Abstract
Merkel cell carcinoma (MCC) is an aggressive, polyomavirus-associated skin cancer. Robust cellular immune responses are associated with excellent outcomes in MCC patients, but these responses are typically absent. We determined the prevalence and reversibility of class I MHC (MHC-I) downregulation in MCC, a potentially reversible immune evasion mechanism. Cell surface MHC-I expression was assessed on 5 MCC cell lines using flow cytometry as well as immunohistochemistry on tissue microarrays representing 114 patients. Three additional patients were included that had received intralesional interferon treatment and had evaluable specimens before and after treatment. mRNA expression analysis of antigen presentation pathway genes from 35 MCC tumors was used to examine mechanisms of downregulation. 84% of MCCs (total n=114) demonstrated reduced MHC-I expression as compared to surrounding tissues, and 51% had poor or undetectable MHC-1 expression. Expression of MHC-I was lower in polyomavirus-positive MCCs as compared to virus-negative MCCs (p<0.01). The MHC-I downregulation mechanism was multifactorial and did not depend solely on HLA gene expression. Treatment of MCC cell lines with ionizing radiation, etoposide, or interferon (IFN) resulted in MHC-I upregulation, with IFNs strongly upregulating MHC-I expression in vitro and in 3 of 3 patients treated with intralesional IFNs. MCC tumors may be amenable to immunotherapy, but downregulation of MHC-I is frequently present in these tumors, particularly those that are polyomavirus-positive. This downregulation is reversible with any of several clinically available treatments that may thus promote the effectiveness of immune stimulating therapies for MCC.
Keywords: Merkel, MHC-I, downregulation, interferon, CD8
INTRODUCTION
Merkel cell carcinoma (MCC) is a skin cancer with 46% disease-associated mortality (1) and increasing impact. Several lines of evidence point to a key role for T-cell immunity in preventing and controlling MCC. Multiple forms of T-cell immune suppression (including immunosuppressive medications, HIV/AIDS, and lymphoid malignancies) have been associated with increased risk of MCC (2), and T-cell immune suppressed patients have poorer outcomes (3–5). Conversely, robust intratumoral CD8+ and CD3+ lymphocyte infiltration is associated with excellent MCC patient survival, however, most tumors lack these responses (6, 7). Greater than 90% of MCC patients have no clinically appreciable systemic immune suppression suggesting that T-cell evasion may instead be local and/or tumor-driven.
In 2008, MCC was associated with a novel but highly prevalent polyomavirus (8), the Merkel cell polyomavirus (MCPyV or MCV). Viral oncoproteins (T-antigens) are expressed in at least three-quarters of MCCs (9–11) and their persistent expression is necessary for MCC cell division (12). Furthermore, these nonhuman oncoproteins are targets for adaptive immune responses in MCC patients, with humoral (13) and more importantly cellular (including CD8+ T-cell) immune responses demonstrable in the blood and in the tumor microenvironment (14, 15). Therefore, these viral antigens suggest that the tumor is immunogenic and must have specifically avoided CD8+ T-cell recognition. They further represent compelling targets for MCC-specific immunotherapy, including adoptive T-cell therapies.
Nucleated cells express major histocompatibility complex class I (MHC-I), a requirement for presenting peptides from intracellular proteins to CD8+ T cells. Multiple viruses (16) and virus-associated cancers (e.g. Kaposi’s sarcoma (17), cervical cancer (18)) are known to directly or indirectly downregulate MHC-I as a mechanism of immune escape.
We hypothesized MCC tumors would exhibit reduced expression of MHC-I as a mechanism of immune escape and that this may be reversible. To investigate this, we studied surface MHC-I expression with immunohistochemistry (IHC) on samples from more than one hundred unique patients. To study the reversibility of MHC-I downregulation, we tested the effects of clinically available treatments including several interferons (IFN), cytotoxic chemotherapy, and radiation therapy (XRT) on MHC-I expression on MCC cell lines. IFNs were of special interest as prior studies in other settings suggest they often promote antiviral immune responses, possess anti-polyomavirus (19, 20) and anti-MCC activity (19, 21), and re-induce MHC-I expression. Moreover, IFNs are broadly clinically available in the United States with current indications for antiviral, immunomodulatory, and anticancer applications. This study demonstrates that MHC-I downregulation is prevalent in Merkel cell carcinoma and can be re-induced using any of several clinically available therapies. Reversal of MHC-I downregulation has the potential to increase exposure of tumor antigens in order to augment endogenous immunity and immunotherapy.
MATERIALS AND METHODS
MCC cell lines
MCC cell lines MKL-1 (22), WaGa (12), UISO (23), MCC13 (24), and MCC26 (25) were maintained in RPMI 1640 medium with 10% fetal calf serum, 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). MCC13, MCC26, and UISO cell lines were obtained from their original creators, MKL-1 from Masa Shuda, and WaGa was created in the Becker laboratory and previously characterized as referenced above. Merkel cell polyomavirus (MCPyV) status of these lines has been previously reported (12) and was determined based on PCR assay and Southern blot. The majority of studies were performed with MKL-1 as it is best characterized, is relatively easy to maintain (although non-adherent), and is well established to be positive for Merkel cell polyomavirus (12). Our aliquots of MKL-1 were confirmed to be MCPyV positive by Western blot (14) using the CM2B4 antibody (9). No other authentication assay was performed.
