understood, we hypothesized that PTEN/Akt signaling may play a role in regulating this process. EGFR, which is known to be activated in CRC leads to induction of PTEN/Akt axis (35). In addition, we have reported that miR-21 mediated induction of colon CSCs is associated with down-regulation and decreased phosphorylation of PTEN (decreased activation) leading to activation of Akt (36, 37). Figure 4E demonstrates a marked increase in pPTEN and decreased pAkt levels over the corresponding controls in response to 20 µM EPA + FuOx (Fig. 5E). The dose of 10 µM EPA + FuOx also caused reduction in the activated (phosphorylated) form of Akt (Fig. 5E). The fact that EPA+FuOx activates PTEN resulting in the decreased Akt activity suggests PTEN/Akt axis is involved in modulating Wnt/β-catenin signaling.
EPA + FuOx treatment reduces the levels of pro-inflammatory metabolites in SCID mice
Both arachidonic acid (AA) and EPA are metabolized in vivo by the cyclooxygenase, lipoxygenase, and epoxygenase pathways to prostaglandins, hydroxy fatty acids as well as leukotrienes, and epoxy fatty acids, respectively. To assess the PUFA metabolic changes in the animals treated with EPA, we analyzed the plasma eicosanomic profiles of both treated and untreated animals by LC-MS. The method included all possible metabolites of AA and EPA from all three enzymatic pathways. Table 1 shows the detected metabolites of both fatty acids in plasma. The results show a lower concentration of AA metabolites in EPA+FuOx treated animals compared to control FuOx treated mice and a significantly lower concentration of inflammatory mediators, LTB4 (and its metabolite 12-Oxo LTB4) and PGE2 (as well as its metabolites, 13,14-dihydro-15-keto PGE2 and bicyclo PGE2) in EPA+FuOx treated animals. Interestingly, a majority of EPA metabolites show similar plasma concentrations between the two groups, despite the fact that EPA is a significantly poorer substrate to the metabolic enzymes compared to AA (38).
Table 1.
Concentrations of eicosanoids in control and EPA+FuOx treated mouse plasma.
| Eicosanoid | Control (FuOx) |
EPA+FuOx | % of control |
|---|---|---|---|
| 6-keto PGF1α | 48.4±11.7 | 20.4±3.8 | 42 |
| TXB2 | 78.9±54.9 | 17.1±0.7 | 22 |
| PGE2 | 24.0±8.4 | 7.7±2.1 | 32 |
| 13,14-dihydro-15-keto PGE2 | 7.9±5.2 | 2.2±1.2 | 27 |
| Bicyclo PGE2 | 21.1±18.0 | 5.0±2.8 | 24 |
| 13,14-dihydro-15-keto PGF2α | 4.6±2.5 | 1.8±0.9 | 39 |
| 13,14-dihydro-15-keto PGD2 | 9.4±6.9 | 2.2±1.1 | 23 |
| PGJ2 | 65.4±33.2 | 33.7±17.7 | 52 |
| 5(6)-EpETrE | 17.2±9.0 | 5.8±1.9 | 34 |
| 8(9)-EpETrE | 17.8±9.9 | 5.0±1.0 | 28 |
| 11(12)-EpETrE | 47.1±22.8 | 14.8±3.3 | 31 |
| 11,12-DiHETrE | 2.8±1.4 | 1.7±0.8 | 62 |
| 14(15)-EpETrE | 19.4±16.7 | 6.4±1.4 | 33 |
| 14,15-DiHETrE | 4.3±2.9 | 3.1±1.0 | 73 |
| LTB4 | 8.4±3.5 | 2.0±0.3 | 24 |
| 12-Oxo LTB4 | 4.1±1.4 | 0.1±0.1 | 3 |
| 5-HETE | 28.7±9.8 | 14.9±2.1 | 52 |
| 5-oxo ETE | 2.7±0.6 | 2.5±0.6 | 90 |
| 8-HETE | 33.8±14.2 | 9.8±0.5 | 29 |
| 9-HETE | 12.2±4.0 | 4.4±0.1 | 36 |
| 11-HETE | 109.8±49.9 | 33.8±6.2 | 31 |
| 12-HETE | 1629.0±855.4 | 340.4±144.8 | 21 |
| 12-Oxo ETE | 5.1±2.5 | 2.0±0.8 | 39 |
| 15-HETE | 42.5±20.2 | 15.4±1.2 | 36 |
| 15-Oxo ETE | 1.1±0.4 | 0.7±0.2 | 66 |
| 8(9)-EpETE | 2.3±1.5 | 1.2±0.4 | 53 |
| 11(12)-EpETE | 1.3±1.0 | 1.4±0.1 | 110 |
| 14(15)-EpETE | 2.2±1.7 | 2.0±0.6 | 90 |
| 17(18)-EpETE | 2.9±1.7 | 1.6±0.4 | 55 |
| 5-HEPE | 4.3±1.7 | 3.5±0.6 | 80 |
| 8-HEPE | 4.2±1.6 | 2.9±0.7 | 69 |
