Abstract
Chronic neuropathic pain is a common consequence of spinal cord injury (SCI), develops over time and negatively impacts quality of life, often leading to substance abuse and suicide. Recent evidence has demonstrated that reactive oxygen species (ROS) play a role in contributing to neuropathic pain in SCI animal models. This investigation examines four compounds that reduce ROS and the downstream lipid peroxidation products, Apocynin, 4-oxo-tempo, U-83836E, and Tirilazad, and tests if these compounds can reduce nocioceptive behaviors in chronic SCI animals. Apocynin and 4-oxo-tempo significantly reduced abnormal mechanical hypersensitivity measured in forelimbs and hindlimbs in a model of chronic SCI-induced neuropathic pain. Thus, compounds that inhibit reactive oxygen species or lipid peroxidation products can be used to ameliorate chronic neuropathic pain.
Keywords: Chronic Neuropathic pain, Oxidative stress, Mechanical Sensitivity, Spinal Cord Injury
Introduction
Chronic pain affects 116 million people per year in the United States at a cost of over 635 billion dollars for treatment fees and lost productivity annually (Institute of Medicine, 2011). Neuropathic pain, a chronic and difficult to diagnose and treat manifestation of pain, often does not respond well to commonly prescribed analgesic treatments (Murphy & Reid, 2001; Vissers, 2006). The lack of effective treatments can leave the patients in constant pain, leading to increased episodes of depression and suicide (Blair et al, 2003; Cairns et al, 1996; Widerstrom-Noga et al. 2001). Understanding the mechanisms behind chronic neuropathic pain will facilitate development of targeted therapies for people suffering from chronic neuropathic pain.
Patients commonly develop chronic neuropathic pain by trauma to nervous tissue, either peripherally or centrally. Specifically, up to two-thirds of all spinal cord injured (SCI) people develop neuropathic pain syndromes (Finnerup and Jensen, 2004; Werhagen et al, 2004). Our lab has developed a SCI animal model that consistently produces chronic neuropathic pain (Hulsebosch et al., 2000; Hulsebosch, 2003) parallels the pathophysiology described in people with SCI (Bunge et al., 1993; Bunge, 1994), and allows the rigorous study of cellular and molecular mechanisms of neuropathic pain after SCI in a controlled environment.
It has been reported that reactive oxygen species (ROS) play an important role in chronic neuropathic pain (Schmidtko et al, 2013). ROS are highly oxidative molecules that naturally occur as a consequence of cellular energy production. Cellular stress or trauma results in higher than normal intracellular concentrations of ROS, which can overpower the homeostatic proteins and cause oxidative damage to the cell. Neurons are especially sensitive to ROS since neurons have greater energy demands to function as compared to glial and other cells in the central nervous system (Bell, 2013).
We previously reported that downstream consequence of ROS, lipid peroxidation (LP) products, may also contribute to neuropathic pain in chronic SCI animals (Gwak et al, 2013). To better investigate the role that oxidation damage plays in chronic neuropathic pain, we examined four compounds that are known to reduce ROS and lipid peroxidation (Stefanska and Pawliczak, 2008; Khalil and Khodr, 2001; Hall, 1992; Hall et al, 2010: Wilcox, 2010). These four compounds are 1) Apocynin, a NADPH oxidase inhibitor, 2) 4-oxo-tempo (also known as TEMPONE), a spin trap nitroxyl radical, 3) U-83836E, a free radical scavenger that inhibits iron-dependent lipid peroxidation, and 4) Tirilazad, a potent peroxyl scavenger and membrane stabilizer. Each of these compounds was tested based on different mechanisms of action involving ROS and lipid peroxidation reduction products. We report that intraspinal administration of Apocynin and 4-oxo-tempo significantly attenuated the abnormal mechanical hypersensitivity that develops following SCI in rats.
Materials and Methods
Experimental Animals
Subjects were male Sprague-Dawley rats, 200-225 g, (Harlan laboratories, Houston, TX), and housed with a reversed day/night cycle of 12 hour periods. Experimental procedures followed all NIH Guidelines for the Care and Use of Laboratory Animals. Thirty-eight total animals were used in the experiments. For each experiment, 16 subjects were randomly divided into two groups for each trial (n = 8/group ), either the compound + vehicle + SCI group or the vehicle + SCI alone group.