Flow cytometric detection of MHC-I expression
Flow cytometry was performed using the w6/32 antibody (26), which detects the expression of MHC-I on the cell surface with an epitope shared by classical and non-classical HLA antigens. K562 cells, which lack cell-surface expression of MHC-I, served as negative controls. One of the known MHC-positive lymphoblastoid cell lines listed above served as a positive control. Cells were treated with XRT, etoposide, carboplatin, or one of three recombinant IFNs: IFN-α-2b (Intron A, Merck, Whitehouse Station, NJ), IFN-β-1b (Betaseron; Bayer, Montville, NJ), or IFN-γ-1b (ActImmune, InterMune, Brisbane, CA). Dosages are listed in the figure legend (see Figure 1).
Figure 1. MHC-I downregulation on MCC cell lines is reversible with multiple treatment modalities.
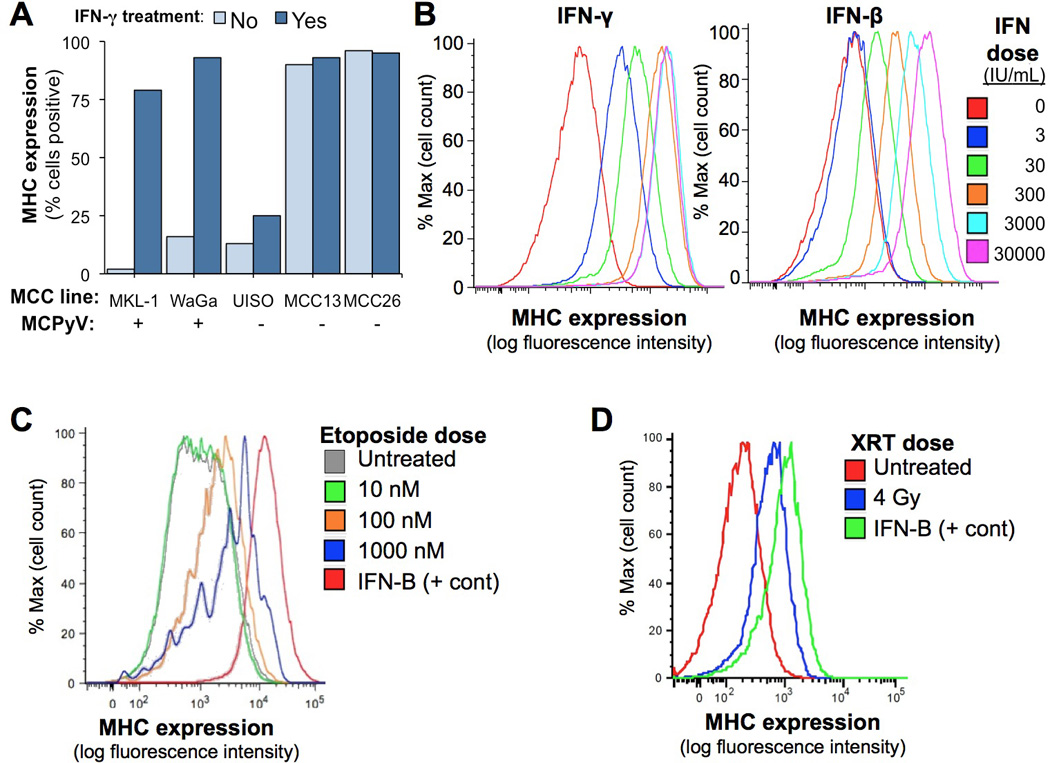
A) Effect of IFNγ on MHC-I surface expression among 5 MCC cell lines as assessed by flow cytometry. Merkel cell polyomavirus (MCPyV) status is indicated by the (+) or (−) sign below each cell line name. Cells were treated with 2000 IU/mL IFNγ for 72 hours. B) Dose-dependent IFNγ and IFN-β induction of MHC-I expression on the MKL-1 MCC cell line. Day 7 data are shown; partial induction was seen as early as treatment day 1. C) Etoposide-induced induction of MHC-I on the MKL-1 cell line. Partial effects were seen as early as day 1; day 4 is shown. IFN-β = 3000 IU/mL D) Radiation-induced induction of MHC-I on the MKL-1 cell line. Day 2 is shown as there were few viable cells thereafter. IFN-β = 300 IU/mL.
Patients and tumors
All materials and data were obtained from the MCC Data and Tissue Repository at the University of Washington/Fred Hutchinson Cancer Research Center (IRB approval #6585). 117 patients were included, with 88 enrolled from the United States, 28 from Germany, and 1 from Japan. Of these, 114 were cases that were part of the tissue microarrays (TMA) screened to determine MHC-I tumor expression; while an additional and non-overlapping 3 cases were not featured on the microarray but instead represented retrospective materials obtained from patients treated with IFN. All patients whose samples were on the microarray with at least one adequate core were included in the study. All patients had MCC as assessed by two or more pathologists. Diagnoses occurred between the years of 1985–2011.
mRNA expression data
mRNA array expression data from a previously published data set representing 35 MCC tumors from 34 distinct patients were utilized (6), GEO accession number GSE22396. Please see previous publication for complete methods description. In brief, Merkel cell carcinoma tumors were macrodissected, RNA was extracted, and cDNAs prepared. cDNAs were applied to the human Rosetta custom Affymetrix 2.0 chip (Affymetrix, Santa Clara, CA) in a single batch at Rosetta Inpharmatics and analysis performed with Resolver software (version 6.0, Rosetta Biosoftware, Seattle, WA).