| 9-HEPE | 7.2±3.7 | 4.0±2.0 | 55 |
| 11-HEPE | 5.0±2.3 | 3.1±0.4 | 61 |
| 12-HEPE | 112.1±67.9 | 60.4±40.0 | 54 |
| 15-HEPE | 6.4±2.4 | 4.0±0.7 | 63 |
| 18-HEPE | 3.9±1.4 | 3.2±0.6 | 80 |
All values are ng/ml plasma (Mean±SEM, n=3). Metabolites of EPA were italicized. Abbreviations: PG – prostaglandin, TX – thromboxane, EpETrE – epoxyeicosatrienoic acid, EpETE – epoxyeicosatetraenoic acid, LT – leukotriene, HETE – hydroxyeicosatetraenoic acid, HEPE – hydroxyeicosapentaenoic acid, ETE – eicosatrienoicacid, DiHETrE – dihydroxyeicosatrienoic acid
Discussion
The main objective of the current investigation was to study the efficacy of EPA as an inhibitor of recurrent colorectal cancer growth and to determine whether EPA in combination with FuOx would be more effective than either agent/regimen alone.
The preventive and therapeutic efficacy of a combination of EPA and DHA or each PUFA alone have been demonstrated in multiple preclinical studies using a variety of rodent models of early stage CRC (39). These studies have consistently demonstrated reduction in CRC incidence (Reviewed by (39)). Our data demonstrate for the first time that EPA acts synergistically with FuOx to markedly inhibit the growth of chemo-resistant colon cancer cells that form bulk of the recurrent tumor. Although the underlying cause for tumor recurrence is not fully understood, one of the reasons is thought to be the presence of CSCs/CSLCs that are resistant to conventional chemotherapy and retain limitless potential to regenerate (1–3, 40). The resistance of CSCs/CSLCs to therapy has been attributed to a multitude of factors, including increased expression of drug transporters and intracellular detoxification enzymes, up-regulation of anti-apoptotic proteins, increased efficiency of DNA repair and alterations in cell kinetics (41). As the CR HT-29 and CR HCT-116 cells exhibit increased stem-like characteristics, as evidenced by increased colonosphere formation, increased drug efflux, an elevated expression of CSC/CSLC markers, higher tumorigenic potential in SCID mice, an increased Wnt/β-catenin and EGFR signaling (4–7), they provide a suitable model to study the efficacy of EPA and/or FuOx in inhibiting recurring colon cancer.
Our current observation that EPA causes a marked reduction in colonosphere formation by CR HT-29 and CR HCT-116 cells, which is further exacerbated by the combination of EPA and FuOx, suggests that this regimen not only inhibits proliferation of CSCs, but also their functional properties. Furthermore, the fact that the same combination treatment also induces disintegration of colonospheres in CR HCT-116 cells suggests that this treatment strategy could be utilized to eliminate/kill colon CSCs that have already extravasated the primary tumor and entered the vascular system. In support of this contention, we have observed a marked reduction in the proportion of CD44+/CD166low CSC phenotype in EPA/FuOx treated CR HT-29 cells and a reduced expression of stem cell markers CD44, ALDH1, CD133, β-catenin. Yang et al recently reported that a combination of EPA and DHA exerts a direct anti-proliferative and pro-apoptotic effect on the cancer stem like cells using SW620 colon cancer cell line and increases their sensitivity to 5-FU (42). Likewise, our data show that while EPA is effective in inhibiting growth of CSC/CSLC-enriched chemo-resistant cells, the combination is even more effective.
