Abstract
BACKGROUND
Hodgkin lymphoma (HL) survivors have significant cardiovascular risk and require long-term surveillance. This study assessed the prevalence of coronary artery disease (CAD) by coronary computed tomographic angiography (CCTA) in adult survivors of childhood HL.
METHODS
Thirty-one HL survivors, 13 (42%) treated with radiation therapy (RT) only and 18 (58%) with multimodal therapy, underwent CCTA, echocardiography, electrocardiography (ECG), and treadmill stress testing. Obstructive CAD was defined as ≥50% occlusion of the left main (LM) or ≥70% occlusion of the left anterior descending (LAD), left circumflex (LCx), or right coronary (RCA) arteries on CCTA. Echocardiograms with resting wall motion abnormalities or ejection fraction <50%; ECGs with Q waves, ST abnormalities without Q waves, or T-wave abnormalities without Q waves; and ≥1-mm J-point depression with horizontal or down-sloping ST segment on stress testing were considered abnormal.
RESULTS
Prevalence of disease in participants (median age, 40 years; range, 26–55; median time from cancer diagnosis, 24 years; range, 17–39) was 39%, with 39 plaques detected in 12 survivors. Three participants (10%) treated with RT only had 4 obstructive lesions; 9 (29%; 5 treated with RT only; 4 with multimodal therapy) had nonobstructive lesions. Approximately 15% of lesions involved the LM; 21%, proximal LAD; 18%, proximal RCA; and 13%, proximal LCx arteries. Of the 12 participants with CAD by CCTA, 7 had positive ECG; 1, positive echocardiography; and 1, a positive stress test.
CONCLUSIONS
CCTA identified CAD in a substantial portion of HL survivors and may be an effective screening modality for this population.
Keywords: cardiovascular, Hodgkin lymphoma, CT angiography, coronary artery disease, radiotherapy
INTRODUCTION
The successful treatment of pediatric Hodgkin lymphoma (HL) has evolved over the last 5 decades from treatment with high-dose radiotherapy (RT) alone to risk- and response-adapted regimens combining chemotherapy with lower radiation doses and smaller nodal fields. Recent protocols are now testing the elimination of radiation altogether.1 During the 1960s and 1970s, radiation doses ≥35 Gy directed at wide nodal fields were frequently curative for patients with localized disease but ineffective for those with advanced systemic disease.2 The introduction of multimodal therapy and refinements in staging, imaging, and response assessments have increased 5-year survival rates for pediatric HL to nearly 97%.3
However, survival rates over time are lower compared to expected rates in the US population, with only 74.1% (95% CI, 71.8%–76.6%) of HL survivors alive at 30 years from diagnosis.4 Cardiovascular disease, the leading noncancer cause of death among survivors of HL, has been associated with exposure to anthracyclines and mediastinal radiation and can manifest as coronary artery disease (CAD), congestive heart failure, and/or cerebrovascular accidents.5,6 The highest risk has been reported in patients <25 years of age at treatment, among whom the 30-year cumulative incidence rates for any cardiovascular disorder and myocardial infarction are 34.5% and 12.9%, respectively.7 These risks increase with longer follow-up.
Monitoring for cardiovascular disease, proposed by several groups,8–10 has focused on cardiomyopathy screening with recommendations for measurement of cardiac function in survivors exposed to cardiac-directed radiation and/or anthracyclines. Additional cardiac imaging recommendations are directed by exposure factors and results of the initial screening. Cardiology consultation is suggested at 5 to 10 years after radiation exposure to evaluate for CAD among those treated with ≥40 Gy alone or ≥30 Gy plus an anthracyline.8
Data describing coronary computed tomographic angiography (CCTA) as a tool to screen for CAD in asymptomatic, high-risk HL survivors are limited. Rademaker et al11 reported CAD in 8 of 9 HL survivors who had been treated with 34 to 45 Gy of mediastinal radiation. None of these patients had electrocardiogram (ECG) findings suggestive of acute or prior ischemia. Küpeli et al12 used CCTA to evaluate 119 asymptomatic HL survivors (mean age, 20 y; range, 6–43 y) who had been treated with RT and/or cardiotoxic chemotherapy ≥2 years previously; coronary artery abnormalities were reported in 19 (16%) patients. The risk was significantly higher among those treated with mediastinal radiation >20 Gy (RR, 6.8; 95% CI, 1.6–28.8) compared to those exposed to ≤20 Gy. Subclinical CAD in a high-risk population of older survivors (mean age, 40 y) further from diagnosis has not yet been investigated with CCTA.
Our aim was to determine the prevalence of CAD detected by CCTA in asymptomatic long-term survivors of HL exposed to chest RT alone or multimodal therapy (RT plus chemotherapy), and to compare these results with standard noninvasive screening tests.