Beta2Microglobulin (B2M) reverse-transcription quantitative PCR
RNA was isolated from MKL-1 cells by RNeasy (Qiagen, CA). RNA quality was confirmed by spectrophotometry. cDNA was generated using the Applied Biosystems High Capacity Reverse Transcription Kit (Applied Biosystems, CA). B2M and 18s (control) transcript quantities were determined by TaqMan® PCR using commercially available reagents (Applied Biosystems, CA) on an ABI 7900 platform in 384-well format (Applied Biosystems, CA) as per manufacturer’s instructions.
Tissue microarrays (TMA)
114 tumors from 114 distinct patients were represented on at least one of 5 TMA slides composed of 0.6 mm cores of formalin-fixed, paraffin-embedded tumors. 77 (67%) were primary lesions, 19 (16%) were nodal metastases, 2 (2%) were recurrences, 8 (7%) were skin metastases, and 8 (7%) lesions were from undetermined sites.
MHC class I immunohistochemistry
The EMR8-5 antibody (MBL International, Woburn, MA) was utilized to determine MHC-I expression. EMR8-5 is reported to recognize the extracellular domains of the following classical HLA molecules: HLA-A*2402, -A*0101, -A*1101, -A*0201, -A*0207, -B*0702, -B*0801, -B*1501, -B*3501, -B*4001, -B*4002, -B*4006, -B*4403, -Cw*0102, -Cw*0801, -Cw*1202, and -Cw*1502 (27). Epitope retrieval was performed with 20 minutes of steam in a pH 6 citrate buffer. Primary antibody was used at 1:100 dilution, blocking was with 15%swine/5%human serum, mouse EnVision secondary detection was utilized (Dako, Carpenteria, CA). Tonsil cores provided on-slide positive tissue staining controls. Normal mouse serum (NMS) was run as a negative isotype control. Further supporting the adequacy of staining were within-tumor controls: strong membranous MHC class I staining was observed as expected on stromal cells and tumor-infiltrating lymphocytes but not on erythrocytes.
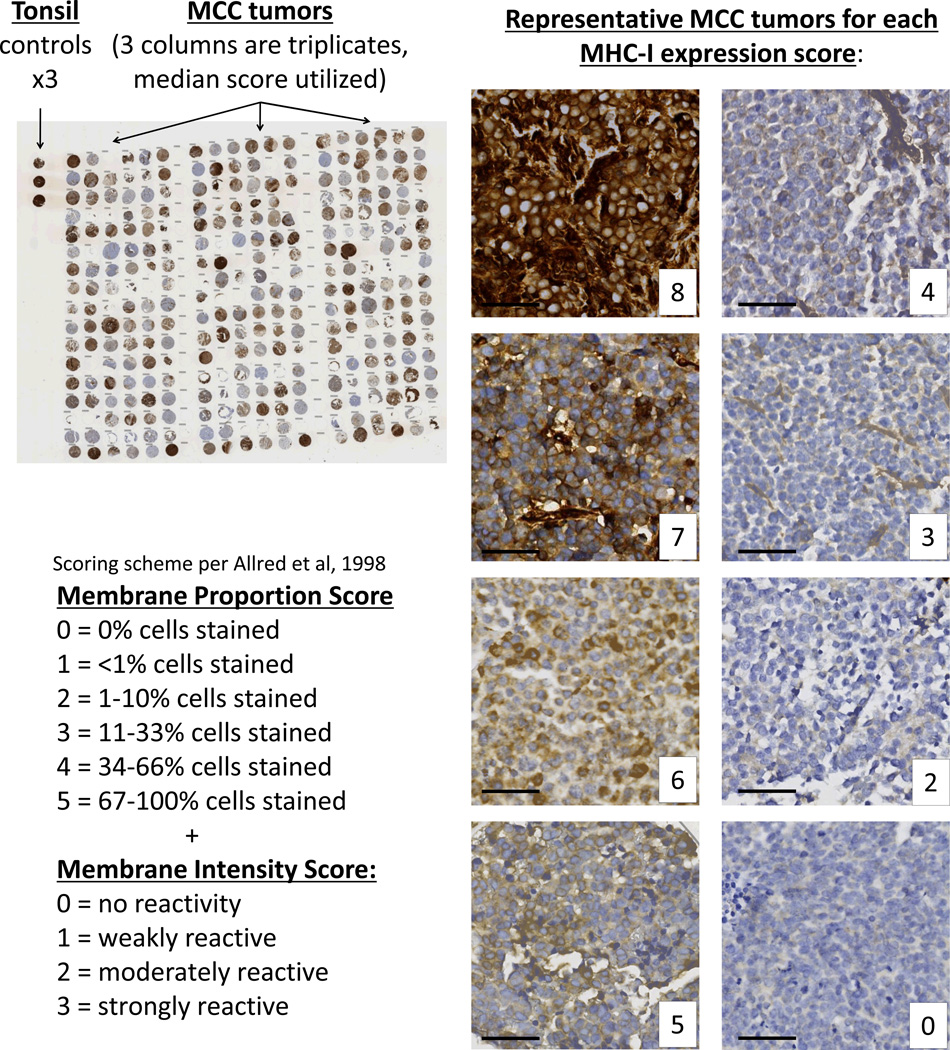
Specimens were assessed for tumor cell membrane staining by three observers who were blinded to the identity of the samples. TMAs were scored using the Allred method (28) as follows: a score between 0 and 8 is determined from the sum of a proportion score (0–5 scale reflecting the fraction of cells with any stain), and a staining intensity score (0–3 scale reflecting the strength of staining among the positive cells). The median of the triplicate tumor cores was determined for each observer, and then the median of the observers' scores was utilized in analyses. In the event that scorers disagreed by more than two points on the combined 0–8 scale, scores from an independent pathologist blinded to the previous reads were used instead, or the specimen was eliminated if the independent observer determined the sample quality to be inadequate. If a patient had more than one lesion represented, a single lesion was included based on priority: primary > nodal metastasis > recurrence > regional skin metastasis > distant metastasis.