An increased expression and activity of Akt has been reported as an essential factor for cell survival during carcinogenesis (43). Aberrant Akt activation is the result of PI3K and PTEN downstream signaling (44), and in turn affects various pathways including inactivation of GSK-3β resulting in dysregulated canonical Wnt/β-catenin signaling (45, 46). Dysregulation of Wnt/β-catenin pathway has been reported to play a pivotal role in the development and progression of CRC. Translocation of β-catenin to the nucleus activates the transcription of its target genes like cyclin D1, c-myc, MMP-7, MT1-MMP, axin-1 etc (47–49). We have reported that the Wnt/β-catenin pathway also plays a crucial role in regulating the growth and maintenance of colon CSCs (7). Although the precise mechanism by which EPA+FuOx inhibit the growth of chemo-resistant CRC, our current observation that EPA/FuOx greatly reduces β-catenin transcriptional activity suggests a crucial role for Wnt/β-catenin signaling in regulating this event. Furthermore, the fact that EPA+FuOx activates PTEN resulting in decreased Akt activity suggests PTEN/Akt axis is involved in modulating Wnt/β-catenin signaling. Additional support comes from the observation that the combination of EPA and FuOx also decreases cyclin D1 and c-myc expression, two downstream effector molecules of Wnt/β-catenin signaling pathway, that are known to be involved in regulating cell proliferation. In addition, the combination of EPA and FuOx was found to reduce the expression of ABCG-2 protein, which has been implicated as a key regulator in the maintenance of stem cells in various human cancer cell lines (4, 34, 50) and contributes towards resistance to chemo-therapy (3, 34). Down-regulation of ABCG-2 in EPA/FuOx treated cells indicates a reduction in drug efflux capability resulting in its sensitization to chemotherapy. An increased apoptosis in EPA/FuOx treated cells as indicated by reduced PARP cleavage further confirms the efficacy of combination therapy on viability of CR cells. These data strongly suggest that EPA/FuOx treatment could be used to target stem cell enriched recurrent colorectal cancer.
Data generated from our in vivo studies utilizing SCID mice xenograft model of colon cancer also support the in vitro observations. When SCID mice bearing xenografts of CR HCT-116 and CR HT-29 cells were administered with either EPA or EPA +FuOx, the tumor growth was greatly reduced. In fact, no significant increase in growth of xenografts by CR HT-29 was observed following administration of EPA and FuOx. Xenografts formed by CR HCT-116 cells were decreased by at least 50% following EPA or the combinatorial treatment. The fact that EPA by itself reduced the growth indicates its chemopreventive efficacy. This is supported by EPA's ability to inhibit colonosphere formation in vitro indicating decreased number of CSCs/CSLCs. The fact that these changes were more apparent following combination treatment, also suggests that EPA could be utilized for preventive as well as therapeutic purposes.
The reduction in tumor growth could be attributed to decrease in tumor cell proliferation, as evidenced by decreased PCNA staining in the treated xenograft (data not shown). Furthermore, our observation that the expression of CK-20, a marker of differentiation, is greatly increased in cells isolated from SCID xenografts of chemo-resistant cells following EPA/FuOx treatment strongly suggests that the current combination therapy induces differentiation leading to increased sensitivity to the combination of EPA/FuOx treatment strategy.
Eicosanomic analysis of plasma from EPA/FuOx treated animals offers a possibility of modulating inflammatory response in these animals. Both AA and EPA are metabolized by the same enzymes to highly physiologically active lipid mediators such as prostaglandins, leukotrienes, hydroxy and epoxy fatty acids. However, metabolites of AA (an ω-6 PUFA) are pro-inflammatory, whereas those derived from EPA (an ω-3 PUFA) are anti-inflammatory and participate in active resolution of inflammation (51–53). The eicosanomic profile (Table 1) shows a lower concentration of AA metabolites in EPA/FuOx treated animals. Concentration of about 75% of the AA metabolites detected was less than 50% in EPA/FuOx treated animals, whereas all EPA metabolites are above 50% or nearly equal between the two groups. While EPA metabolites are expected to be higher in the animals fed with the fatty acid, it has a 2–3 fold metabolic disadvantage compared to AA (38). Moreover, the apparent suppression of AA metabolites in EPA treatment is not uniform across all lipid mediators. While inflammatory mediators such as LTB4 and PGE2 are lower in EPA treated animals, 5-OxoETE, another neutrophil chemotactic lipid mediator of the 5-lipoxygenase pathway is similar in both groups. It is noteworthy that 5-OxoETE is increased in cancer cells under oxidative stress (54). On the other hand, the anti-inflammatory lipid mediators of the epoxygenase pathway, e.g. 11(12)-EpETE, (55) are elevated upon EPA treatment (Table 1). While it is difficult to conclude on the limited sample size data presented from this pilot study, the data offers intriguing possibility that EPA treatment alters the balance of pro- and anti-inflammatory lipid mediators towards an improved outcome of chemotherapy.