Spinal Cord Injury Procedures
The animals were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg). Anesthesia is considered complete when there was no withdrawal response to noxious foot pinch. When the animal was fully anesthetized, its back was shaved, and a laminectomy was performed exposing spinal segment T10. We produced contusion spinal injury using the Infinite Horizon impactor (150kdyne, 1 second dwell time). Following the injury, the musculature was sutured, the skin autoclipped and the animals allowed to recover from anesthesia. The animals were eating and drinking within 3 hrs of surgery. Antibiotic treatment began immediately after surgery with a subcutaneous injection of 0.3 cc of Baytril (22.7 mg/ml) followed by a second injection 7hrs later; after which, Baytril injections were given twice daily for 7 days and once daily for 3 more days to prevent urinary tract infections. Bladders were manually expressed twice daily. Automatic bladder emptying is achieved in all spinally contused rats by 10 days post contusion. Post injury animals were housed in ALAC approved facilities. Records were kept on each animal with respect to its day of injury, weight at the time of injury, the computer record of the impact parameters, drug regimen measurements, and samples taken.
Behavior Assays
Mechanical threshold to paw withdraw in the forelimbs and hindlimbs of the animals were tested using a range of von Frey filaments (North Coast, 0.2 to 25 gram force) using a modified Dixon up-down method with the paw withdrawal threshold was calculated. During the von Frey assay, the animals were also monitored for supraspinal behaviors such as head turning, changes in body posture, avoidance, vocalizations, paw licks, and aggressive behavior toward the von Frey filament. These supraspinal responses are monitored in order to ensure non-reflexive pain behavior is being measured (Christensen and Hulsebosch, 1997). Responses of the mechanical sensitivity assays were taken pre surgery, for the purposes of a baseline measure, and compared to responses measured every week, beginning 14 days after injury, to monitor the animals’ development of hypersensitivity. Animals that did not display an increase in mechanical sensitivity of at least 40 percent of baseline values were excluded from the experiment. Of the 38 animals that had the SCI surgery, two animals were excluded for not meeting the criteria. Locomotor activity in open field conditions was ranked. There were no indications that the compounds tested had a sedative effect on the animals at the dosages tested, and the locomotor scores were unchanged.
Intrathecal Injections
The animals were given intrathecal lumbar injections with Apocynin (0.01mg/kg, 0.05mg/kg, 0.1mg/kg, Tocris Bioscience), Tirilazad mesylate (0.01mg/kg, 0.05mg/kg, 0.1mg/kg, Cayman Chemical), U-83836E (0.05mg/kg, 0.5mg/kg, 1.0mg/kg, Cayman Chemical), or 4-oxo-tempo (0.05mg/kg, 0.5mg/kg, 1.0mg/kg, Sigma-Aldrich) at L3-L4 of the spinal column to avoid damage to the spinal cord. The pH of each solution of inhibitors was set to 7.2-7.4 pH to match that of the animals’ cerebrospinal fluid. The animals were anesthetized by inhalation anesthesia 4% isoflurane. Fifty microliters of vehicle (10% DMSO, in 0.9% Saline) or vehicle plus a test compound, was injected during a one minute period into the intrathecal space. Fifty microliters was chosen to ensure that the solution diffused to cervical segments. The animals were alert and mobile moments after the inhalation anesthesia was removed, displaying no signs of distress or neural damage.
Experimental design
Behavioral testing started 42 days post injury, during the animal’s nocturnal cycle, which is when the animals are most active. The animals were first tested before injection, and then 30, 60, and 120 minutes after injection. This design was repeated for each compound trial with a minimum of one day in between trials to allow for drug washout, washout was determined by behavioral analysis and knowledge of the inhibitors halflives. Animals were randomly assigned to either the vehicle or compound groups for each trial.
Statistical Analysis
Statistical analyses were performed using SPSS software (ver. 14, SPSS Inc., Chicago, IL). The significant value for each test was α < 0.05. A within-group repeated measures ANOVA was used to analyze the data for the same group over time. Data values are expressed and graphed as mean with standard error of the mean (mean ± SEM).
