Abstract
Serum C-reactive protein (CRP) is a sensitive marker of systemic inflammation. Since there is a well-recognized relationship between local inflammation and colorectal cancer, we aimed to evaluate whether serum CRP levels were associated with the occurrence of colorectal adenomas and serrated polyps using data from a large adenoma prevention trial. 930 participants with a history of colorectal adenomas were enrolled in a randomized trial of calcium supplementation (1200 mg/day) for the prevention of colorectal adenomas. Outcomes in this analysis are metachronous adenomas (and advanced neoplasms specifically), and serrated polyps at follow-up colonoscopy. High sensitivity CRP levels were measured 1 year following baseline colonoscopy. Multivariate analysis was performed to estimate risk ratios (RR) using Poisson regression, controlling for potential confounders. We measured serum CRP levels in 689 participants(mean CRP 3.62mg/L ± 5.72). There was no difference in CRP levels with respect to calcium vs. placebo treatment assignment (p=0.99). After adjustment for potential confounders, we found no association between CRP level and risk of recurrent adenoma or advanced lesion (Quartile 4 vs. Quartile 1: RR (95% CI) = 0.99 (0.73, 1.34) and 0.92 (0.49, 1.75) respectively). Similarly, no association was seen between CRP levels and riskof serrated polypsor proximal serrated polyps(Quartile 4 vs. Quartile 1: RR (95% CI) = 1.32 (0.85, 2.03) and 1.19 (0.54, 2.58) respectively. In conclusion, this large prospective colorectal adenoma chemoprevention study found no significant relationship between CRP levels and occurrence of adenomas, advanced neoplasms, or serrated polyps.
Keywords: C-Reactive Protein [MeSH], Inflammation [MeSH], Colon Polyps [MeSH], serrated polyps, serrated neoplasia
Introduction
Inflammation is associated with colorectal carcinogenesis(1). Serum C-reactive protein (CRP) is a sensitive yet nonspecific marker of systemic inflammation that is elevated in association with chronic inflammatory conditions(2),coronary heart disease (3), obesity (4), and various cancers (5).Several case-control studies have reported elevated circulating CRP concentrations in CRC patients compared to healthy controls (6, 7). Additionally, a recent systematic review of prospective studies reported an increased risk of CRC associated with CRP elevation(8).
However, published data are less conclusive regarding the association between serum CRP and colorectal adenomas. While some studies have reported that CRP is associated with higher risk of colorectal adenomas (9, 10), others have found null (11-13), or even inverse associations(14). To our knowledge, no study has previously examined the association between inflammatory markers and serrated polyps (SPs), yet some of these lesions are now understood to be important CRC precursors as well.
A correlation of serum CRP levels with colonoscopic findings would suggest an important role of inflammation in the early phases of colorectal carcinogenesis. CRP could be a useful clinical biomarker for risk stratification and/or screening test selection. In this context, we aimed to investigate whether CRP elevation is associated with an increased risk of adenoma or SPoccurrence using data from a large polyp chemoprevention trial.
Materials and Methods
Study design and participants
The details of the study design have beenpublished elsewhere(15). Briefly,the Calcium Polyp Prevention Study was conducted at 6 centers across the US between November 1988 and December 1996. Subjects were recruited for participation if they had an index colonoscopy with at least 1 large-bowel adenoma within 3 months (and no remaining polyps in the large bowel), were >21 to <80 years old, in good health, and without a history of familial polyposis or invasive large-bowel cancer. Baseline data collection included demographics (age, gender, race), smoking status, and measurement of height and weight. Body mass index (BMI) was calculated as[weight (kg)]/[height (m)]2and obesity was defined as a BMI ≥30 kg/m2. 930 subjects underwent randomization to calcium carbonate (3 g daily; 1.2 g elemental calcium) or placebo in blinded fashion. Participants underwent follow-up colonoscopies at approximately one and four years after the baseline examination to determine occurrence of colorectal polyps (Figure 1). All participants provided written informed consent and this study was approved by the Institutional Review Boards at all study sites.
Figure 1. Study schematic showing timing of C-reactive protein measurement and study colonoscopies.
