Abstract
Purpose
Childhood acute lymphoblastic leukemia (ALL) is treated with potentially neurotoxic drugs and neurologic complications in long-term survivors are inadequately studied. This study investigated neurologic morbidity and its effect on quality of life in long-term survivors of childhood ALL.
Methods
Prospective, single institution, cross-sectional, institutional review board-approved study of long-term ALL survivors. Participants were recruited from institutional clinics. Participants answered an investigator-administered questionnaire followed by evaluation by a neurologist. Quality of life (QOL) was also assessed.
Results
Of the 162 participants recruited over a 3-year period, 83.3 % reported at least one neurologic symptom of interest, 16.7 % had single symptom, 11.1 % had two symptoms, and 55.6 % had three or more symptoms. Symptoms were mild and disability was low in the majority of participants with neurologic symptoms. Median age at ALL diagnosis was 3.9 years (0.4–18.6), median age at study enrollment was 15.7 years (6.9–28.9), and median time from completion of ALL therapy was 7.4 years (1.9–20.3). On multivariable analyses, female sex correlated with presence of dizziness, urinary incontinence, constipation, and neuropathy; use of≥10 doses of triple intrathecal chemotherapy correlated with uri-nary incontinence, back pain, and neuropathy; cranial radiation with ataxia; history of ALL relapse with fatigue; and CNS leukemia at diagnosis with seizures. Decline in mental QOL was associated with migraine and tension type headaches, while physical QOL was impaired by presence of dizziness and falls. Overall, good QOL and physical function was maintained by a majority of participants.
Conclusions
Neurologic symptoms were present in 83 % long-term ALL survivors. Symptoms related morbidity and QOL impairment is low in majority of survivors. Female sex, ≥10 doses of intrathecal chemotherapy, and history of ALL relapse predispose to impaired QOL.
Implications for Cancer Survivors
This study will educate survivors and their care providers regarding cancer or treatment-related neurologic symptoms and morbidity. This study will help them understand factors contributing to impaired QOL when present.
Keywords: Childhood, Acute lymphoblastic leukemia, Neurologic outcome, Quality of life
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy and requires aggressive CNS-directed chemotherapy to achieve cure rates of over 85 % [1]. Acute neurologic complications are not uncommon and include seizures, encephalopathy, neuropathy, and stroke-like episodes [2]. Long-term cognitive deficits and their association with white matter changes are also well documented. However, long-term neurologic complications of ALL and its treatment are currently inadequately studied and reported. Utilizing self-reported questionnaire and sibling control, the Childhood Cancer Survivor Study (CCSS) reported elevated risk of coordination problems, sensory-motor problems, seizures, and headaches in more than 4,000 long-term survivors of ALL [3]. However, symptoms were not fully defined and quality of life (QOL) was not determined. Furthermore, self-reporting questionnaires may under- or over- estimate presence of symptoms [4].
With increasing pool of ALL survivors, pediatricians and primary care givers are expected to manage and take care of their medical issues. This requires understanding long-term effects of cancer and its treatment on survivor's health. Results of CCSS were not available at the time of study development. We hypothesized that childhood ALL survivors will have a high prevalence of neurologic symptoms and signs. We defined neurologic symptom as something a patient will experience or report to her primary physician and which a primary physician will consider for neurologist referral. Neurologic sign was defined as something a neurologist will find on neurological examination of a survivor, such as weakness, incoordination, loss of sensation etc. Some symptoms such as dizziness or back pain could be appropriate for multi-specialty referral but are seen in a neurologist office as well. Non-neurologic pain such as from joints or ligaments other than the back was not included.
Long-term survivors of childhood cancer continue follow-up at our institution until their 18th birthday and must achieve a 10-year cancer free status before they are discharged from care, even if it means follow-up beyond their 18th birthday. This and <10 % loss to follow-up rate provided us a large cohort in which to study neurologic complications and their impact on QOL.