In summary, the present data indicate the EPA could be a potential preventive and therapeutic treatment modality for recurrent colon cancer, which is known to be highly enriched in chemotherapy-resistant CSCs/CSLCs, as evidenced by the reduction in stem cell characteristics such as colonosphere formation and increased disintegration, decreased number of CD44+/CD166low cells, decreased expression of stem cell markers, increased CK20 levels and reduction in drug efflux in vitro and in vivo. These changes are associated with inhibition of PTEN/Akt axis leading to reduced Wnt/β-catenin signaling, induction of apoptosis and reduction in the pro-inflammation markers.
Acknowledgments
Financial Support: This study was supported by grants from the NIH (AG014343 to A. Majumdar) and the Department of Veteran Affairs (I101BX001927 to A.Majumdar). Eicosanomic analysis was supported in part by a grant from National Center for Research Resources, NIH (S10RR027926 to KRM.
Abbreviations used
- MTT
3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide
- 5-FU
5-Fluorouracil
- FuOx
5-Fluorouracil+Oxaliplatin
- AA
Arachidonic acid
- CSCs/CSLCs
Cancer stem/stem-like cells
- CR
Chemo (FuOx)-resistant
- CRC
Colorectal cancer
- EPA
Eicosapentaenoic acid
- Ox
Oxaliplatin
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose.
References
- 1.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 2.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 3.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Padhye S, Sarkar FH, et al. Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res. 2011;28:827–838. doi: 10.1007/s11095-010-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2:321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Sarkar FH, Majumdar AP. Down-regulation of miR-21 Induces Differentiation of Chemoresistant Colon Cancer Cells and Enhances Susceptibility to Therapeutic Regimens. Transl Oncol. 2013;6:180–186. doi: 10.1593/tlo.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azrad M, Turgeon C, Demark-Wahnefried W. Current Evidence Linking Polyunsaturated Fatty Acids with Cancer Risk and Progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Research WCrFAIfC. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: 2007. [Google Scholar]
- 10.Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am J Epidemiol. 2010;171:969–979. doi: 10.1093/aje/kwq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, Hoseini M, Parizade SM, Farhoudi F, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009;64:321–327. doi: 10.2143/AC.64.3.2038016. [DOI] [PubMed] [Google Scholar]
- 13.Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204:476–482. doi: 10.1016/j.atherosclerosis.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Clarke RG, Lund EK, Latham P, Pinder AC, Johnson IT. Effect of eicosapentaenoic acid on the proliferation and incidence of apoptosis in the colorectal cell line HT29. Lipids. 1999;34:1287–1295. doi: 10.1007/s11745-999-0480-7. [DOI] [PubMed] [Google Scholar]
- 15.Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 16.Latham P, Lund EK, Johnson IT. Dietary n-3 PUFA increases the apoptotic response to 1,2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis. 1999;20:645–650. doi: 10.1093/carcin/20.4.645. [DOI] [PubMed] [Google Scholar]
- 17.Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, et al. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis. 2004;25:2303–2310. doi: 10.1093/carcin/bgh265. [DOI] [PubMed] [Google Scholar]
- 18.Fan YY, Davidson LA, Callaway ES, Goldsby JS, Chapkin RS. Differential effects of 2- and 3-series E-prostaglandins on in vitro expansion of Lgr5+ colonic stem cells. Carcinogenesis. 2014;35:606–612. doi: 10.1093/carcin/bgt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawcroft G, Volpato M, Marston G, Ingram N, Perry SL, Cockbain AJ, et al. The omega-3 polyunsaturated fatty acid eicosapentaenoic acid inhibits mouse MC-26 colorectal cancer cell liver metastasis via inhibition of PGE2-dependent cell motility. Br J Pharmacol. 2012;166:1724–1737. doi: 10.1111/j.1476-5381.2012.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, et al. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFbetaR2) in colon cancer cells. Carcinogenesis. 2012;33:68–76. doi: 10.1093/carcin/bgr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6:7. doi: 10.1186/1750-2187-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nangia-Makker P, Tait L, Shekhar MP, Palomino E, Hogan V, Piechocki MP, et al. Inhibition of breast tumor growth and angiogenesis by a medicinal herb: Ocimum gratissimum. Int J Cancer. 2007;121:884–894. doi: 10.1002/ijc.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, Levi E, et al. