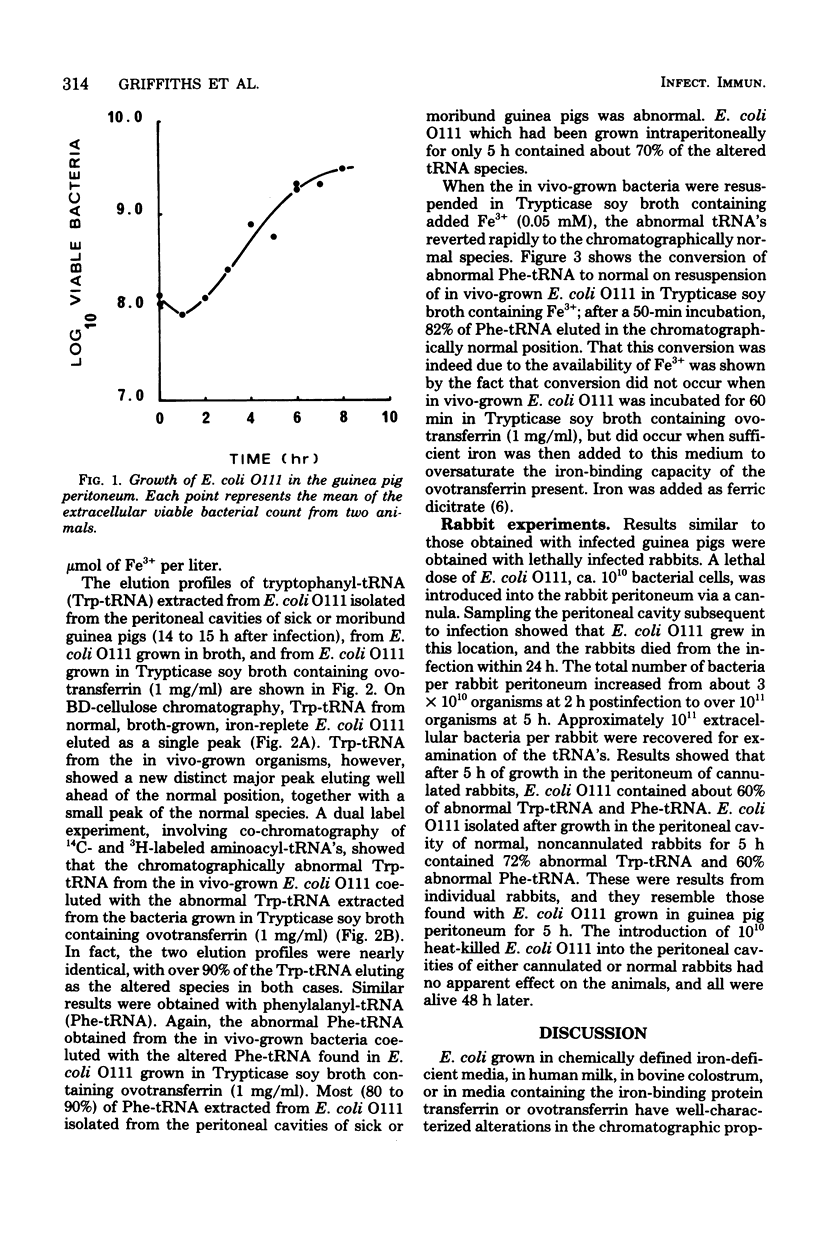
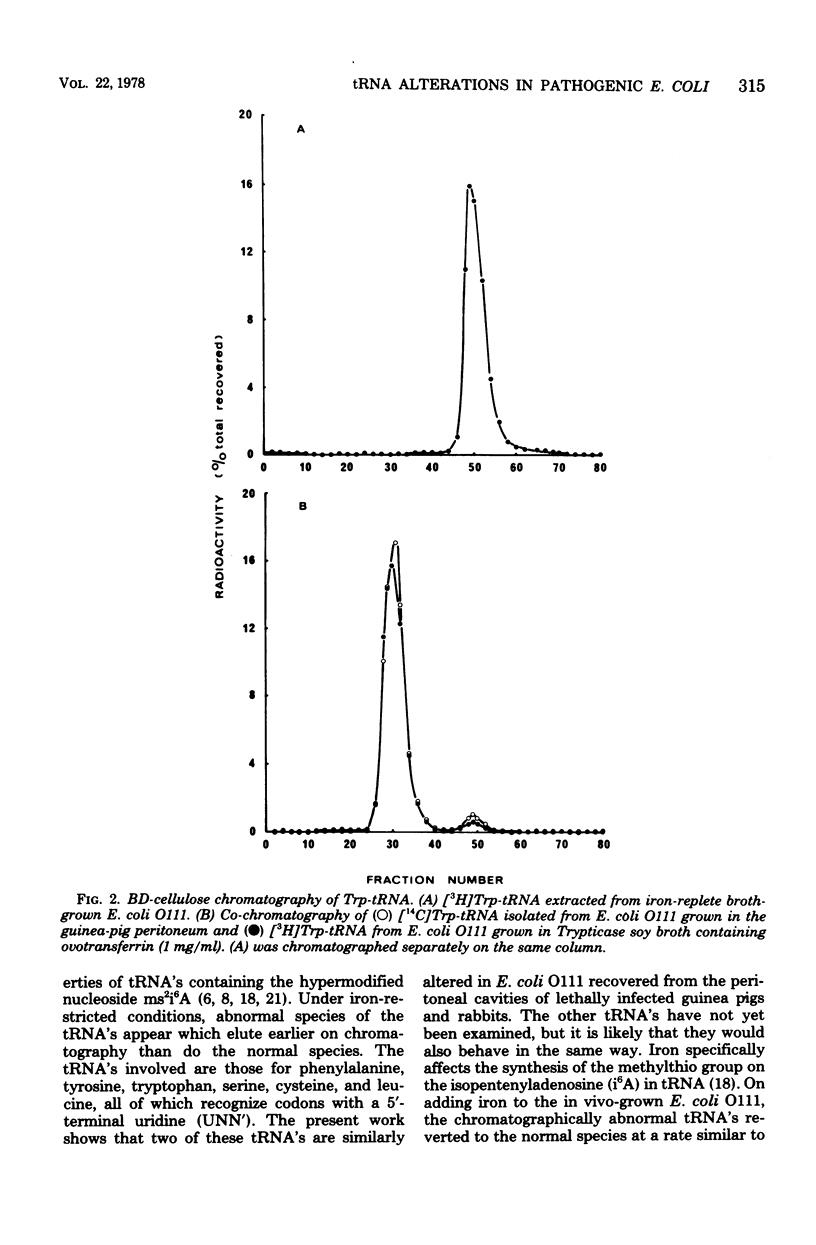
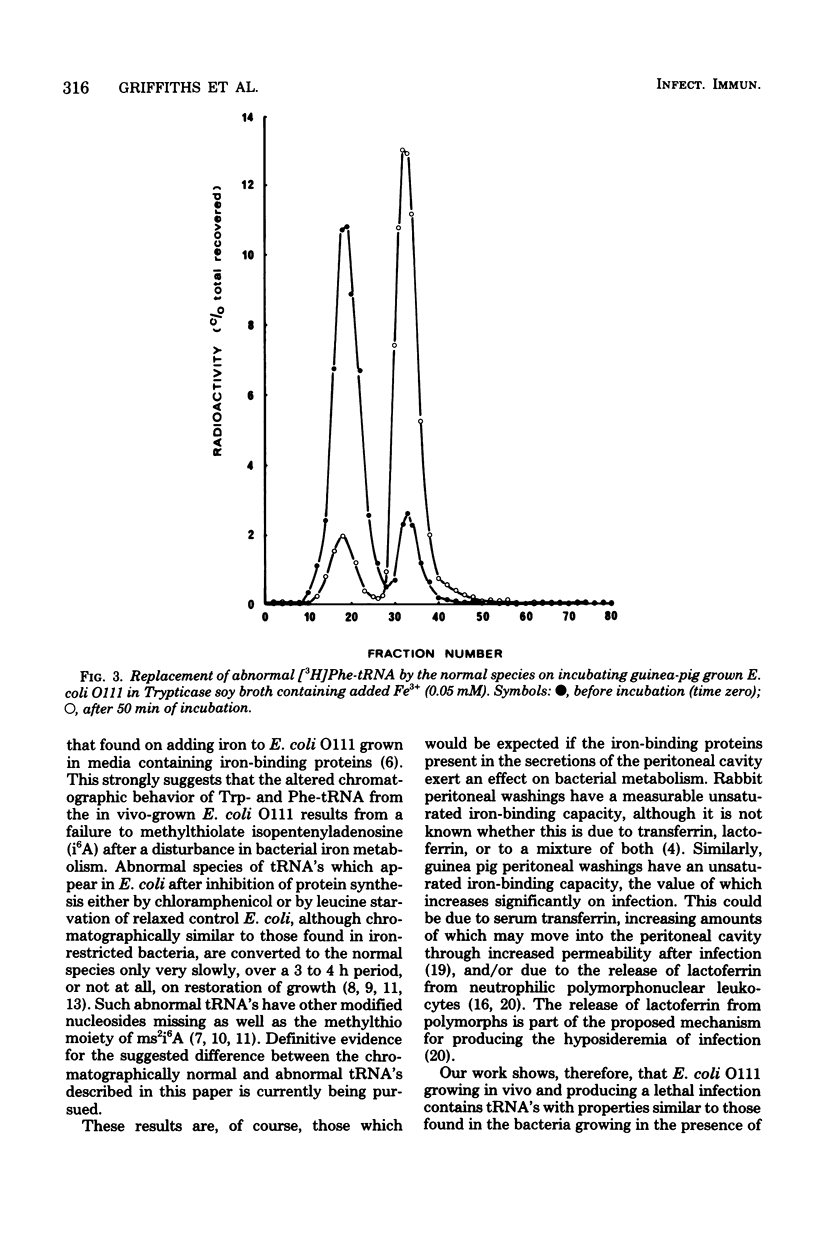
Abstract
Escherichia coli grown in chemically defined iron-deficient media or in fluids containing the iron-binding proteins transferrin, lactoferrin, or ovotransferrin have well-characterized alterations in the chromatographic properties of tRNA's containing the modified nucleoside 2-methylthio-N6-(delta2-isopentenyl)-adenosine. The present work shows that similar tRNA alterations occur in E. coli O111 recovered from the peritoneal cavities of lethally infected guinea pigs and rabbits. Adding iron to these in vivo-grown bacteria resulted in the rapid conversion of chromatographically abnormal tRNA's to the normal species. The work strongly suggests that host iron-binding proteins, present in mucosal and other secretions, can affect the metabolism of invading organisms. The idea that the tRNA alterations are connected with the adaptation of E. coli to growth under the iron restricted conditions imposed by iron-binding proteins in tissue fluids, and thus with bacterial pathogenicity, is therefore made particularly attractive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezkorovainy A., Zschocke R. H. Structure and function of transferrins. I. Physical, chemical, and iron-binding properties. Arzneimittelforschung. 1974 Apr;24(4):476–485. [PubMed] [Google Scholar]