MATERIALS AND METHODS
Cases for this study were participants in the St. Jude Lifetime Cohort (SJLIFE) Study, an ongoing study of patients previously treated at St. Jude Children’s Research Hospital (SJCRH) that was designed to facilitate longitudinal evaluation of health outcomes among adults previously treated for a pediatric malignancy.13 Medical histories and detailed treatment information are abstracted from the medical records, and participants undergo a core assessment battery and risk-based medical screening based on the Children’s Oncology Group’s Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers.8 Informed consent was obtained from all participants, and the protocol was approved by the SJCRH Institutional Review Board.
A convenience sample of HL survivors participating in the cohort was recruited for this pilot protocol. Eligible patients were ≥15 years past HL diagnosis, ≤55 years old, and had received treatment with chest RT alone or multimodal therapy. Participants were excluded if they had an implanted medical device, irregular cardiac rhythm, or CT contrast allergy; were unable to hold their breath for CT imaging or walk on a treadmill; or were pregnant. Additionally, participants with a history of congenital heart disease, congestive heart failure, myocardial infarction, or coronary artery revascularization (percutaneous or surgical) were not included.
Survivors with a body mass index (BMI) ≥25 kg/m2 and <30 kg/m2 were considered overweight; those with a BMI ≥30 kg/m2 were considered obese. Hypertension was defined as blood pressure ≥140/90 mmHg and/or treatment with an antihypertensive. Dyslipidemia was defined as any abnormality on a fasting lipid panel (total cholesterol >200 mg/dL; LDL-C >130 mg/dL; HDL-C <40 mg/dL; triglycerides >150 mg/dL) and/or treatment with a lipid-lowering agent.
Coronary Artery Evaluation With CCTA
CCTA was performed using a 64-detector CT scanner (Light Speed VCT; GE Healthcare, Milwaukee, Wis). A power injector (EZEM Empower; Bracco Diagnostics, Monroe Township, NJ) was used to deliver contrast media through an 18-gauge needle in an antecubital vein. Depending on patient weight, 80 to 120 mL of iohexol 350 mg iodine/mL (Omnipaque 350; GE Healthcare) was administered.
A test bolus was used to determine the timing of scan delay and image acquisition. Primary scanning parameters were as follows: 64 detectors; individual detector width, 0.625 mm; gantry rotation time, 350 ms; pitch, 0.24; tube voltage, 120 kV; and tube current, 550 mA during 40% to 80% RR interval, decreased to as low as 200 mA during the remainder of the RR interval for retrospective technique.
Prospective low-dose scanning was performed if the patient’s heart rate was regular and <65 beats/min. Patients with a prescan heart rate of ≥65 beats/min were given 50 mg of metoprolol orally 1 hour before scanning. Retrospective technique with tube current modulation was used if heart rate was >65 and ≤85 beats/min. CCTA was not performed if the heart rate was >85 beats/min. On one occasion, an initial prospective scan was unsatisfactory, and a second scan was performed with retrospective technique. Thirteen scans used prospective technique; 18 used retrospective technique.
CCTA Image Analysis
The coronary tree was segmented according to the modified American Heart Association classification.14 Original source images, selected maximum-intensity projections, and curved multiplanar reconstructions were individually evaluated by one examiner (SDF). Obstructive CAD was defined as ≥50% occlusion of the left main (LM) coronary artery or ≥70% occlusion of the left anterior descending (LAD) artery, left circumflex (LCx) artery, or right coronary artery (RCA).
Other Screening Methods
Two-dimensional Doppler ultrasound echocardiography (VIVID-7; GE Healthcare) was performed per the American Society of Echocardiography guidelines.15 Resting wall motion abnormalities or ejection fraction <50% was considered abnormal.
Twelve-lead ECG was performed and centrally coded per the Novacode classification system. Tracings were considered positive for CAD if they coded high likelihood of Q-wave MI (Q-wave MI with major Q waves or Q-wave MI with moderate Q waves with ST-T abnormalities), moderate likelihood of Q-wave MI (possible Q-wave MI with moderate Q-waves without ST-T abnormalities or possible Q-wave MI with minor Q-waves with ST-T abnormalities), or isolated ischemic abnormalities (ST abnormalities without Q-waves or T-wave abnormalities without Q-waves).16
Treadmill stress testing was performed per the Balke protocol, where the walking speed was held constant at 3 km/h while the grade was increased by 2.5% every 2 minutes. J-point depression ≥1 mm with horizontal or down-sloping ST segment was considered positive for CAD.
Statistical Analysis
The utility of CCTA in detecting CAD was evaluated by comparing the prevalence of abnormalities detected by CCTA and other screening tests such as echocardiography, ECG, and treadmill stress testing.