Methods
This was an institutional review board-approved, prospective, cross-sectional study of childhood ALL survivors. Informed consent was obtained from participants when 18 years of age or older, and from parents when younger. An introductory letter was mailed to all potential participants. Patients were then recruited during their annual follow-up visits. Eligibility criteria included the following: treatment on institutional protocols, at least 5 years from the time of ALL diagnosis, at least 1 year from completion of all cancer therapy, English as a primary language, and absence of a pre-existing cognitive disorder preventing study evaluation. Presence of symptoms of interest prior to ALL diagnosis did not preclude enrollment.
Of the 432 survivors meeting eligibility criteria that visited the hospital between December 2005 to October 2008, 260 could be approached to participate. Of these, 232 (89.2 %) consented but 58 could not be scheduled during their annual visit due to patient's (n=8) or physician's (n=50) schedule. An additional 12 survivors did not show for their appointment. Thus, we recruited 162 survivors which constitute 37 % of the possible 432 eligible survivor and 80 % of 202 eligible and available survivors. All 162 were treated on institutional protocols, Total 11 (6 %), Total 12 (7 %), Total 13 (54 %), Total 14 (10 %), and Total 15 (23 %). We collected information on intrathecal and high dose intravenous methotrexate doses. Such data was not collected for vincristine as there was small difference in cumulative doses per protocol; 34 to 38 doses of 1.5 mg/m2 (maximum 2.0 mg) in all protocols except somewhat higher 44 doses in Total 15. No one was excluded due to a pre-existing condition. Medical records were reviewed and a questionnaire exploring neurologic symptoms was administered by trained investigators. Each symptom was initially explored with standardized question(s); affirmative responses were followed by further questions to define the symptom and assess related disability. Parents could qualify participant's response when appropriate.
Symptoms and signs of interest
Dizziness
Participants were asked a screening question if they suffered dizziness, such as a feeling of lightheadedness, out of balance sensation, or spinning sensation. A positive answer required answering further questions including the formal Dizziness Handicap Inventory questionnaire [5]. This 25-item scale evaluates the impact of dizziness on physical, functional, and emotional function. Scores achieved on this scale can vary from 0 (no disability) to 100 (marked disability).
Fatigue
The presence of fatigue was ascertained according to criteria proposed by Cella et al. [6]. All participants were asked a screening question if they experienced significant fatigue, diminished energy, or increased need to rest that is not related to recent activity. Those answering in affirmative answered 10 additional screening questions; affirmative responses to at least 5 qualified for a diagnosis of fatigue. The Brief Fatigue Inventory was then administered to participants determined to have fatigue [7]. This is a seven item questionnaire, each item is scored 0–10, and a mean score is calculated. A mean score of >7 indicates severe fatigue, 0 as no fatigue and 1–6 as mild to moderate fatigue.
Falls, stroke, bladder and bowel function
Standard clinical questions were used to assess bladder and bowel function and the impact of reported dysfunction on dayto-day activity.
Headache
Each participant was asked “Do you suffer from headaches lasting at least an hour and not attributable to a specific cause or illness, such as fever, trauma, medications etc.” Affirmative answer required answering 24 additional questions designed to define headache type and severity. The diagnosis of headache and its different types were based on the International Classification of Headache Disorders-2nd Edition [8]. Probable migraine headaches were considered as migraine and infrequent and frequent tension type headaches were categorized as episodic tension type headache. Chronic headaches were diagnosed if present for more than 15 days each month for the last three consecutive months. Headache-related disability was determined by the Migraine Disability Assessment Scale (MIDAS) questionnaire and its pediatric version when appropriate [9, 10]. A score of 0–5 on MIDAS is considered minimal or no disability, 6–10 as mild, 11–20 as moderate, and >21 as severe disability.
Seizures
Participants were asked about history of seizures and its phenotype. The study neurologist identified the seizure type according to the International League against Epilepsy criteria [11]. Seizure severity was categorized by the Liverpool Seizure Severity Score [12]. This 12 item questionnaire is scored 0–100, with low scores suggesting minimal or no disability and highest score suggesting marked seizure related disability.
Neuropathy
A neuropathy symptom score was obtained on every participant [13]. This is a 16-item questionnaire that assesses cranial nerve function and sensory, motor, and autonomic symptoms of neuropathy. Presence and type of neuropathy was determined based on both the questionnaire responses and evaluation by the study neurologist. Common Terminology Criteria for Adverse Events v4.0 (CTCAE) toxicity grades were applied.