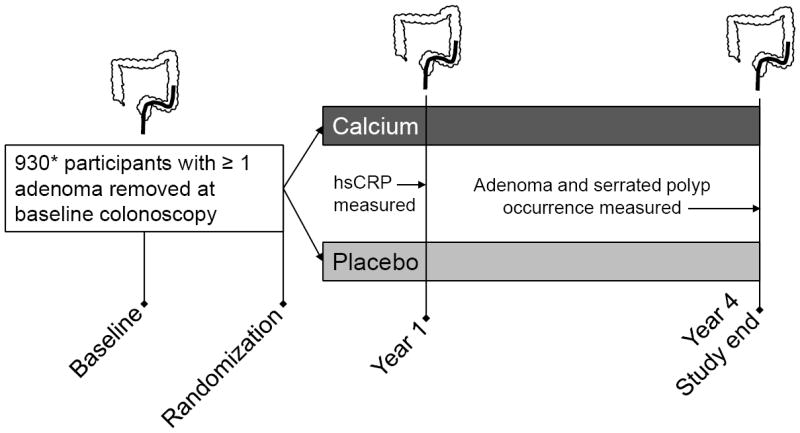
*689/930 subjects had both a year 4 colonoscopy and a CRP measurement and were included in the study. Reasons for missing CRP data included refusal of blood draw or blood sample not obtained (n=17), unevaluable specimen or lab error (n=11), and unavailable or missing samples (n=115).hsCRP: high sensitivity C-reactive protein
C-reactive protein measurement
At enrollment and around the time of each of the two follow-up colonoscopies, we obtained specimens of venous blood. Serum was initially stored at -20°C or below, pending shipment to the central laboratory (Dartmouth) for storage at -70°C until analysis. High sensitivity CRP measurements were performed on blood samples obtained at the year 1 follow up visit. At the time of blood collection, participants had been taking the study drug for approximately 9 months. Serum CRP was measured using CRPHS Tina-quant testing kits on a Roche/Hitachi modular P analyzer with a limit of detection: 0.03 mg/L (0.00003 g/L).
Outcome measurement
For this study, only participants who had colonoscopies at year 4 were included and the risk period was the time between years 1 and 4 following baseline colonoscopy. At each colonoscopy, the endoscopist recorded the size and location of all mucosal lesions. All polyps seen after randomization were removed and examined histologically by the study pathologist. Advanced neoplasms were defined as lesions with >25% villous histology, size ≥1 cm or high-grade dysplasia/cancer.Although current understanding is that SPscan be categorized as hyperplastic polyps (HP), sessile serrated adenomas (SSA) and traditional serrated adenomas (TSA)(16), at the time this study was done, essentially all serrated lesions were interpreted as HPs. Proximal (or right-sided)SPs were defined as polyps occurring in the cecum, ascending and transverse colon. We also performed a secondary cross-sectional analysis of year 1 colonoscopy results.
Statistical analysis
CRP was analyzed as a categorical variable (dichotomous, split at the median, and into quartiles). Bivariate comparisons were performed using Student’s t-tests or Chi-squared tests. Generalized linear models were performed using a Poisson distribution to estimate risk ratios (RR) and 95% confidence intervals (CI)adjusted for age, sex, center, baseline adenoma history, treatment arm, and surveillance colonoscopy interval. Quartile 1 (Q1) and median or below CRP levels were the referent groups used in modeling. Statistical analyses were performed using STATA (College Station, TX, USA) and SAS (Cary, NC, USA).
Results
Participant characteristics
Baseline characteristics of participants are presented in Table 1. A total of 832 participants underwent a year 4 follow up colonoscopy;689 (83%) of these had CRP data and could be included in this analysis. There was no difference between the study treatment groups and those excluded for lack of CRP data with respect to age, gender, BMI, or smoking status (data not shown).
Table 1.