Radiation exposure from the CCTA scan was only available for 19 study participants. The two primary factors affecting exposure were the type of gating used and the patient’s body habitus. Fisher’s exact test was used to assess whether the frequency distribution of prospective and retrospective ECG-gated coronary artery acquisitions differed significantly between the 2 groups, and a 2-sample t-test, assuming unequal variances, was used to assess whether the BMI distribution significantly differed between the 2 groups (19 with CCTA radiation dose vs 12 for whom dose was unavailable).
RESULTS
Forty-five SJLIFE participants agreed to additional participation in this study. Thirteen could not complete the study because of contrast allergy (n = 2), tachycardia (n = 5), renal failure (n = 1), failed IV access (n = 2), or refusal (n = 3). Of the 31 survivors (69%) enrolled, 13 were treated with RT alone and 18 were treated with multimodal therapy. The median age of patients was 40 years (range, 26–55 y) at the time of evaluation, and the median time from initial diagnosis was 24 years (range, 17–39 y) (Table 1). Those treated with RT only were slightly older (median, 42 y; range, 37–55 y) than those treated with multimodal therapy (median, 38 y; range, 26–46 y). Patients treated with RT only were also further from therapy (median, 33 y; range, 21–39 y) than those treated with multimodal therapy (median, 22 y; range, 18–32 y). Cardiovascular risk factors are detailed in Table 1. The majority of patients were considered low risk, based on National Cholesterol Education Program Adult Treatment Panel (NCEP ATPIII) risk scoring for asymptomatic adults,17 and only one would have met recommendations for coronary artery calcium screening.18
TABLE 1.
Demographic and Treatment Characteristics of HL Survivors
| Patient Characteristic | Total (n = 31) n (%) |
Radiotherapy Only (n = 13) n (%) |
Multimodal Therapy (n = 18) n (%) |
|---|---|---|---|
| Sex | |||
| Male | 12 (39) | 5 (39) | 7 (39) |
| Female | 19 (61) | 8 (62) | 11 (61) |
| Age, y | |||
| 25–34 | 1 (3) | 0 (0) | 1 (6) |
| 35–44 | 25 (81) | 9 (69) | 16 (89) |
| 45–55 | 5 (16) | 4 (31) | 1 (6) |
| Age at cancer diagnosis, y | |||
| 0–9 | 6 (22) | 4 (36) | 2 (11) |
| 10–14 | 6 (19) | 4 (29) | 2 (11) |
| 15–19 | 19 (59) | 5 (36) | 14 (78) |
| Years since diagnosis | |||
| 15–24 | 18 (58) | 3 (23) | 15 (83) |
| 25–34 | 8 (26) | 5 (39) | 3 (17) |
| 35–39 | 5 (16) | 5 (38) | 0 (0) |
| Race/ethnicity | |||
| Non-Hispanic white | 26 (84) | 12 (92) | 14 (78) |
| Non-Hispanic black | 5 (16) | 1 (8) | 4 (22) |
| Treatment | |||
| Anthracycline exposure | |||
| <150 mg/m2 | 6 (33) | NA | 6 (33) |
| 150–249 mg/m2 | 9 (50) | NA | 9 (50) |
| ≥250 mg/m2 | 3 (17) | NA | 3 (17) |
| Chest radiotherapy | |||
| <20 Gy | 3 (10) | 0 (0) | 3 (17) |
| 20–29 Gy | 13 (42) | 0 (0) | 13 (72) |
| ≥30 Gy | 15 (48) | 13 (100) | 2 (11) |
| Cardiovascular risk factors | |||
| Overweight (BMI 25–29) | 13 (42) | 5 (39) | 7 (39) |
| Obesity (BMI ≥30) | 5 (16) | 1 (8) | 4 (22) |
| Diabetes mellitus | 1 (3) | 0 (0) | 1 (6) |
| Hypertension | 7 (23) | 2 (15) | 5 (28) |
| Dyslipidemia | 15 (48) | 6 (46) | 9 (50) |
| Smoker | |||
| Current | 6 (19) | 3 (23) | 3 (17) |
| Past | 7 (23) | 3 (23) | 4 (22) |
| Never | 18 (58) | 7 (54) | 11 (61) |
Abbreviations: BMI, body mass index; HL, Hodgkin lymphoma.
All survivors were exposed to RT. Those treated with RT alone received ≥30 Gy, whereas only 2 (11%) patients in the multimodal group received doses that high; most (n = 16, 89%) received 20 to 29 Gy. Survivors in the multimodal group had a median anthracycline exposure of 191 mg/m2 (range, 96–316 mg/m2).
A total of 39 coronary artery lesions were identified in 12 patients: 59% of lesions were calcified; 26%, noncalcified; and 15%, mixed (Figs. 1, 2). Four lesions were obstructive (10%), whereas 35 lesions (90%) were nonobstructive. Sixty-seven percent of lesions (obstructive and nonobstructive) were located proximally in the coronary artery tree, and 74% involved the left-sided circulation (Table 2, Fig. 2). Six lesions (15%) were identified in the LM coronary artery; 5 resulted in <25% diameter reduction, but 1 had 50% to 70% stenosis. Eight lesions (21%) were identified in the proximal LAD artery, 5 (13%) in the proximal LCx artery, and 7 (18%) in the proximal RCA.