Results
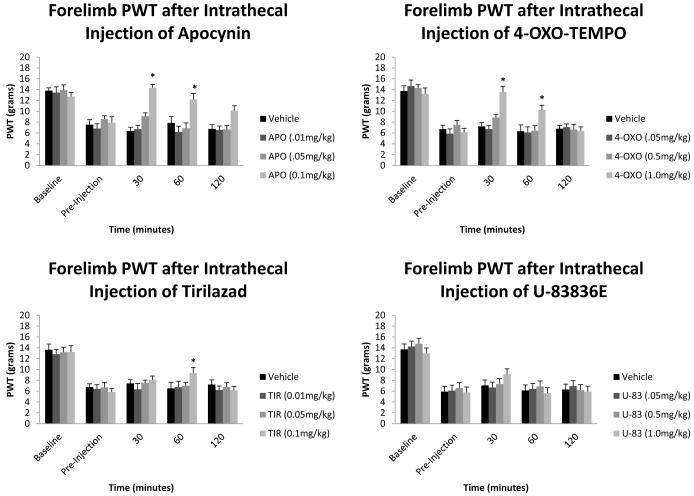
Figure 1 represents the changes in the forelimb paw withdraw thresholds (PWT, grams) before SCI, before intrathecal injection, 30, 60, and 120 minutes after intrathecal injection. All forelimb withdraw thresholds values in the pre-injection groups were significantly different compared to baseline values. The 30 minute, 60 minute, and 120 minute values for the vehicle groups were not significantly different from the preinjection values. The apocynin group values were significantly different (α < 0.05) from their pre-injection value at 30 minutes (14.35 ± 0.96), and 60 minutes (12.19 ± 0.81) after injection for the highest dosage of 0.1mg/kg. The 1.0mg/kg 4-oxo-tempo values for 30 minutes (13.56 ± 0.92), and 60 minutes (10.26 ± 0.74) were significantly different from the 4-oxo-tempo preinjection values. The Tirilazad group was not significantly different from the preinjection value 30 minutes after injection, however, 60 minutes after injection (9.32 ± 0.85) was significantly different for the 0.1mg/kg dosage as compared to the preinjection values.
Figure 1.
Forelimb paw withdraw thresholds (PWT) after intrathecal injection of reactive oxygen species (ROS) and lipid peroxidation (LP) inhibitors. All baseline mean values are similar to each other with no significant difference from between groups. All pre-injection mean values are significantly different to the baseline mean values. 0.1mg/kg Apocynin and 1.0mg/kg 4-oxo-tempo show the best recovery of mechanical sensitivity with near baseline measurements 30 and 60 minutes after injection. 0.1mg/kg Tirilizad also showed a significant effect, however this did not occur until 60 minutes after injection. Data are plotted as mean ± SEM. *p<0.05 compared to baseline mean values.
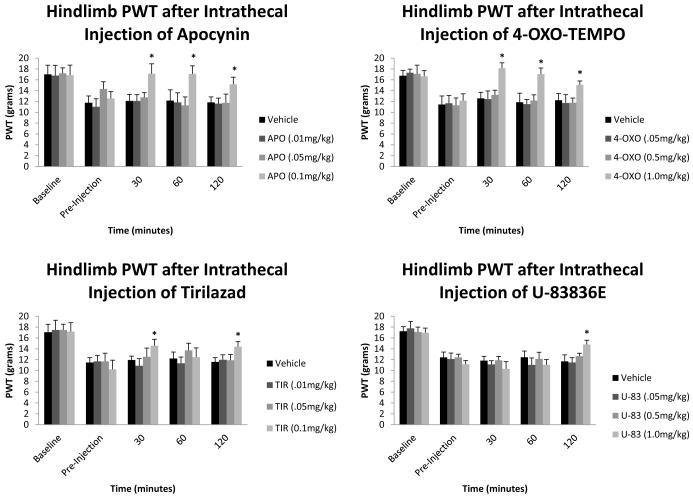
Figure 2 represents the changes in the hindlimb paw withdraw thresholds (PWT, grams) before SCI, before intrathecal injection, 30, 60, and 120 minutes after intrathecal injection. All hindlimb paw withdraw thresholds values in the pre-injection groups were significantly different from baseline values. The 30 minute, 60 minute, and 120 minute values for the vehicle group were not significantly different from the preinjection values. The 0.1mg/kg apocynin dosage group values of 30 minutes (17.14 ± 0.86), 60 minutes (17.11 ± 0.91), and 120 minutes (15.17 ± 0.87) were significantly different from the preinjection values. The 1.0mg/kg 4-oxo-tempo dosage group values of 30 minutes (18.15 ± 0.67), 60 minutes (17.05 ± 1.07), and 120 minutes (15.07 ± 0.87) were all significantly different from the 4-oxo-tempo preinjection values. The 0.1mg/kg tirilazad group values for 30 minutes (14.38 ± 0.48), and 120 minutes (14.23 ± 0.95) after injection were all significantly different from the preinjection values. The 1.0mg/kg U-83836E group value for 120 minutes after injection (14.57 ± 1.47) was the only value significantly different from its preinjection values.