Circulating serum high sensitivity C-reactive protein level by baseline subject characteristics
| Baseline characteristic | n | Serum CRP mean (SD) | p value |
|---|---|---|---|
| Age | 0.01 | ||
| <50 | 96 | 1.20 (2.96) | |
| 50-59 | 176 | 1.57 (3.32) | |
| 60-69 | 316 | 1.85 (3.80) | |
| ≥70 | 101 | 2.10 (3.37) | |
|
| |||
| Gender | 0.01 | ||
| Male | 496 | 1.58 (3.52) | |
| Female | 193 | 2.07 (3.46) | |
|
| |||
| Race/Ethnicity | <0.0001 | ||
| White | 588 | 1.69 (3.40) | |
| Black | 48 | 3.14 (3.63) | |
| Hispanic | 23 | 1.80 (2.78) | |
| Other | 30 | 0.66 (4.54) | |
|
| |||
| BMI | <0.0001 | ||
| <25 | 212 | 1.21 (3.57) | |
| 25-29 | 309 | 1.62 (3.31) | |
| 30+ | 166 | 2.86 (3.33) | |
|
| |||
| Smoking Status | 0.03 | ||
| Never | 230 | 1.48 (3.47) | |
| Former | 337 | 1.72 (3.56) | |
| Current | 122 | 2.15 (3.43) | |
|
| |||
| Study treatment | 0.99 | ||
| Placebo | 349 | 1.70 (3.62) | |
| Calcium | 340 | 1.70 (3.43) | |
|
| |||
| Number of baseline adenomas | 0.30 | ||
| 1 | 380 | 1.77 (3.54) | |
| 2 | 155 | 1.48 (3.56) | |
| 3 or more | 154 | 1.77 (3.45) | |
|
| |||
| Advanced adenoma at baseline | 0.95 | ||
| No | 442 | 1.70 (3.46) | |
| Yes | 247 | 1.71 (3.64) | |
|
| |||
| Hyperplastic polyp at baseline | 0.20 | ||
| No | 510 | 1.64 (3.48) | |
| Yes | 179 | 1.89 (3.63) | |
p values reflect differences in log-transformed CRP between categories, and were obtained using Student’s t-tests
2 subjects had unknown BMI
BMI: body mass index; SD: Standard deviation; CRP: C-reactive protein.
Serum CRP levels
The mean and median CRP serum levels among all participants were 3.62 and 1.79mg/L respectively (Standard deviation 5.72; interquartile range 0.75, 3.94). For reference, CRP levels >10mg/L suggest active infection or inflammation, so most participants in this study had low levels.Age, female sex, African American race, obesity, and smoking were associated with higher mean CRP levels (Table 1). CRP levels did not differ significantly based on baseline adenoma or SP status. There was no difference in CRP level(log-transformed) with respect to randomization to calcium vs. placebo treatment (p=0.99).
Serum CRP andadenoma recurrence
The RR for adenomas andadvanced neoplasms did not vary with CRP levels in crude analyses. After adjustment for age, sex, treatment group, study center, colonoscopy interval, and adenoma history, there was also no statistically significant association between CRP level and risk of metachronous adenomas(Q4 vs. Q1: RR = 0.99 (95% CI 0.73, 1.34)), or advanced neoplasms (Q4 vs. Q1: RR = 0.92 (95% CI 0.49, 1.75))(Table 2). Analysis of dichotomized CRP levels revealed similar results. There was also no association between CRP levels and adenomas found on the year 1 colonoscopy (Supplementary table 1). Risk for advanced neoplasms at the year 1 exam did appear to be increased among subjects whose CRP was in the highest quartile, though this estimate was imprecise with wide confidence intervals. In all analyses,additional adjustment for BMI did not materially affect RR’s found (data not shown).
Table 2.
Risk ofconventional adenomas and serratedpolypsduring follow-up (years 2-4) associated with categories of circulating serum high sensitivity C-reactive protein levels.
| CRP levels | # events/N (%) | Crude RR (95% CI) | Adjusted* RR (95% CI) |
|---|---|---|---|
| All Adenomas | |||
|
| |||
| Dichotomous | |||
| ≤median | 121/337 (35.9%) | 1.00 (REF) | 1.00(REF) |
| >median | 126/335 (37.6%) | 1.05 (0.86, 1.28) | 0.99 (0.80, 1.21) |
| Quartiles | |||
| Q1: ≤0.74mg/L | 55/164 (33.5%) | 1.00 (REF) | 1.00 (REF) |