Back pain
Clinical questions were used to evaluate the presence of back pain. The Modified Hanover Low Back Pain Disability Questionnaire assessed its impact on physical function [14]. This is a 9-item questionnaire scored 0–9, where higher numbers represent greater disability.
Attention deficit disorder
As previously published [15], participants responded to an 18-item questionnaire probing symptoms of attention deficit hyperactivity disorder consistent with the Vanderbilt Attention-Deficit Hyperactivity Disorder Parent Rating Scale [16]. DSM-IV criteria were then applied to determine if a participant had attention deficit, hyperactivity, or both.
Ataxia
The Scale for Assessment and Rating of Ataxia (SARA) was used to determine the presence and severity of ataxia [17]. This is an 8 item scale with a score range of 0 (no ataxia) to 40 (most severe ataxia).
Quality of life
Health elated QOL was assessed with The Medical Outcome Survey Short Form-36 (SF36) [18] and could be collected in 141 participants. Physical and emotional component summary scale scores and individual subscales were utilized. Each component summary scale includes four subscales: physical functioning (all physical activities), role physical (problems with work or daily activities because of physical condition), bodily pain, and general health comprise physical component summary scale; while vitality, social functioning, role emotional (problem with work or daily activities as a result of emotional problems), and mental health comprise the mental component summary scale. Summary and individual scales have a mean population score of 50 and standard deviation of 10. A score of <40 on summary or individual scales indicates poor QOL.
Neurologic evaluations
Each participant underwent evaluation by one of the study neurologists (RBK or EBM) that included further assessment of pertinent symptoms and a thorough standardized neurologic evaluation. A Modified Mini Mental Status examination was also conducted [19]. This is scored on a scale of 0 to 100 where lower scores suggest impaired function.
Statistical analysis
Descriptive statistics and frequencies of neurologic symptoms and signs were calculated (Tables 1 and 2). Univariate logistic regression analysis was used for each neurologic morbidity (symptom or sign of interest) to explore its association with demographic (sex, age at cancer diagnosis) and medical variables (history of leukemia relapse, CNS leukemia at diagnosis, radiation, high dose intravenous methotrexate use, intrathecal methotrexate doses, and body mass index). Variables with a p value<0.1 were selected into the multiple logistic regression model. Time since ALL diagnosis was included as a continuous variable in the multivariable model. The factor was considered to be associated with outcome if the p value was 0.05 or less (Table 3). For the analysis of quality of life, frequency, mean, range, and standard deviation were provided for QOL scales (Table 4). Similarly, univariate logistic regression analysis was used to find the associations between abnormal QOL scales and neurologic symptoms listed in Table 2. Variables with a p value<0.1 were selected into the multiple logistic regression model and Table 5 presents the symptoms with p values 0.05 or less. All analyses were done using SAS 9.2 (SAS Institute, Cary, NC).
Table 1.
Demographic features and study variables of the cohort (n =162)
| Variable | Frequency (%) |
|---|---|
| Sex | |
| Male | 90 (56 %) |
| Female | 72 (44 %) |
| Race | |
| White | 146 (90 %) |
| Age ≤3 years at cancer diagnosis | 57 (35 %) |
| History of leukemia relapse | 8 (5 %) |
| CNS leukemia at diagnosisa | 39 (24 %) |
| CNS radiation | 23 (14 %) |
| Stem cell transplantation | 0 (0 %) |
| Intravenous methotrexate dose | 25 (15 %) |
| ≥ 5 g/m2 | |
| Number of intrathecal chemotherapy dosesb | |
| Median (range) | 9 (9-23) |
| 9-12 | 100 (61.7 %) |
| ≥13 | 62 (38.3 %) |
| Body mass index at diagnosis | |
| Overweight | 39 (24 %) |
| Obese | 44 (27 %) |
| Hypertension | |
| Pre | 15 (9 %) |
| Definite | 6 (4 %) |
| Median age at cancer diagnosis (range) | 3.9 years (0.4-18.6) |
| Median age at study enrollment (range | 15.7 years (6.9-29.0) |
| Median time from cancer diagnosis (range) | 10.2 years (range 5-22.7) |
| Time from last treatment | |
| Median (range) | 7.4 years (1.9-20.3) |
| 25th quartile | 4.0 years |
| 75th quartile | 10.6 years |
| Median hemoglobin level (range) | 13.9 g/dL (11.4-17.2) |
Both CNS2 and CNS3
All participants received triple intrathecal therapy with cytarabine, methotrexate, and hydrocortisone
Table 2.