Figure 2.
Hindlimb paw withdraw thresholds (PWT) after intrathecal injections of reactive oxygen species (ROS) and lipid peroxidation (LP) inhibitors. All baseline mean values are similar to each other with no significant difference from between groups. All pre-injection mean values are significantly different to the baseline mean values. 0.1mg/kg Apocynin and 1.0mg/kg 4-oxo-tempo show the best recovery of mechanical sensitivity, with both being significantly different at 30, 60, and 120 minutes after injection. 0.1mg/kg Tirilizad is also significantly different 30 and 120 minutes after injection. 1.0mg/kg U-83836E was significantly different 120 minutes after injection. Data are plotted as mean ± SEM. *p<0.05 compared to baseline mean values.
Discussion
Neuropathic pain is difficult to treat, because patients do not respond well to commonly prescribed analgesics (Murphy & Reid, 2001; Vissers, 2006). Underlying mechanisms of neuropathic pain are important to determine in order to create treatments that target these mechanisms and treat neuropathic pain. The present study tests four compounds that all reduce mechanical hypersensitivity in chronic SCI animals to different extents. However, the chemical mechanism of action is different for each compound and there may be off-target effects. By looking at the efficacy of each compound, insight into key processes can be gained in the ROS/LP pathway that affects chronic mechanical hypersensitivity. Apocynin and 4-oxo-tempo are effective and significantly reduced mechanical sensitivity in our model of chronic neuropathic pain. Apocynin, a NADPH oxidase inhibitor, reduces ROS by limiting the production of superoxides, which are the precursors of ROS. Apocynin has been shown to reduce inflammation in many other animal illness models as well as in animal models of nervous tissue damage (Valencia et al, 2012; Ghosh et al, 2012; Seo et al, 2012; Impellizzeri et al, 2011). Apocynin’s efficacy is likely due to its mechanism, since reducing superoxide would have a broad effect on the downstream ROS production. 4-oxo-tempo and other nitroxide scavengers, such as TEMPOL have been used in treating animal models of hypertension (Wilcox, 2010) and are potent scavengers of ROS radicals, particularly peroxynitrite (Carroll et al, 2000). The efficacy of 4-oxo-tempo is likely related to the broad effect that direct scavenging of oxidative radicals can have on chronic SCI animals, and may implicate peroxynitrite being a major contributor to generating chronic pain in SCI animals.
There was limited efficacy of both tirilazad and U-83836E, both of which are in the lazariod family of compounds. The mechanisms of both tirilazad and U-83836E target downstream lipid peroxidation process, by either scavenging the peroxyl radicals or stabilizing the bilayer membrane. Tirilazad effects peaked at the 60 minute measurements; this is most likely because the lipid peroxidation scavenging and membrane stabilization process has a longer time course when compared to the mechanisms of the other compounds. All the compounds seemed to persist longer in the hindlimbs when compared to the forelimb time course. This might have been due to the close proximity of the injection site to the lumbar enlargement, which subserves the hindlimbs. The limited activity of the lipophilic compounds may also be due to the limitations of solubility of the compounds. The experiments were limited to dosages that would remain soluble in the vehicle and at the pH 7.2-7.4, which is the same as that of the animals’ cerebrospinal fluid. It is possible that higher dosages of the lipophilic compounds may be more efficacious in reducing mechanical sensitivity in SCI animals; however, it is difficult to solubilize these compounds at higher dosages without altering the pH of the solution or using solvents that may adversely affect spinal tissue. By investigating the analgesic properties of these compounds we are able to gain better insight into the mechanism that ROS and lipid peroxidation play in chronic neuropathic pain. A broad approach to reducing superoxides and other downstream effects of ROS via scavenging is more likely to produce an analgesic effect than that of inhibiting lipid peroxidation products which could also reduce mechanical hypersensitivity, but it requires longer intervals to be effective. This study demonstrates the novel finding that compounds that inhibit or reduce reactive oxygen species or lipid peroxidation products reduce mechanical sensitivity in chronic spinal cord injury animals and should be considered when developing treatments for chronic neuropathic pain.