| Q2: 0.74-1.785mg/L | 66/173 (38.2%) | 1.14 (0.86, 1.51) | 1.11 (0.83, 1.49) |
| Q3: 1.785-3.90mg/L | 66/169 (39.1%) | 1.16 (0.88, 1.55) | 1.09 (0.82, 1.46) |
| Q4: >3.90mg/L | 60/166 (36.1%) | 1.08 (0.80, 1.44) | 0.99 (0.73, 1.34) |
|
| |||
| Advanced Neoplasms† | |||
|
| |||
| Dichotomous | |||
| ≤median | 30/330 (9.1%) | 1.00 (REF) | 1.00 (REF) |
| >median | 36/325 (11.1%) | 1.22 (0.77, 1.93) | 0.98 (0.62, 1.55) |
| Quartiles | |||
| Q1: ≤0.74mg/L | 16/162 (9.9%) | 1.00 (REF) | 1.00 (REF) |
| Q2: 0.74-1.785mg/L | 14/168 (8.3%) | 0.84 (0.43, 1.67) | 0.85 (0.43, 1.67) |
| Q3: 1.785-3.90mg/L | 17/164 (10.4%) | 1.05 (0.55, 2.01) | 0.89 (0.47, 1.70) |
| Q4: >3.90mg/L | 19/161 (11.8%) | 1.19 (0.63, 2.25) | 0.92 (0.49, 1.75) |
|
| |||
| Serrated Polyps | |||
|
| |||
| Dichotomous | |||
| ≤median | 69/343 (20.1%) | 1.00 (REF) | 1.00 (REF) |
| >median | 75/346 (21.7%) | 1.08 (0.81, 1.44) | 1.10 (0.81, 1.48) |
| Quartiles | |||
| Q1: ≤0.74mg/L | 32/167 (19.2%) | 1.00 (REF) | 1.00 (REF) |
| Q2: 0.74-1.785mg/L | 37/176 (21.0%) | 1.10 (0.72, 1.67) | 1.19 (0.77, 1.84) |
| Q3: 1.785-3.90mg/L | 35/173 (20.2%) | 1.06 (0.69, 1.62) | 1.10 (0.71, 1.71) |
| Q4: >3.90mg/L | 40/173 (23.1%) | 1.21 (0.80, 1.83) | 1.32 (0.85, 2.03) |
|
| |||
| Proximal Serrated Polyps | |||
|
| |||
| Dichotomous | |||
| ≤median | 24/343 (7.0%) | 1.00 (REF) | 1.00 (REF) |
| >median | 23/346 (6.7%) | 0.95 (0.55, 1.65) | 1.00 (0.59, 1.71) |
| Quartiles | |||
| Q1: ≤0.74mg/L | 11/167 (6.6%) | 1.00 (REF) | 1.00 (REF) |
| Q2: 0.74-1.785mg/L | 13/176 (7.4%) | 1.12 (0.52, 2.44) | 1.17 (0.55, 2.48) |
| Q3: 1.785-3.90mg/L | 11/173 (6.4%) | 0.97 (0.43, 2.17) | 1.01 (0.46, 2.22) |
| Q4: >3.90mg/L | 12/173 (6.9%) | 1.05 (0.48, 2.33) | 1.19 (0.54, 2.58) |
CRP: C-reactive protein; RR: risk ratio; CI: confidence interval; Q: quartile
Adjusted for treatment arm, age, sex, center, baseline adenoma history, and surveillance colonoscopy interval; RRs obtained by GLM with Poisson distribution.
Advanced neoplasm defined as an adenoma ≥10mm, and/or with villous histology, or high grade dysplasia/cancer
Serum CRP and occurrence of serrated polyps
Higher CRP levels were also not associated with increased risk of SPs in crude analyses. Multivariable analysis also did not demonstrate an association between CRP level and risk of any SP(Q4 vs. Q1: RR = 1.32 (95% CI 0.85, 2.03)) or right-sided SP (Q4 vs. Q1: RR = 1.19 (95% CI 0.54, 2.58)) occurrence during follow-up (Table 2). CRP levels greater than the median were also not associated with occurrence of any SPor proximal SPs specifically.There was also no association between CRP levels and SPs found on the year 1 colonoscopy (Supplementary table 1).
Discussion
In this large multi-center prospective study, we found that CRP levels were not associated with an increased risk of recurrence of conventional adenomas. We also did not find any association between CRP levels and occurrence of SPs during follow up. In addition, we found no effect of calcium supplementation on CRP levels, similar to other studies(17, 18).
CRP is an acute-phase protein that is synthesized in the liver in response to proinflammatory cytokine signaling, primarily via interleukin-6 and tumor necrosis factor alpha. (19). An important role of CRP is to bind phosphocholine on pathogens as well as apoptotic or necrotic host cells, which in turn activates the complement system and recruits phagocytes(19).Serum CRP rises rapidly in response to tissue injury or infection, but CRP elevation (generally at low levels) is also seen in chronic inflammatory or neoplastic states, a process that is likely mediated by different signaling mechanisms(20).