Frequency of neurologic symptoms and signs (n=162)
| Variable | Yes |
|---|---|
| Dizziness | 54 (33.3 %) |
| 1 to 11 episodes/year | 36 (22.2 %) |
| ≥12 episodes/year | 18 (11.1 %) |
| Fatigue | 35 (21.6 %) |
| Mild | 21 (13 %) |
| Moderate to severe | 14 (8.6 %) |
| Falls | 25 (15.4 %) |
| Occasional | 18 (11.1 %) |
| Frequent | 7 (4.3 %) |
| Urinary urgency | 14 (8.6 %) |
| Urinary hesitancy | 13 (8 %) |
| Urinary incontinence | 24 (14.9 %) |
| Constipation | 34 (21 %) |
| Fecal incontinence | 3 (1.9 %) |
| Any headache | 76 (46.9 %) |
| Migraine headache | 51 (31.5 %) |
| Tension type headache | 49 (30.2 %) |
| Headache disability | 34 (21 %) |
| Mild | 22 (64.7 %) |
| Moderate to severe | 12 (35.3 % |
| Seizures | 17 (10.5 %) |
| On anti-epileptic drug | 3 (2 %) |
| Neuropathy | 102 (63 %) |
| Back pain | 37 (22.8 %) |
| Attention deficit | 17 (10.5 %) |
| Incoordination | 44 (27 %) |
| Mild swallowing difficulty | 5 (3 %) |
| Normal cranial nerves | 162 (100 %) |
| Distal sensory impairment | 2 (1 %) |
| Involuntary movementsa | 1 (0.6 %) |
| Distal leg motor weakness | 3 (2 %) |
| Absent muscle stretch reflexes | 24 (15 %) |
| Karnofsky ≥90 | 161 (99 %) |
| Mini mental status exam ≤70 | 4 (2 %) |
Tremor
Table 3.
Positive associations for neurologic morbidity on multivariate logistic regression analyses (n=162)
| Neurologic symptom | Variable | Effect | Odds ratio | 95 % Confidence interval | p value | |
|---|---|---|---|---|---|---|
| Dizziness | Gender | Female vs. male | 2.01 | 1.01 | 4.0 | 0.05 |
| H/O leukemia relapse | Yes vs. no | 4.81 | 1.02 | 22.71 | 0.05 | |
| Fatigue | H/O leukemia relapse | Yes vs. no | 8.35 | 1.16 | 59.93 | 0.03 |
| Urine incontinence | Gender | Female vs. male | 4.49 | 1.64 | 12.26 | <0.0001 |
| Age at Dx | Older vs. younger | 0.84 | 0.71 | 0.99 | 0.04 | |
| IT-chemotherapya | ≥10 vs. <10 doses | 3.61 | 1.31 | 9.95 | 0.01 | |
| Constipation | Gender | Female vs. male | 2.33 | 1.05 | 5.16 | 0.04 |
| Seizure | CNS cancer | Yes vs. no | 3.03 | 1.05 | 8.73 | 0.04 |
| Ataxia | Radiation | Yes vs. no | 4.78 | 1.64 | 13.93 | <0.0001 |
| Back pain | IT-chemotherapya | ≥10 vs. <10 doses | 0.44 | 0.2 | 0.96 | 0.04 |
| Neuropathy | Gender | Female vs. male | 2.23 | 1.12 | 4.41 | 0.02 |
| IT-chemotherapya | ≥10 vs. <10 doses | 2.0 | 1.02 | 3.93 | 0.04 | |
vs. versus, Dx ALL diagnosis, IT intrathecal
All participants received triple intrathecal therapy with cytarabine, methotrexate, and hydrocortisone
Table 4.