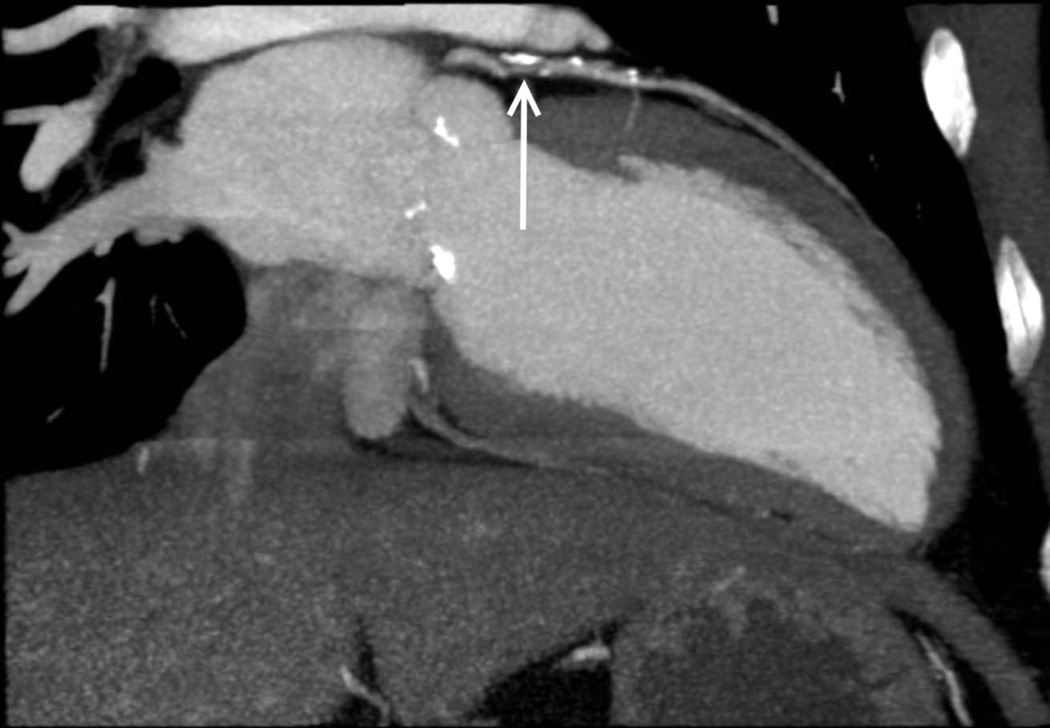
Figure 1.
Coronary computed tomographic angiography multiplanar reconstruction in the 2-chamber orientation demonstrating an obstructive lesion of the proximal left anterior descending artery (arrow) secondary to mixed (calcified and noncalcified) atherosclerotic plaque.
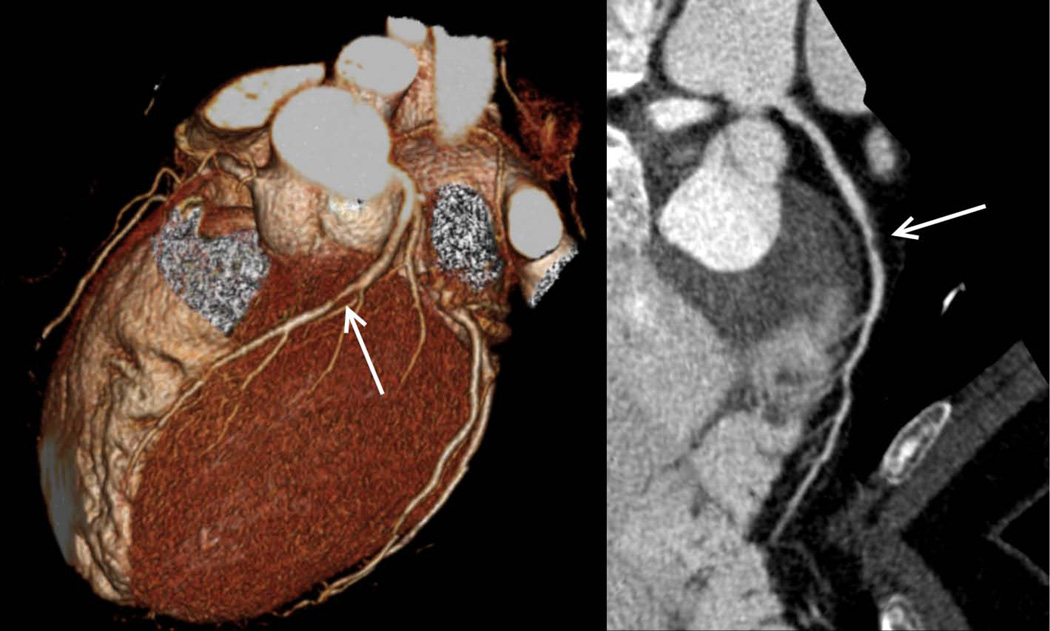
Figure 2.