MCPyV immunohistochemistry
Two TMAs, containing samples from 82 patients, had previously been stained for MCPyV T-antigen (13) with the CM2B4 antibody (9). An Allred score of 2 or greater defined MCPyV positivity.
CD8 immunohistochemistry
CD8 infiltration data were available and previously reported for 77 tumors (6).
Statistical analysis
Linear regression was utilized for two-way comparisons in Figures 2A-B. The non-parametric Wilcoxon-Rank-Sum test was employed in Figure 4B to compare MHC-I expression between virus-positive and virus-negative tumors. The Wilcoxon-Rank-Sum test was also utilized to compare CD8+ cell infiltrates between MHC-I strongly expressing tumors (defined as Allred score of maximal 8) and weakly or non-expressing tumors (Allred score of 7 or less). The paired t-test was employed for the comparison of MHC-I expression before and after treatment for IFN-treated tumors (Figure 5). A p value of less than 0.05 was considered significant. Analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
Figure 2. Mechanism of MHC-I downregulation in MCC tumors.
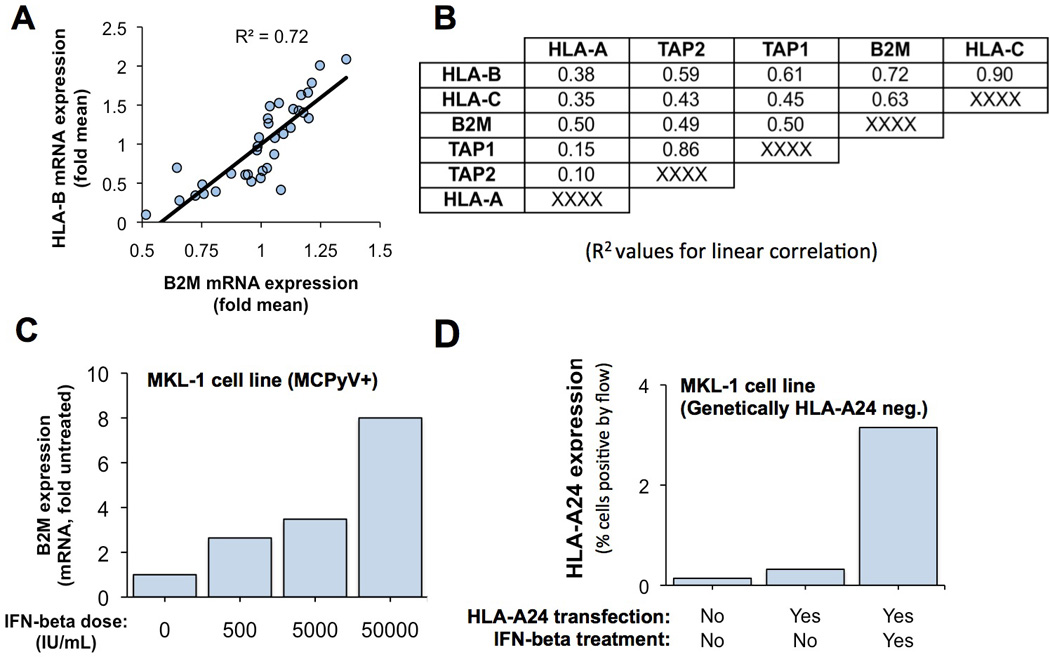
A and B) mRNA expression of MHC class I HLA genes was highly correlated to mRNA expression of B2M and antigen-processing genes among 35 MCC tumors. Values in panel B represent R-squared values for linear correlation comparing relative expression of gene at left to gene at top (B2M example is indicated with a double box). C) Treatment of MKL-1 MCC cells with IFN-β is associated with induction of B2M mRNA expression as determined by real-time, reverse transcription PCR. D) Transfection of HLA-A24 under a constitutive promoter (CMV) was insufficient to restore expression of MHC class I in MKL-1 cells suggesting deficiencies in surface MHC-I expression were not solely due to poor HLA gene expression. However, surface expression of MHC class I was induced when HLA-A24 was combined with IFN.