Quality of life in ALL survivors (n=141)
| Scalea | ≤40a | Mean (range) | Standard deviation |
|---|---|---|---|
| Mental summary scale | 11 | 53.8 (21.4-67.7) | 9.2 |
| Vitality | 34 | 49.7 (20.9-70.8) | 12.4 |
| Social functioning | 25 | 49.7 (7.8-62.3) | 11.7 |
| Role emotional | 11 | 52.9 (9.2-55.9) | 9.9 |
| Mental health | 7 | 57.5 (19.0-64.1) | 9.2 |
| Physical summary scale | 11 | 52.4 (27.3-62.0) | 7.3 |
| Physical functioning | 11 | 53.1 (14.9-57.0) | 7.7 |
| Role physical | 15 | 53.0 (17.7-56.9) | 8.5 |
| Bodily pain | 14 | 54.2 (24.1-62.1) | 9.6 |
| General health | 13 | 53.0 (21.0-63.9) | 9.3 |
Population mean is 50 with standard deviation of 10. A score of ≤40 indicates impaired quality of life
Table 5.
Positive associations of neurologic symptoms with QOL by multivariate logistic regression analyses (n=141)
| QOL scale | Symptom | Odds ratio | 95 % CI | p value |
|---|---|---|---|---|
| Mental health summary score | Migraine | 19.0 | 1.2-297.1 | 0.04 |
| Tension type | 17.3 | 1.0-316.9 | 0.05 | |
| Vitality | Fatigue | 5.7 | 1.6-20.0 | 0.01 |
| Back pain | 3.2 | 1.0-10.7 | 0.05 | |
| Social functioning | No correlation | |||
| Role emotional | No correlation | |||
| Mental health | Fatigue | 12.0 | 1.8-79.8 | 0.01 |
| Tension type headache | 13.1 | 1.3-132.0 | 0.03 | |
| Physical health summary score | Dizziness | 9.2 | 1.4-62.3 | 0.02 |
| Fall | 11.9 | 1.9-72.9 | 0.01 | |
| Physical function | Falls | 4.4 | 1.0-19.3 | 0.05 |
| Role physical | Fatigue | 6.4 | 1.4-29.0 | 0.02 |
| Bodily pain | No correlation | |||
| General health | Fatigue | 4.9 | 1.3-19.0 | 0.02 |
QOL quality of life, CI 95 % confidence interval
Results
All 162 participants answered the questionnaire and completed the evaluation by a neurologist. Comparison of participants and non-participants is already published [15]. There was no difference between participants and non-participants (n=270) in gender distribution (p=0.89), age at ALL diagnosis (p= 0.99), ALL risk group (p=0.53), B or T cell lineage (p=0.94), high dose methotrexate (p=0.26) or intrathecal methotrexate use (p=1.00), prednisone (p=0.98) or dexamethasone use (p= 0.28), and treatment with cranial radiation (p=0.29). QOL data was collected on 141 participants. Chemotherapy alone was used in 86 %, and all received triple intrathecal therapy with methotrexate, cytarabine, and hydrocortisone.
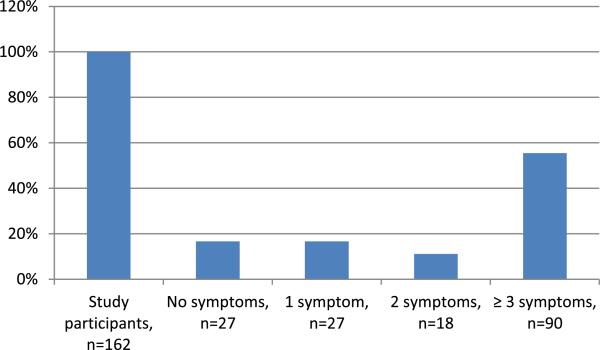
Clinical and demographic variables are provided in Table 1. There were 90 male and 72 female participants with median age of 3.9 years (range 0.4–18.6 years) at ALL diagnosis and 15.7 years (range 6.9–29 years) at study enrollment. Median time for ALL diagnosis was 10.2 years (range 5–22.7 years) and from last cancer treatment was 7.4 years (range 1.9– 20.3 years). Majority (90 %) were of the white race. Table 2 provides prevalence of neurologic symptoms; at least one symptom of interest was present in 135 patients (83.3 %) and three or more in 90 (55.6 % [Fig. 1]).