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014;9:e84369. doi: 10.1371/journal.pone.0084369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Nangia-Makker P, Wang Y, Raz T, Tait L, Balan V, Hogan V, et al. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer. 2010;127:2530–2541. doi: 10.1002/ijc.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nangia-Makker P, Raz T, Tait L, Shekhar MP, Li H, Balan V, et al. Ocimum gratissimum retards breast cancer growth and progression and is a natural inhibitor of matrix metalloproteases. Cancer Biol Ther. 2013;14:417–427. doi: 10.4161/cbt.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nautiyal J, Kanwar SS, Majumdar AP. EGFRs) in aging and carcinogenesis of the gastrointestinal tract. Curr Protein Pept Sci. 2010;11:436–450. doi: 10.2174/138920310791824110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uygur R, Aktas C, Tulubas F, Uygur E, Kanter M, Erboga M, et al. Protective effects of fish omega-3 fatty acids on doxorubicin-induced testicular apoptosis and oxidative damage in rats. Andrologia. 2013 doi: 10.1111/and.12173. [DOI] [PubMed] [Google Scholar]
- 29.Zugno AI, Chipindo HL, Volpato AM, Budni J, Steckert AV, de Oliveira MB, et al. Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience. 2014;259:223–231. doi: 10.1016/j.neuroscience.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 30.Maddipati KR, Zhou SL. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins & Other Lipid Mediators. 2011;94:59–72. doi: 10.1016/j.prostaglandins.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Markworth JF, Vella L, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, et al. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2013;305:R1281–R1296. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 33.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert Opin Drug Metab Toxicol. 2009;5:1529–1542. doi: 10.1517/17425250903228834. [DOI] [PubMed] [Google Scholar]
- 35.Nautiyal J, Kanwar SS, Majumdar AP. EGFRs) in Aging and Carcinogenesis of the Gastrointestinal Tract. Curr Protein Pept Sci. 2010 doi: 10.2174/138920310791824110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy S, Majumdar AP. Signaling in colon cancer stem cells. J Mol Signal. 2012;7:11. doi: 10.1186/1750-2187-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy S, Yu Y, Padhye SB, Sarkar FH, Majumdar APN. Difluorinated-Curcumin (CDF) Restores PTEN Expression in Colon Cancer Cells by Down-Regulating miR-21. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 39.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 40.Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828–2838. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 41.Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev. 2012;38:589–598. doi: 10.1016/j.ctrv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Yang T, Fang S, Zhang HX, Xu LX, Zhang ZQ, Yuan KT, et al. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells in vitro. J Nutr Biochem. 2013;24:744–753. doi: 10.1016/j.jnutbio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaler P, Godasi BN, Augenlicht L, Klampfer L. The NF-kappaB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1beta. Cancer Microenviron. 2009 doi: 10.1007/s12307-009-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaish V, Sanyal SN. Role of Sulindac and Celecoxib in the regulation of angiogenesis during the early neoplasm of colon: exploring PI3-K/PTEN/Akt pathway to the canonical Wnt/beta-catenin signaling. Biomed Pharmacother. 2012;66:354–367. doi: 10.1016/j.biopha.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Kolligs FT, Bommer G, Goke B. Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66:131–144. doi: 10.1159/000066755. [DOI] [PubMed] [Google Scholar]
- 48.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Wu WK, Wang XJ, Cheng AS, Luo MX, Ng SS, To KF, et al. Dysregulation and crosstalk of cellular signaling pathways in colon carcinogenesis. Crit Rev Oncol Hematol. 2013;86:251–277. doi: 10.1016/j.critrevonc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Katayama R, Koike S, Sato S, Sugimoto Y, Tsuruo T, Fujita N. Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export. Cancer Sci. 2009;100:2060–2068. doi: 10.1111/j.1349-7006.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 52.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan CN, Petasis NA. Resolvins and Protectins in Inflammation Resolution. Chemical Reviews. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant GE, Rubino S, Gravel S, Wang X, Patel P, Rokach J, et al. Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid. Carcinogenesis. 2011;32:822–828. doi: 10.1093/carcin/bgr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu J-Y, Stephen Lee KS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proceedings of the National Academy of Sciences. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]