Coronary computed tomographic angiography 3D reconstruction (left) and curved multiplanar reconstruction (right) from the same patient revealing a nonobstructive lesion (50%–70% stenosis) of the mid left anterior descending artery (arrows) secondary to noncalcified atherosclerotic plaque.
TABLE 2.
Coronary Artery Disease Detected by CCTA
| Location of Plaque | N = 39 (%) |
|---|---|
| Left main artery | 6 (15) |
| Left anterior descending artery | |
| Proximal | 8 (21) |
| Middle | 6 (15) |
| Distal | 1 (3) |
| Diagonals | 2 (5) |
| Left circumflex artery | |
| Proximal | 5 (13) |
| Distal | 0 (0) |
| Right coronary artery | |
| Proximal | 7 (18) |
| Middle | 2 (5) |
| Distal | 2 (5) |
Abbreviation: CCTA, coronary computed tomography angiography.
The overall prevalence of obstructive and nonobstructive disease detected by CCTA was 39%. Four obstructive lesions were identified in 3 (10%) participants, all treated with RT only. These lesions were proximally located (Table 3, Fig. 3). One individual had stenosis of the LAD and LCx arteries, 1 had RCA disease, and 1 had a 50% to 70% narrowing of the LM coronary artery. All of these patients subsequently underwent conventional angiography, with confirmation of disease in all 3. Only 1 reported a history of angina. Two underwent surgical revascularization, and 2 subsequently died of cardiovascular disease (1 with and 1 without revascularization).
TABLE 3.
Obstructive Lesions Detected by CCTA
| Age (y) |
Sex | Radiation (cGy) |
Anthracyline (mg/m2) |
CCTA | Echocardiogram | Stress Test | Electrocardiogram | 10-Year CV Risk |
Status |
|---|---|---|---|---|---|---|---|---|---|
| 43 | F | 3500 | 0 | 50%–70% LM 25%–50% proximal LAD 25%–50% mid LAD 25%–50% proximal LCx 50%–70% proximal RCA |
EF 54%; no wall motion abnormalities |
Normal | Major Q-wave abnormality |
1% | 3-vessel CABG; Dead of CAD |
| 40 | M | 3600 | 0 | >70% proximal LAD <25% mid LAD 25%–50% D1 >70% proximal LCx 25%–50% proximal RCA <25% mid RCA |
EF 51%; no wall motion abnormalities |
Abnormal | Major Q-wave abnormality |
2% | 6-vessel CABG; Alive |
| 53 | F | 3900 | 0 | >70% proximal RCA <25% LM <25% proximal LAD 25%–50% mid LAD <25% distal LAD <25% proximal LCx |
EF 51%; no wall motion abnormalities |
Normal | Major Q-wave abnormality |
1% | Dead of CAD |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; CCTA, coronary computed tomographic angiography; CV, cardiovascular; D1, first acute diagonal branch of the LAD; EF, ejection fraction; LAD, left anterior descending artery; LCx, left circumflex artery; LM, left main artery; RCA, right coronary artery.