Fig. 1.
Prevalence of neurologic symptoms in 162 study participants
Dizziness
Dizziness was reported by 54 (33.3 %) participants with 36 (22.2 %) reporting <12 episodes and 18 (11.1 %) ≥12 episodes in the last year; 3 of the 18 with ≥12 episodes had constant symptoms. Impairment of balance in these episodes occurred some of the time in 23 and often in 1 participant. Median duration of dizzy symptoms was 4.65 years (range 0.1– 14.7 years). History of leukemia relapse and female sex were associated with dizziness.
Dizziness interfered with function (CTCAE grade-2) in five participants with 1 reporting interference with activities of daily living (CTCAE grade-3). Similarly, low scores were reported on physical, functional, and emotional components of the dizziness handicap inventory with three participants restricting activities of daily living and 14 scored low on the emotional subscale, suggesting some dizziness related frustration.
Fatigue
Fatigue was determined in 35 (21.6 %) participants: 21 (13 %) with mild (CTCAE grade-1), 11 (6.8 %) with moderate (CTCAE grade-2), and 3 (1.8 %) with severe fatigue (CTCAE grade-3). This was confirmed by examining scores on the Brief Fatigue Inventory where three participants scored in the severe range (mean score ≥7) and 12 had moderate fatigue (mean score >4). History of leukemia relapse increased the risk of fatigue.
Falls
Falls were reported by 25 (15.4 %) participants with 7 (4 %) experiencing them often. Associated injury was reported some of the time by 14 (9 %) and often by 6 (3 %) participants. Fear of falls resulted in restriction of work, play, or leisure activities in 7 (4 %) participants. No risk factor was identified.
Cerebrovascular accidents
No patient was determined to have a transient ischemic attack or stroke.
Bladder and bowel
Increased urinary urgency was reported by 14 (8.6 %) participants and hesitancy by 13 (8 %), moderate in 3 and mild in 10. Urinary incontinence was present in 24 participants (14.9 %): rarely with stress (straining) in 5, often with stress in 1, rarely without stress in 12, and often without stress in 6. Restriction of outside home activities was reported sometimes, often, or all the time by one participant each. No risk factor was detected for urinary urgency, while female sex and ≥10 doses of intrathecal chemotherapy increased the risk of urinary incontinence; older age at ALL diagnosis reduced the risk of urinary incontinence.
Constipation was reported by 34 (21 %) participants. Most reported it as mild except two participants who required occasional and one frequent enema use. Fecal incontinence was reported by three (1.9 %) participants, two with rare soiling and one with frequent soiling and needing diaper use. Female gender increased constipation risk.
Headache
The International Headache Society criteria for headache presence were satisfied by 76 (47 %) participants. Migraine headaches were present in 51 (31 %) and 25 (15 %) experienced auras. Tension type headache was diagnosed in 49 (30 %). Both migraine and episodic tension type headaches were present in 24 (15 %) and 18 (11 %) participants had chronic daily headaches. MIDAS score in those with headaches suggested absence of disability in 42, mild disability in 22, moderate disability in 7, and severe disability in 5 participants. No risk factor was identified for migraine headaches, tension type headaches, or for headache-related disability.
Seizures
Seizures were reported by 17 (11 %) participants: seizures developed prior to ALL diagnosis and as a presenting symptom of ALL in two each, three developed seizures during first induction, three in consolidation phase, and the rest after 6-months of treatment. Median time from ALL diagnosis to first seizure was 6.2 months (range -11.4 to 48.8 months). Seizure types included complex partial in nine, generalized tonic-clonic in five, atonic in one, simple partial in one, and myoclonic in one participant. Only three participants were on anti-seizure drugs at enrollment and one reported multiple seizures a month. Two participants reported a seizure in the prior 4 weeks: one scored 60 on the Liverpool Disability Scale suggesting significant disability while the other scored zero suggesting no disability. Presence of CNS leukemia at ALL diagnosis correlated with seizures.