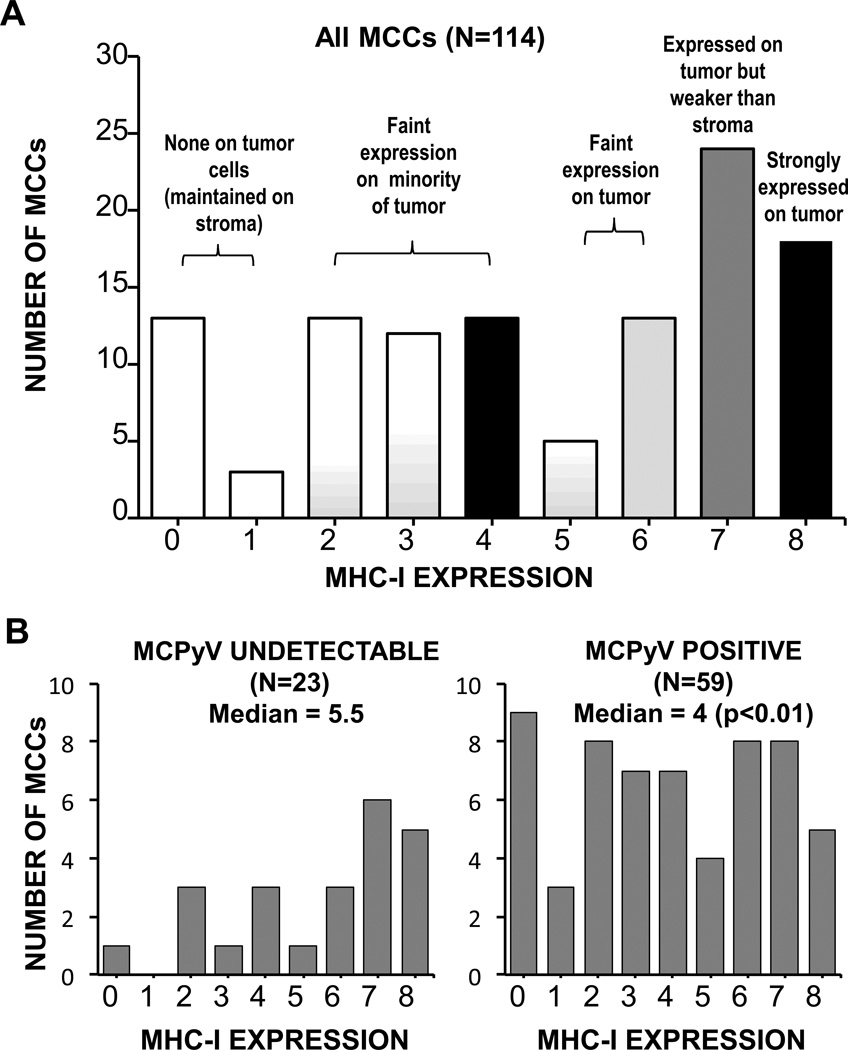
Figure 4. MHC-I downregulation is frequent in human MCC tumors.
A) MHC-I protein expression among 114 human MCC tumors as determined by immunohistochemistry and Allred scoring. MHC-I was downregulated (Allred score ≤ 7) on 84% of MCCs. B) Merkel cell polyomavirus-expressing tumors exhibit less MHC-I expression. Among a subset of tumors with available MCPyV IHC (n=82), Merkel cell polyomavirus T antigen-positive tumors had significantly poorer MHC-I expression as compared to tumors with undetectable viral proteins (p<0.01).
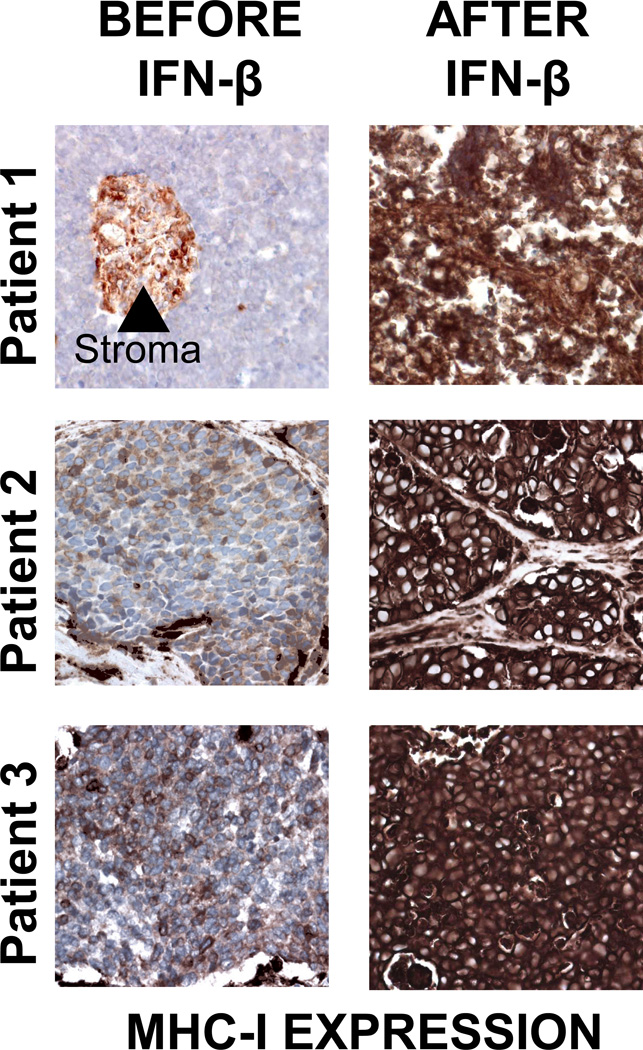
Figure 5. Treatment of human MCC tumors with intralesional IFN-β is associated with MHC-I upregulation.
Clinical details of patient 1 (not including immunologic studies) were reported previously (30). After IFN- β monotherapy, patient 1 subsequently experienced 8+ years of disease-free survival. Three patients that received intralesional IFN injections as part of their MCC treatment who had specimens available from before and after IFN treatment are shown. A significant increase in MHC-I expression was observed on tumor cells after treatment (p=0.04; paired t-test).