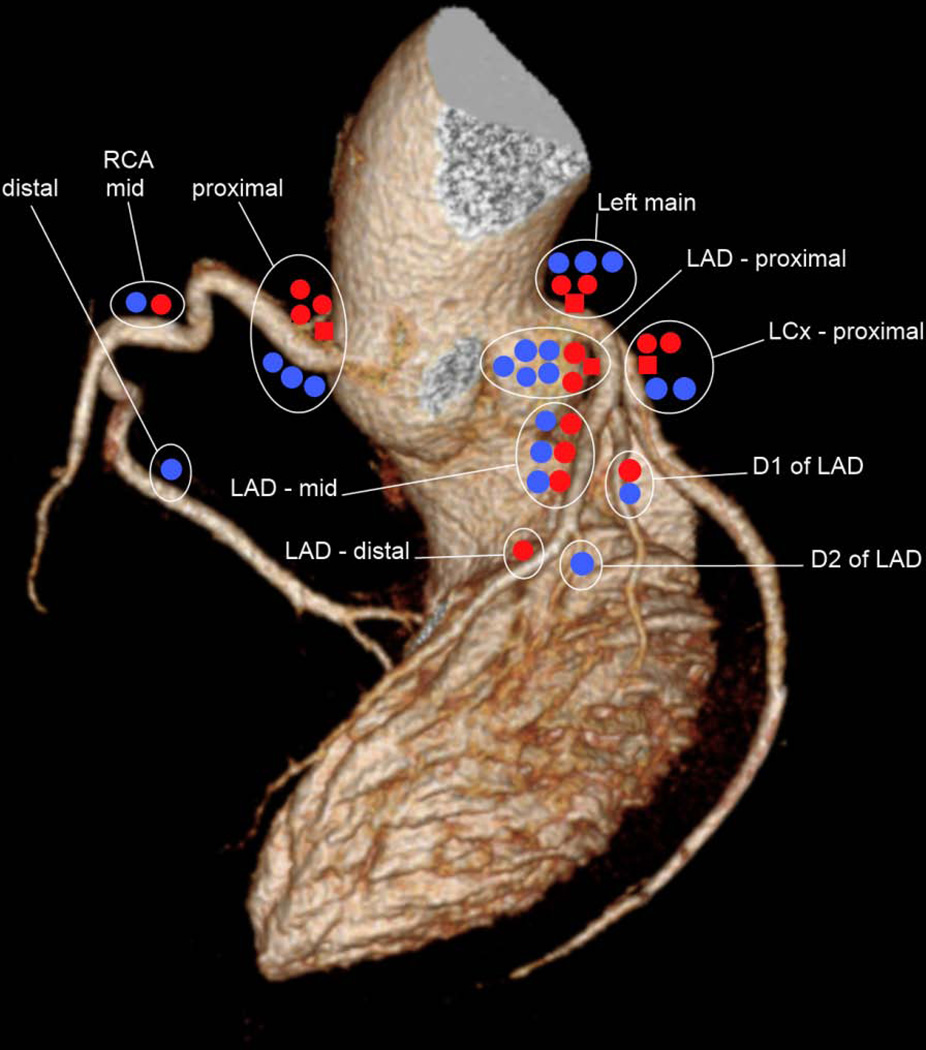
Figure 3.
Schematic plot of the distribution of lesions identified by coronary computed tomographic angiography along coronary territories, demonstrating clustering of lesions in the proximal coronary arteries. Red = Hodgkin lymphoma survivors treated with radiotherapy only; Blue = Hodgkin lymphoma survivors treated with multimodal therapy; ■= obstructive plaque;●, ●= nonobstructive plaque.
Nine survivors (29%), 5 treated with RT only and 4 with multimodal therapy, had nonobstructive lesions (Table 4), with most stenoses having <25% to 50% diameter reduction. One individual had a 50% to 70% mid LAD stenosis. One individual had a calcified lesion with >70% stenosis of the RCA ostium identified by CCTA. Coronary angiography revealed only nonobstructive disease, although review of the images revealed a small vessel with coronary spasm during the procedure, potentially confounding the findings. None of these patients had clinically evident CAD.
TABLE 4.
Nonobstructive Lesions Detected by CCTA
| Age (y) |
Sex | Radiation (cGy) |
Anthracyline (mg/m2) |
CCTA | Echocardiogram | Stress Test | Electrocardiogram | 10-Year CV Risk |
Status |
|---|---|---|---|---|---|---|---|---|---|
| 42 | M | 3511 | 0 | >70% proximal RCA <25% LM |
EF 59%; no wall motion abnormalities |
Normal | Negative | 1% | Alive |
| 37 | M | 3600 | 0 | <25% proximal LAD 25%–50% mid LAD <25% D2 |
EF 59%; no wall motion abnormalities |
Normal | Negative | 3% | Dead of SMN |
| 43 | M | 3500 | 0 | <25% LM <25% proximal LAD 25%–50% proximal LCx <25% proximal RCA |
EF 60%; no wall motion abnormalities |
Normal | Major isolated ST-T abnormalities |
14% | Alive |
| 39 | F | 3850 | 0 | <25% LM | EF 64%; no wall motion abnormalities |
Normal | Negative | 1% | Alive |
| 55 | F | 3840 | 0 | <25% proximal LAD | EF 62%; no wall motion abnormalities |
Normal | Major Q-wave abnormality |
1% | Alive |
| 41 | M | 2075 | 149 | <25% LM 50%−70% mid LAD 25%−50% proximal D1 <25% proximal RCA |
EF 54%; no wall motion abnormalities |
Normal | Negative | 1% | Alive |
| 42 | F | 2050 | 136 | 25–50% proximal LAD <25% proximal LCx <25% proximal RCA 25%−50% distal RCA |
EF 66%; no wall motion abnormalities |
Invalid | Isolated ST/T abnormalities; LVH |
5% | Alive |
| 37 | F | 1920 | 147 | <25% proximal LAD | EF 68%; no wall motion abnormalities |
Normal | Negative | 1% | Alive |
| 46 | M | 2000 | 170 | <25% mid LAD <25% mid RCA |
EF 69%; no wall motion abnormalities |
Normal | Isolated ST/T abnormalities |
1% | Alive |
Abbreviations: CABG, coronary artery bypass graft; CCTA, coronary computed tomographic angiography; CV, cardiovascular; D1 and D2, first and second acute diagonal branches of the LAD; EF, ejection fraction; LAD, left anterior descending artery; LCx, left circumflex artery; LM, left main artery; LVH, left ventricular hypertrophy; RCA, right coronary artery; SMN, second malignant neoplasm.