- Bullen J. J., Leigh L. C., Rogers H. J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology. 1968 Oct;15(4):581–588. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Ward C. G., Wallis S. N. Virulence and the role of iron in Pseudomonas aeruginosa infection. Infect Immun. 1974 Sep;10(3):443–450. doi: 10.1128/iai.10.3.443-450.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Alterations in tRNAs containing 2-methylthio-N6-(delta2-isopentenyl)-adenosine during growth of enteropathogenic Escherichia coli in the presence of iron-binding proteins. Eur J Biochem. 1978 Jan 16;82(2):503–513. doi: 10.1111/j.1432-1033.1978.tb12044.x. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Bacteriostatic effect of human milk and bovine colostrum on Escherichia coli: importance of bicarbonate. Infect Immun. 1977 Feb;15(2):396–401. doi: 10.1128/iai.15.2.396-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. C., Mann M. B. Comparative fingerprint and composition analysis of the three forms of 32P-labeled phenylalanine tRNA from chloramphenicol-treated Escherichia coli. Biochemistry. 1974 Nov 5;13(23):4704–4710. doi: 10.1021/bi00720a004. [DOI] [PubMed] [Google Scholar]
- Juarez H., Skjold A. C., Hedgcoth C. Precursor relationship of phenylalanine transfer ribonucleic acid from Escherichia coli treated with chloramphenicol or starved for iron, methionine, or cysteine. J Bacteriol. 1975 Jan;121(1):44–54. doi: 10.1128/jb.121.1.44-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Fournier M. J. Modification-deficient transfer ribonucleic acids from relaxed control Escherichia coli: structures of the major undermodified phenylalanine and leucine transfer RNAs produced during leucine starvation. Biochemistry. 