RESULTS
MHC class I expression is downregulated but re-inducible on MCC cell lines
The cell-surface expression of MHC-I was determined by flow cytometry on five MCC cell lines (Figure 1A). Two of three polyomavirus-negative MCC cell lines demonstrated maintained MHC-I expression. In contrast, two of two polyomavirus-positive cell lines were MHC-I negative.
IFNs are well-characterized mediators of antiviral immune responses, with upregulation of MHC-I being one of their classical functions. We therefore tested their effects on MHC-I expression in MCC cell lines. Treatment with IFNγ resulted in significantly increased expression of MHC-I in both MCPyV-positive cell lines (Figure 1A). Among the MCPyV-negative cell lines, a modest increase was observed in UISO cells, while MHC-I was already present at high levels at baseline in the remaining two cell lines. While virus-positive cell lines are well established to be similar in nature to human MCC tumors, it is less clear that the virus-negative cell lines are biologically representative as they lack many key hallmarks of MCC including cytokeratin-20 expression (29).
We also investigated whether other clinically available treatments could reverse MHC-I downregulation. IFN-β (Figure 1B) and IFN-α (data not shown) each strongly induced MHC-I in a dose-dependent fashion, although higher dosages were needed to achieve the same effect as for IFNγ. Etoposide, a standard MCC chemotherapeutic, also induced MHC-I expression (Figure 1C), while platins (cisplatin and carboplatin) did not (data not shown). Finally, XRT resulted in modest MHC-I upregulation (Figure 1D), and this effect was dose-dependent (data not shown).
Mechanism of MHC-I downregulation and interferon-mediated reversal
Delivery of MHC-I onto the cell surface requires not only the expression of the relevant MHC-I heavy chain gene, but also of B2M and numerous antigen-processing genes. Among the 35 MCCs (6), expression levels of MHC-1 mRNAs were highly correlated to those of B2M and genes involved in peptide processing and presentation such as components of the transporter associated with antigen processing (TAP) complex (Figure 2A, 2B). This implies simultaneous downregulation of multiple components of this pathway in MCC tumors. Furthermore, IFN treatment of MKL-1 cells was associated with upregulated mRNA expression of pathway components other than HLA genes (eg. B2M, Figure 2C), suggesting the effects of IFN on MHC-I expression in MCC are not limited to upregulating MHC-I heavy chain genes.
To determine the importance of these non-HLA components on the observed upregulation of MHC-I on the surface of MCC tumor cells, MKL-1 cells (HLA-A*2402 negative) were transfected with HLA-A*2402 driven by a constitutive cytomegalovirus (CMV) promoter (Figure 2D). Transfection of HLA-A*2402 alone was not sufficient to restore MHC-I expression on the surface of MKL-1 cells. However, when IFN- β -1b was added to the HLA-A*2402 transfection, surface HLA-A*2402 expression was induced (Figure 2D).
MHC class I cell surface expression is reduced in the majority of human Merkel cell carcinomas
MHC-I expression was determined by IHC on TMAs of MCC tumors from 114 patients (Figure 3). 84% of MCC tumors demonstrated MHC-I downregulation on tumor cells as compared to stroma, and 51% demonstrated marked downregulation (Figure 4A and Supplemental Table 1).
Figure 3. MHC class I immunohistochemistry of Merkel cell carcinoma tumors.
A total of 114 MCC tumors were represented on 5 tissue microarrays and were stained with the EMR8-5 antibody that recognizes HLA-A, -B, and -C. These were scored for proportion of cells expressing MHC class I and intensity of the expression utilizing the Allred scoring system ranging from 0 to a maximal score of 8. Representative MCC tumors at each combined Allred score are shown at right. Black bar represents 50 micrometers.
Among patients with primary tumors represented on TMAs (n=77), median expression was 5 (corresponding to faint expression on most tumor cells) and 83% had some downregulation in MHC-I expression. A trend toward lower MHC-I expression was observed among patients who were instead represented with a nodal (n=19) or distant skin (n=8) metastasis (median of 4 and 2.5, and downregulation of 89% and 100% of tumors, respectively), however this difference did not achieve statistical significance.
To determine whether MHC-I expression was associated with intratumoral CD8+ lymphocyte-infiltration, we compared CD8+ infiltration with MHC-I expression for 77 MCC cases with both data types available. There was no statistically significant difference in CD8+ infiltration between cases with strong MHC-I expression (Allred score of 8) or reduced/absent MHC-I expression (Allred score 0–7).
Merkel cell carcinomas with detectable MCPyV exhibit less MHC-I expression
Approximately 80% of MCCs express MCPyV-derived oncoproteins, and these oncoproteins have been demonstrated to be substrates for CD8+ T cells (14). We hypothesized that these tumors would be particularly likely to have lost MHC-I expression. Indeed, MHC-I expression was significantly lower in MCCs with detectable virus (median score of 4 vs. 5.5, Figure 4B, p<0.01).
MCCs treated with interferon-beta had greater MHC-I expression post treatment
Two cases have been reported in which intralesional IFN-β injection has been successful as primary therapy for MCC (30, 31). We obtained slides from before and after injection from one of these cases (30), as well as from an additional two cases that have been treated with IFN-β (and later went on to receive other therapies including surgery and XRT). As this study represented a retrospective case review, these patients were not part of a standardized protocol, but all analysis was carried out after IRB approval. Lesion shrinkage was observed in all three cases with intralesional IFN injection.