Acknowledgements
This work was supported by grants from The Dunn and The West Foundations, Mr. Liddell, Mission Connect of TIRR and NIH grants NS 11255 and 39161. The authors have no conflicts of interest to declare.
ARRIVE guidelines have been followed:
Yes
=> if No, skip complete sentence
=> if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
Footnotes
Conflicts of interest: none
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already included
References
- Bell KF. Insight into a neuron’s preferential susceptibility to oxidative stress. Biochem Soc Trans. 2013;41(6):1541–5. doi: 10.1042/BST20130245. [DOI] [PubMed] [Google Scholar]
- Blair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Clinical implications of recent advances in neurotrauma research. In: Salzman SK, Faden AI, editors. The Neurobiology of Central Nervous System Trauma. Oxford Univ. Press; New York: 1994. pp. 328–339. [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. In: Seil FJ, editor. Advances in Neurology. Vol. 59. Raven Press; New York: 1993. pp. 75–89. [PubMed] [Google Scholar]
- Cairns DM, Adkins RH, Scott MD. Pain and depression in acute traumatic spinal cord injury: origins of chronic problematic pain? Arch Phys Med Rehabil. 1996;77(4):329–35. doi: 10.1016/s0003-9993(96)90079-9. [DOI] [PubMed] [Google Scholar]
- Carroll RT, Galatsis P, Borosky S, Kopec KK, Kumar V, Althaus JS, Hall ED. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) Inhibits Peroxynitrite-Mediated Phenol Nitration. Chem. Res. Toxicol. 2000;13:294–300. doi: 10.1021/tx990159t. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Jensen TS. Spinal cord injury pain: mechanisms and treatment. Eur J Neurol. 2004 Feb;11(2):73–82. doi: 10.1046/j.1351-5101.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. Journal of Neuroinflammation. 2012;9:241. doi: 10.1186/1742-2094-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hassler SN, Hulsebosch CE. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 2013;154(9):1699–708. doi: 10.1016/j.pain.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Hall E. Novel inhibitors of iron-dependent lipid peroxidation for neurodegenerative disorders. Ann Neurol. 1992;32:137–42. doi: 10.1002/ana.410320724. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu G-Y, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Mechanisms and treatment strategies for chronic central neuropathic pain after spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2003;8:76–91. [Google Scholar]
- Impellizzeri D, Mazzon E, Esposito E, Paterniti I, Bramanti P, Cuzzocrea S. Effect of Apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radical Research. 2011;45(2):221–36. doi: 10.3109/10715762.2010.526604. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Consensus Report. 2011 [Google Scholar]
- Kallenborn-Gerhardt W, Schroder K, Geisslinger G, Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther. 2013;137(3):309–17. doi: 10.1016/j.pharmthera.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radical Biology & Medicine. 2001;31(4):430–39. doi: 10.1016/s0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- Murphy D, Reid DB. Pain treatment satisfaction in spinal cord injury. Spinal Cord. 2001;39(1):44–46. doi: 10.1038/sj.sc.3101094. [DOI] [PubMed] [Google Scholar]
- Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radical Biology & Medicine. 2010;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Seo J, Park JY, Choi J, Kim TK, Shin JH, Lee JK, Han PL. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. Neurobiology of Disease. 2012;32(28):9690–99. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanska J, Pawliczak R. Apocynin: Molecular Aptitudes. Mediatores of Inflammation. 2008;2008:1–10. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AG, Singh IN, Wang J, Carrico KM, Hall ED. Mitochondiral protection after traumatic brain injury by scavenging lipid peroxyl radicals. J. Neurochemistry. 2010;114:271–80. doi: 10.1111/j.1471-4159.2010.06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia A, Sapp E, Kimm JS, McClory H, Reeves PB, Alexander J, Ansong KA, Masso N, Frosch MP, Kegel KB, Li X, DiFiglia M. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntinton’s disease. Human Molecular Genetics. 2012;22(6):1112–31. doi: 10.1093/hmg/dds516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vissers KC. The clinical challenge of chronic neuropathic pain. Disabil Rehabil. 2006;28(6):343–9. doi: 10.1080/09638280500287270. [DOI] [PubMed] [Google Scholar]
- Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury-relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004;42:665–673. doi: 10.1038/sj.sc.3101641. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Chronic pain after spinal injury: interference with sleep and daily activities. Arch Phys Med Rehabil. 2001;82:1571–1577. doi: 10.1053/apmr.2001.26068. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacology & Therapeutics. 2010;126:119–45. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]