Radiation exposure from CCTA as measured in dose-length product was 661 ± 350 mGy cm (mean ± SD) among 19 patients for whom dose was archived. The BMI of this subset of patients was no different from those for whom dose was not archived (25.6 kg/m2 vs 27.8 kg/m2; P = 0.25), and the ratio of prospective to retrospective ECG-gated coronary artery acquisitions was not significantly different between the 2 groups (P = 1.0), suggesting that the patient subset radiation dose is a reasonable estimate for the entire studied population.
Other than ECG abnormalities, few positive findings were identified by the other testing modalities. Among the 3 individuals with obstructive lesions identified by CCTA, all had positive findings on resting ECG; 1 had evidence of ischemia on treadmill stress testing, and none had an abnormal resting echocardiogram. None of the patients with nonocclusive lesions had positive stress testing or echocardiography; 4 of the patients with nonocclusive plaques had positive findings on resting ECG. Among those participants with no evidence of CAD on CCTA (n = 19), no abnormalities on stress testing were identified. On echocardiogram, 2 patients had an ejection fraction <50% (46% and 47%); 2 others had ST-T segment abnormalities on resting ECG.
DISCUSSION
In this population of HL survivors, we found evidence of obstructive and nonobstructive coronary plaque on CCTA, without significant corresponding changes on echocardiography or treadmill stress testing, although all 3 patients with obstructive CAD had positive ECGs. Most of the coronary artery plaques were calcified, with two-thirds located in proximal arterial segments and 15% in the LM coronary artery.
Radiation-induced CAD is a known late effect following treatment for HL, with few noninvasive screening options and little consensus on the best method for long-term monitoring.19 Historically, screening has included echocardiography with variable follow-up after completion of therapy. The median age of patients with CAD in this study was 40 years, which is significantly younger for diagnosis of CAD than in a noncancer population,20–22 suggesting that this patient population requires more vigilant screening. Multidetector CCTA is a potentially useful screening modality for these patients. In an investigation of adults without known CAD who were evaluated for nonemergent chest pain, researchers reported sensitivity, specificity, and positive and negative predictive values of 94%, 83%, 48%, and 99%, respectively, for CCTA in detecting ≥70% stenosis.23 Ha et al20 used CCTA to evaluate healthy young adults (≤40 y) with no history of cancer and found that 11% of study participants had evidence of occult CAD, but no cases were obstructive. In our similarly young population, 10% of patients had obstructive disease; an additional 29% had nonobstructive lesions. Both obstructive and nonobstructive plaques have been associated with future adverse cardiovascular events.24,25 Alternatively, lack of coronary plaque on CCTA is associated with low probability of a future event (negative likelihood ratio, 0.008; 95% CI, 0.0004–0.17).25 Sixty-one percent of our population had no identifiable plaque. CCTA screening may therefore help clinicians identify and risk-stratify survivors who would benefit from closer surveillance or earlier medical interventions.
Studies designed to assess ischemic heart disease in this patient population have been limited. Rademaker et al11 described CAD in 8 of 9 irradiated HL survivors (5 diagnosed as children, ≤21 y); most had diffuse disease but only 2 had obstructive disease as defined in our study. Unlike our study, the study by Rademaker et al also assessed coronary calcium scores and found that 6 survivors had scores >90th percentile for age and sex and 4 survivors had values 15 years beyond their current age. In a cohort of irradiated and nonirradiated survivors of childhood HL, Küpeli et al12 found that 19 survivors (16%) had coronary abnormalities of varying degrees, albeit only 2 with obstructive disease as defined in our study. This population was younger than ours, with a median age of 23 years (range, 14–43 y) and a median of 10 years (range, 5–31 y) from diagnosis, suggesting potential progression of CAD over time. In this study, 53% of those with coronary abnormalities and 47% of those without were also reported to have echocardiographic irregularities, although specific information about these abnormalities was not provided. In our study, we focused on resting wall motion abnormalities or ejection fraction <50% and found no positive echocardiograms in the 12 patients with CAD. However, 2 of the 19 patients without CAD had mildly reduced global left ventricular ejection fraction, suggesting a cardiomyopathy not attributable to epicardial coronary disease.
Interestingly, many of the ECG recordings in our study were suggestive of ischemic heart disease. Frequently used to assess for arrhythmias or conduction disorders in HL survivors, ECGs may have added benefit for CAD screening. Among our small population, the positive and negative predictive values of ischemic ECG findings were 63% and 25%, respectively. Formal assessment in the larger SJLIFE cohort is ongoing and likely to be more informative.