We hypothesized that intralesional IFN-β injection would be associated with increased MHC-I expression on the tumor cells. Before treatment, MHC-I on tumor cells was strikingly lower than on surrounding tissues (AllRed score (max of 8) was 0, 3, and 4 respectively, for the three cases; Figure 5). However, after IFN treatment, strong expression of MHC-I was observed (Allred score of 8 in each case; p = 0.04).
DISCUSSION
Merkel cell carcinoma is an often-lethal skin cancer associated with a persistent requirement for expression of viral oncoproteins (T-antigens) (8, 12). Although T-cell responses are associated with excellent disease-specific outcomes (6, 32) and viral T-antigens have been demonstrated to elicit specific CD8+ T-cell responses in MCC patients (14), the majority of tumors lack intratumoral CD8+ infiltration suggesting cytotoxic T cell avoidance. In the present study, we found that the majority of 114 MCC tumors exhibit poor expression of MHC-I. Although this is in keeping with findings in other virus-associated malignancies (16), this observation is important because it represents an obstacle to native immune responses and to adaptive immunotherapies. Our finding that MHC-I downregulation in MCC appears to be reversible has clinical significance for therapeutic approaches that target immune stimulation.
Merkel cell carcinoma is an especially appealing target for immunotherapy given the associations between immune responses and outcomes as well as the targetable viral oncoproteins present in most cases. In this study, the most effective in vitro agents for MHC-I upregulation were IFNs. Furthermore, these highly active biologic compounds have been shown to inhibit the growth of MCC cell lines (19, 21) and are associated with downregulation (but not complete loss) of MCPyV T-antigen protein. However, clinical experience with IFNs in MCC has been mixed, with some reported cases (30, 31) of successful intralesional IFN- β treatment, and others reported failures of systemic IFN-α treatment (33–35). More study is needed to determine if and how these compounds can complement other traditional and immune therapies.
We observed an inverse association between Merkel cell polyomavirus T-antigen expression and MHC class I expression in human MCC tumors. It remains to be determined whether MCPyV T-antigens are able to mediate MHC class I downregulation; our study was limited by the inability to test this in vitro due to significant cell death with MCPyV knockdown. It is possible that MCPyV is directly downregulating MHC class I expression, alternately it is possible that MCPyV-positive tumors are more likely to be MHC-I negative due to selection against MHC-I-expressing tumors. However, the presence of tumors with high expression of both MHC-1 and MCPyV as well as those with lack of MHC-1 and detectable MCPyV expression suggest that it is not the only factor at play in MHC-1 downregulation.
Tumors that undergo significant downregulation of cell-surface MHC class I should become targets for natural killer (NK) cell recognition. However, the persistence of these tumors suggests NK cell evasion by MCCs. Further work is needed to determine the mechanism of NK cell evasion by MCC tumors with low or no MHC class I. Plausible mechanisms would include upregulation of inhibitory receptors or downregulation of NK-activating receptors such as NKG2D (36). Should NK responses be deficient, therapies aimed at augmenting NK responses may represent an alternate immunotherapeutic approach to T cell-directed therapies in MHC-I-negative MCC tumors.
In summary, Merkel cell carcinoma is an aggressive skin cancer with persistent expression of immunogenic viral oncoproteins. Clinically, improved CD8+ T cell immune responses are associated with excellent outcomes. Given this, MCC is an appealing target for novel and established immunotherapies. MHC class I downregulation represents one mechanism of immune evasion employed by a majority of MCCs. This presents an obstacle to both native immune responses and T-cell or vaccine-based immunotherapies, but may be reversed with multiple clinically available treatments. Therapies aimed at restoring T-cell responses represent a promising avenue for MCC treatment.
Supplementary Material
Acknowledgements
We thank Liz Donato, Julie Randolph-Habecker, Farinaz Shokri, Piper Treuting and Miranda Schmidt for assistance with immunohistochemistry studies, Janell Schelter for assistance with expression analysis, and Helen Leonard for donation of cell lines.
Financial support: American Cancer Society grant RSG-08-115-01-CCE (PN), NIH RC2CA147820 (PN), NIH K24 CA139052-0 (PN), NIH T32 CA80416-10 (KP), F30ES017385 (KP), TL1RR025016 (AT), Michael Piepkorn Endowment, Poncin and MCC Patient Gift Funds at the University of Washington.
Footnotes
Conflicts of interest: Employment or Leadership Position: Michele A. Cleary and James S. Hardwick, Merck and Co. Consultant or Advisory Role: Juergen Becker, Bristol Myers Squibb, Glaxo Smith Kline, EMD Serono, Novartis, Roche. Stock Ownership: None. Honoraria: Juergen Becker, EMD Serono. Research Funding: None. Expert Testimony: None. Other Renumeration: None.