Whether CRP levels are associated with the development of adenomas and SPsis an important question, since these are the chief knownprecursor lesions of sporadic CRC. If elevated serum CRP levels predict polyp risk, this could have important implications for CRC screening and prevention. However, the existing literature on this topic is inconsistent. A recent Japanese study reported that CRP levels> 90th percentile were associated with a >2-fold increase in the odds of large colorectal adenomas(9). A cross-sectional Chinese study also reported that elevated CRP levels were associated with increased risk of advanced adenomas and multiple adenomas(10). However, several studies have reported no significant association between CRP and adenomas(11-13).Interestingly, one study suggested that CRP levels may be inversely associated with risk of colorectal neoplasia; using data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial, Gunter et al. reported that participants with the highest CRP levels had the lowest risk of incident colorectal adenoma (Q4 vs. Q1: OR 0.65 (95% CI 0.41, 1.03, p for trend = 0.03)(14).
A possible explanation for discordant risk of colorectal adenomas seen in different studies may be related to differences in CRP metabolism among different subjects. A number of studies have demonstrated that CRP levels vary based on genetic variations in the CRP gene (21, 22).A few studies have investigated CRP gene polymorphisms and risk of colorectal neoplasia. Ognjanovic et al. reported that certain single nucleotide polymorphisms (SNP) in the CRP gene (rs1205C and rs1130864A) were associated with both higher CRP levels and a decreased adenoma risk(13).In contrast, Poole et al. also reporteddifferent CRP polymorphisms that were associated with increased risk of both adenomas and hyperplastic polyps(23).CRP gene polymorphisms have also been associated with the risk of CRC, and specificallyCPG-island methylator phenotype (CIMP) cancers (24).Given these findings, it is possible that heterogeneity of CRP alleleswithin the study population and the associated differences in CRP elevations and corresponding risk of polyps could explain our and other reported null associations between CRP levels and CRC precursors.
We are not aware of any other studies that have examined the relationship between CRP serum levels and SPs. Because a keymechanism of SP development is thought to be inhibition of apoptosis(25) and CRP plays a role in apoptosis, this relationship could shed light on the molecular pathology of the serrated pathway. In this study, we found no substantial relationship between CRP levels and occurrence of SPs or right-sided SPs in particular. Nevertheless, it is possible that CRP might be associated with more advanced SPs such as SSAs or TSAs. Additionally, relatively small numbers of SPs limited our ability to detect a statisticallysignificant association.
This was a large, prospective study of participants with a history of colorectal adenomas. CRP measurements were obtained in standardized fashion from serum collected at 1 year after their baseline colonoscopy, which we believed to be an optimal time to measure a potential biomarker of colonic neoplasia. All pathology from follow up colonoscopies was centrallyreviewed, to optimize the fidelity of outcome measurements (i.e. adenoma and SP occurrence). Furthermore, we used a high-sensitivity CRP assay because it measures very low levels of CRP, an important consideration in our cohort of relatively healthy colonoscopy screenees. However, CRP may only be associated with risk of adenoma recurrence at very high levels, and thus the distribution of our data (most subjects had very low CRP measurements) could obscure this relationship. We considered the possibility that obesity confounded the association between CRP and polyp occurrence. However, when we adjusted for BMI, our estimates did not change substantially.It is possible that change or rise in CRP levels may correlate with polyp occurrence, but we were unable to determine this based on our data. Finally not all participants in the parent trial provided CRP samples, which could introduce selection bias, though the non-participation rate was relatively small, and those with missing CRP data were similar with respect to age, gender, race, BMI and smoking status compared to those included in this analysis.
In summary, in this large prospective study, we found no significant relationship between CRP levels and occurrence of adenomasor serrated polyps. It is possible however, that CRP is a more useful marker of colorectal neoplasia during latter stages of carcinogenesis.
Supplementary Material
Acknowledgments
Financial support: This work was supported in part, by NIH training grant T32DK007634 (SDC). The original study was supported in part by grants from the NIH(CA37287, U01 CA046927, and CA23108, PI: Baron).