Unlike previous studies, our study included concurrent treadmill stress testing, which identified a single abnormal test. Given initial concerns regarding maximally stressing at-risk HL survivors, our study did not use a Bruce protocol, thus potentially limiting the overall yield from treadmill stress testing. However, the use of more intensive stress testing in these patients should be approached with caution, as this group has a higher incidence of stenotic aortic valvular disease and underlying conduction abnormalities such as left bundle branch block,5 which could make Bruce protocol stress testing challenging. The Balke protocol has a moderate workload and is suitable for patients with left ventricular systolic dysfunction.
The findings of our study should be interpreted within the context of evolving therapies for HL. The most severe disease (obstructive lesions) was identified among those with the highest radiation exposures. Doses have been reduced, if not eliminated, in newer protocols, which will hopefully lead to reduced risk of CAD among survivors of HL. However, data from irradiated breast cancer survivors would suggest that there is no safe exposure, with a 7.4% increase in coronary events per gray of radiation to the heart.26 Progression and the long-term outcome of this subclinical disease may not yet be evident.
A number of limitations should be considered when interpreting our study results. The small sample size limits the power for detailed analyses of treatment exposures and the potential contribution of traditional cardiovascular risk factors. Reflecting the trend in the general population, many of the patients in our cohort were overweight, hypertensive, or had dyslipidemia, potentially contributing to or significantly worsening CAD.27 Our study did not characterize the atherosclerotic plaque beyond the presence or absence of calcium. More detailed descriptions of plaque character (eg, lipid component, surface integrity, and degree of vascular remodeling) are not part of routine clinical CCTA scanning and analysis. Historically, CCTA has required limited but not insignificant radiation exposures (on average, ~12 mSv).28 Recent technical advances, however, have reduced this exposure, in some cases to <1 mSv.29
Our population of childhood HL survivors had a significantly higher burden of CAD (39%) on CCTA than what has been reported among the similarly aged general population (8.5%–11%).18–20 The lesions clustered in a proximal distribution, thereby increasing the risk for larger myocardial infarctions and coronary death. Given the rarity of ischemic heart disease among young people, few of these asymptomatic HL survivors would typically have been screened for this condition. CCTA may offer an effective noninvasive tool for the management of at-risk cancer survivors, permitting the identification of those who might benefit from lifestyle and/or medication interventions and those for whom lower levels of monitoring may be appropriate.
Acknowledgments
Funding sources: Supported by Cancer Center Support (CORE) Grant (CA21765) to St. Jude Children’s Research Hospital and the American Lebanese Syrian Associated Charities (ALSAC), Memphis, Tennessee.
Footnotes
Financial disclosures:
None.
References
- 1.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA. 2012;307:2609–2616. doi: 10.1001/jama.2012.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma--a report from the Children's Oncology Group. J Clin Oncol. 2012;30:3174–3180. doi: 10.1200/JCO.2011.41.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 6.Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of Hodgkin's Disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 7.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 8.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Sieswerda E, Postma A, van Dalen EC, et al. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23:2191–2198. doi: 10.1093/annonc/mdr595. [DOI] [PubMed] [Google Scholar]
- 10.Wallace WH, Thompson L, Anderson RA. Long term follow-up of survivors of childhood cancer: summary of updated SIGN guidance. BMJ. 2013;346:f1190. doi: 10.1136/bmj.f1190. [DOI] [PubMed] [Google Scholar]
- 11.Rademaker J, Schoder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin's lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol. 2008;191:32–37. doi: 10.2214/AJR.07.3112. [DOI] [PubMed] [Google Scholar]
- 12.Kupeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma. J Clin Oncol. 2010;28:1025–1030. doi: 10.1200/JCO.2009.25.2627. [DOI] [PubMed] [Google Scholar]
- 13.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31:157–187. [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 18.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 20.Ha EJ, Kim Y, Cheung JY, Shim SS. Coronary artery disease in asymptomatic young adults: its prevalence according to coronary artery disease risk stratification and the CT characteristics. Korean J Radiol. 2010;11:425–432. doi: 10.3348/kjr.2010.11.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin KN, Chun EJ, Lee CH, Kim JA, Lee MS, Choi SI. Subclinical coronary atherosclerosis in young adults: prevalence, characteristics, predictors with coronary computed tomography angiography. Int J Cardiovasc Imaging. 2012;28(Suppl 2):93–100. doi: 10.1007/s10554-012-0143-0. [DOI] [PubMed] [Google Scholar]
- 22.Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001–2011. JAMA. 2012;308:2577–2583. doi: 10.1001/jama.2012.70830. [DOI] [PubMed] [Google Scholar]
- 23.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 24.van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53:623–632. doi: 10.1016/j.jacc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57:1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]