REFERENCES
- 1.Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer JD, Shanafelt TD, Otley CC, Roenigk RK, Cerhan JR, Kay NE, et al. Chronic Lymphocytic Leukemia Is Associated With Decreased Survival of Patients With Malignant Melanoma and Merkel Cell Carcinoma in a SEER Population-Based Study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 4.Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642–646. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor Infiltrating Immune Cells and Outcome of Merkel Cell Carcinoma: A Population-based Study. Clin Cancer Res. 2012;18:2872–2881. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 8.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122:4645–4653. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel Cell Polyomavirus-Specific CD8+ and CD4+ T-cell Responses Identified in Merkel Cell Carcinomas and Blood. Clin Cancer Res. 2011;17:6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez BP, Wang C, Viscidi RP, Peng S, He L, Wu TC, et al. Strategy for eliciting antigen-specific CD8+ T cell-mediated immune response against a cryptic CTL epitope of merkel cell polyomavirus large T antigen. Cell Bioscience. 2012;2:36. doi: 10.1186/2045-3701-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9:503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 17.Haque M, Ueda K, Nakano K, Hirata Y, Parravicini C, Corbellino M, et al. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J Gen Virol. 2001;82:1175–1180. doi: 10.1099/0022-1317-82-5-1175. [DOI] [PubMed] [Google Scholar]
- 18.Koopman LA, van Der Slik AR, Giphart MJ, Fleuren GJ. Human leukocyte antigen class I gene mutations in cervical cancer. J Natl Cancer Inst. 1999;91:1669–1677. doi: 10.1093/jnci/91.19.1669. [DOI] [PubMed] [Google Scholar]
- 19.Willmes C, Adam C, Alb M, Volkert L, Houben R, Becker JC, et al. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012;72:2120–2128. doi: 10.1158/0008-5472.CAN-11-2651. [DOI] [PubMed] [Google Scholar]
- 20.Co JK, Verma S, Gurjav U, Sumibcay L, Nerurkar VR. Interferon- alpha and - beta restrict polyomavirus JC replication in primary human fetal glial cells: implications for progressive multifocal leukoencephalopathy therapy. J Infect Dis. 2007;196:712–718. doi: 10.1086/520518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasagakis K, Kruger-Krasagakis S, Tzanakakis GN, Darivianaki K, Stathopoulos EN, Tosca AD. Interferon-alpha inhibits proliferation and induces apoptosis of merkel cell carcinoma in vitro. Cancer Invest. 2008;26:562–568. doi: 10.1080/07357900701816477. [DOI] [PubMed] [Google Scholar]
- 22.Rosen ST, Gould VE, Salwen HR, Herst CV, Le Beau MM, Lee I, et al. Establishment and characterization of a neuroendocrine skin carcinoma cell line. Lab Invest. 1987;56:302–312. [PubMed] [Google Scholar]
- 23.Van Gele M, Van Roy N, Ronan SG, Messiaen L, Vandesompele J, Geerts ML, et al. Molecular analysis of 1p36 breakpoints in two Merkel cell carcinomas. Genes Chromosomes Cancer. 1998;23:67–71. doi: 10.1002/(sici)1098-2264(199809)23:1<67::aid-gcc10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Leonard JH, Dash P, Holland P, Kearsley JH, Bell JR. Characterisation of four Merkel cell carcinoma adherent cell lines. Int J Cancer. 1995;60:100–107. doi: 10.1002/ijc.2910600115. [DOI] [PubMed] [Google Scholar]
- 25.Leonard JH, Bell JR, Kearsley JH. Characterization of cell lines established from Merkel-cell ("small-cell") carcinoma of the skin. Int J Cancer. 1993;55:803–810. doi: 10.1002/ijc.2910550519. [DOI] [PubMed] [Google Scholar]
- 26.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, et al. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 27.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, et al. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res. 2013;19:1816–1826. doi: 10.1158/1078-0432.CCR-12-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 29.Guastafierro A, Feng H, Thant M, Kirkwood JM, Chang Y, Moore PS, et al. Characterization of an early passage Merkel cell polyomavirus-positive Merkel cell carcinoma cell line, MS-1, and its growth in NOD scid gamma mice. J Virol Methods. 2012;187:6–14. doi: 10.1016/j.jviromet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima H, Takaishi M, Yamamoto M, Kamijima R, Kodama H, Tarutani M, et al. Screening of the specific polyoma virus as diagnostic and prognostic tools for Merkel cell carcinoma. J Dermatol Sci. 2009;56:211–213. doi: 10.1016/j.jdermsci.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita E, Hayashi N, Fukushima A, Ueno H. [Evaluation of treatment and prognosis of Merkel cell carcinoma of the eyelid in Japan] Nippon Ganka Gakkai Zasshi. 2007;111:459–462. [PubMed] [Google Scholar]
- 32.Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872–2881. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 33.Krasagakis K, Almond-Roesler B, Zouboulis CC, Tebbe B, Wartenberg E, Wolff KD, et al. Merkel cell carcinoma: report of ten cases with emphasis on clinical course, treatment, and in vitro drug sensitivity. J Am Acad Dermatol. 1997;36:727–732. doi: 10.1016/s0190-9622(97)80325-8. [DOI] [PubMed] [Google Scholar]
- 34.Biver-Dalle C, Nguyen T, Touze A, Saccomani C, Penz S, Cunat-Peultier S, et al. Use of interferon-alpha in two patients with Merkel cell carcinoma positive for Merkel cell polyomavirus. Acta Oncol. 2011;50:479–480. doi: 10.3109/0284186X.2010.512924. [DOI] [PubMed] [Google Scholar]
- 35.Bajetta E, Zilembo N, Di Bartolomeo M, Di Leo A, Pilotti S, Bochicchio AM, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer. 1993;72:3099–3105. doi: 10.1002/1097-0142(19931115)72:10<3099::aid-cncr2820721035>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C, et al. Cancer-Induced Alterations of NK-Mediated Target Recognition: Current and Investigational Pharmacological Strategies Aiming at Restoring NK-Mediated Anti-Tumor Activity. Front Immunol. 2014;5:122. doi: 10.3389/fimmu.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.