Footnotes
Conflicts of interest: None to declare for all authors
References
- 1.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 2.Amos RS, Constable TJ, Crockson RA, Crockson AP, McConkey B. Rheumatoid arthritis: relation of serum C-reactive protein and erythrocyte sedimentation rates to radiographic changes. Br Med J. 1977;1:195–7. doi: 10.1136/bmj.1.6055.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unek IT, Bayraktar F, Solmaz D, Ellidokuz H, Sisman AR, Yuksel F, et al. The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clinical medicine & research. 2010;8:89–95. doi: 10.3121/cmr.2010.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 6.Dymicka-Piekarska V, Matowicka-Karna J, Gryko M, Kemona-Chetnik I, Kemona H. Relationship between soluble P-selectin and inflammatory factors (interleukin-6 and C-reactive protein) in colorectal cancer. Thrombosis research. 2007;120:585–90. doi: 10.1016/j.thromres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Zaloudik J, Lauerova L, Janakova L, Talac R, Simickova M, Nekulova M, et al. Significance of pre-treatment immunological parameters in colorectal cancer patients with unresectable metastases to the liver. Hepato-gastroenterology. 1999;46:220–7. [PubMed] [Google Scholar]
- 8.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123:1133–40. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 9.Otake T, Uezono K, Takahashi R, Fukumoto J, Tabata S, Abe H, et al. C-reactive protein and colorectal adenomas: Self Defense Forces Health Study. Cancer Sci. 2009;100:709–14. doi: 10.1111/j.1349-7006.2009.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu HM, Lin JT, Chen TH, Lee YC, Chiu YH, Liang JT, et al. Elevation of C-reactive protein level is associated with synchronous and advanced colorectal neoplasm in men. The American journal of gastroenterology. 2008;103:2317–25. doi: 10.1111/j.1572-0241.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan AT, Sima CS, Zauber AG, Ridker PM, Hawk ET, Bertagnolli MM. C-reactive protein and risk of colorectal adenoma according to celecoxib treatment. Cancer Prev Res (Phila) 2011;4:1172–80. doi: 10.1158/1940-6207.CAPR-10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsilidis KK, Erlinger TP, Rifai N, Hoffman S, Hoffman-Bolton J, Helzlsouer KJ, et al. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer causes & control : CCC. 2008;19:559–67. doi: 10.1007/s10552-008-9117-x. [DOI] [PubMed] [Google Scholar]
- 13.Ognjanovic S, Yamamoto J, Saltzman B, Franke A, Ognjanovic M, Yokochi L, et al. Serum CRP and IL-6, genetic variants and risk of colorectal adenoma in a multiethnic population. Cancer Causes Control. 2010;21:1131–8. doi: 10.1007/s10552-010-9540-7. [DOI] [PubMed] [Google Scholar]
- 14.Gunter MJ, Cross AJ, Huang WY, Stanczyk FZ, Purdue M, Xue X, et al. A prospective evaluation of C-reactive protein levels and colorectal adenoma development. Cancer Epidemiol Biomarkers Prev. 2011;20:537–44. doi: 10.1158/1055-9965.EPI-10-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 16.Snover D, Ahnen D, Burt R, Odze R. Serrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposis. In: Bozman F, Carneiro F, Hruban R, Theise N, editors. WHO Classification of Tumours of the Digestive System. Berlin: Springer-Verlag; 2010. [Google Scholar]
- 17.Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, et al. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res (Phila) 2011;4:1645–54. doi: 10.1158/1940-6207.CAPR-11-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grey A, Gamble G, Ames R, Horne A, Mason B, Reid IR. Calcium supplementation does not affect CRP levels in postmenopausal women--a randomized controlled trial. Osteoporos Int. 2006;17:1141–5. doi: 10.1007/s00198-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 19.Volanakis JE. Human C-reactive protein: expression, structure, and function. Molecular immunology. 2001;38:189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 20.Kushner I, Samols D, Magrey M. A unifying biologic explanation for “high-sensitivity” C-reactive protein and “low-grade” inflammation. Arthritis care & research. 2010;62:442–6. doi: 10.1002/acr.20052. [DOI] [PubMed] [Google Scholar]
- 21.Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, et al. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation. 2006;114:2458–65. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- 22.Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296:2703–11. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 23.Poole EM, Bigler J, Whitton J, Sibert JG, Potter JD, Ulrich CM. C-reactive protein genotypes and haplotypes, polymorphisms in NSAID-metabolizing enzymes, and risk of colorectal polyps. Pharmacogenet Genomics. 2009;19:113–20. doi: 10.1097/FPC.0b013e32831bd976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slattery ML, Curtin K, Poole EM, Duggan DJ, Samowitz WS, Peters U, et al. Genetic variation in C-reactive protein in relation to colon and rectal cancer risk and survival. Int J Cancer. 2011;128:2726–34. doi: 10.1002/ijc.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higuchi T, Jass JR. My approach to serrated polyps of the colorectum. J Clin Pathol. 2004;57:682–6. doi: 10.1136/jcp.2003.015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