1977 May 17;16(10):2213–2220. doi: 10.1021/bi00629a027. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Fournier M. J. Unbalanced growth and the production of unique transfer ribonucleic acids in relaxed-control Escherichia coli. J Bacteriol. 1975 Dec;124(3):1382–1394. doi: 10.1128/jb.124.3.1382-1394.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Webb E., Fournier M. J. Unique phenylalanine transfer ribonucleic acids in relaxed control Escherichia coli: genetic origin and some functional properties. Biochemistry. 1976 May 4;15(9):1848–1857. doi: 10.1021/bi00654a010. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Huang P. C. Behavior of chloramphenicol-induced phenylalanine transfer ribonucleic acid during recovery from chloramphenicol treatment in Escherichia coli. Biochemistry. 1973 Dec 18;12(26):5289–5294. doi: 10.1021/bi00750a011. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Gefter M. L. An iron-dependent modification of several transfer RNA species in Escherichia coli. J Mol Biol. 1969 Dec 28;46(3):581–584. doi: 10.1016/0022-2836(69)90197-1. [DOI] [PubMed] [Google Scholar]
- Stoner H. B., Bullen J. J., Cushnie G. H., Batty I. Fatal intraperitoneal infection with Clostridium welchii Type A in passively immunised guinea-pigs. The effect on vascular permeability. Br J Exp Pathol. 1967 Jun;48(3):309–318. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Stent G. S. Physiologically induced changes in the property of phenylalanine tRNA in Escherichia coli. J Mol Biol. 1968 Nov 28;38(1):25–40. doi: 10.1016/0022-2836(68)90126-5. [DOI] [PubMed] [Google Scholar]