Attention deficit and hyperactive disorder
As reported previously [15], 10.5 % of the participants fulfilled the criteria for any subtype of attention deficit and hyperactivity disorder. Treatment with cranial radiation was associated with inattention.
Neuropathy
Any neuropathy was present in 102 (62.9 %) participants. Glove and stocking type sensory neuropathy was present in 65 (40 %), CTCAE grade-1 in 63 and grade-2 in 2 participants. Distal weakness with motor neuropathy was present in three (2 %) participants. Autonomic symptoms by questionnaire were present in 47 (29 %) and cranial nerve dysfunction in 10 (6 %) participants. Female sex and ≥10 doses of intrathecal methotrexate correlated with neuropathy.
Back pain
Recurrent back pain was reported by 37 (23 %) participants. School back pack was carried by 19 (12 %) with 11 reporting it to be heavy. Back pain was present for <6 months in 9, 12 months in 2, 2 years in 7, and >2 years in 19. Nine participants had back pain for ≥15 days of each month. Back pain intensity was ≥6 on a pain scale of 1–10 in 21 participants. Twenty had sought no medical help for their back pain. No or low impairment of function according to the Hanover low back pain disability scale was present in 27, moderate in 3, and significant impairment in 7 participants. No risk factor was identified.
Neurologic examination
Most participants had a normal examination (Table 2) except for a mild ataxia and incoordination in 44 (27 %) participants. CNS radiation was a risk factor for ataxia. Amongst 150 participants rated on the Modified Mini Mental Score, a score of ≥90 was obtained by 132 (81 %) participants. A 150-ml water drinking test was normal in 157 (97 %) and mildly abnormal in 5 (3 %) participants. Distal motor weakness was found in 3 (2 %) and sensory impairment in 2 (1 %) participants. Karnofsky performance score was ≥90 in all except one participant who had a score of 50.
Quality of life
Mental and Physical component summary scale scores were ≤40 in 11 (10 %) amongst 141 tested participants (Tables 4 and 5). On multivariable analyses, QOL impairment on the mental component summary scale score correlated with migraine and tension type headaches. Dizziness and history of falls correlated with impaired QOL on the physical component summary scale score. Presence of fatigue impaired sub-scales of vitality, mental health, role physical (work and daily activities), and general health. Subscale of vitality was impaired by presence of back pain.
Discussion
This is the first study of childhood ALL survivors featuring neurologic outcomes assessed by an investigator-administered questionnaire followed by a structured evaluation by a neurologist. This single institution study confirms the CCSS finding of high prevalence of neurologic symptoms in childhood ALL survivors with cancer relapse as a risk factor. However, with more robust methodology, we show a much higher rate of neurologic complications (83 versus 44 %) and new findings such as bladder and bowel impairment, headache types, back pain, and their impact on QOL. We also found higher prevalence of incoordination, seizures, dizziness, sensory neuropathy, and headache when compared to CCSS which had a slightly older cohort (median age 15.7 versus 20.2 years) [3]. In addition to longer follow-up and larger cohort size in CCSS, the difference between the two studies is possibly explained by lack of physician contact in the CCSS and administration of the questionnaire by an investigator in our study. The CCSS questionnaire may also have missed minor symptoms as for example only severe headaches were reported.
We studied multiple variables as risk factors for neurologic symptoms and morbidity. Like CCSS, we noted increased risk of seizures in ALL survivors when compared to the general population [20]. CNS leukemia was a risk factor for seizures in our study and was not available in CCSS. History of ALL relapse also correlated with presence of dizziness and fatigue. Other risk factors included ≥10 doses of intrathecal chemotherapy correlating with urinary incontinence and neuropathy, while radiation treatment was a risk factor for ataxia. CNS leukemia, history of ALL relapse, and CNS radiation may imply greater use of CNS-directed therapy. Both radiation treatment and high dose methotrexate are recognized to cause white matter injury [21]. We also noted an association between history of ALL relapse and use of ≥10 doses of intrathecal chemotherapy (p=0.03), suggesting that ALL relapse may be the most important risk factor by causing increased exposure to neurotoxic drugs. Age was not a predictor of neurologic morbidity except younger age correlating with urinary incontinence.
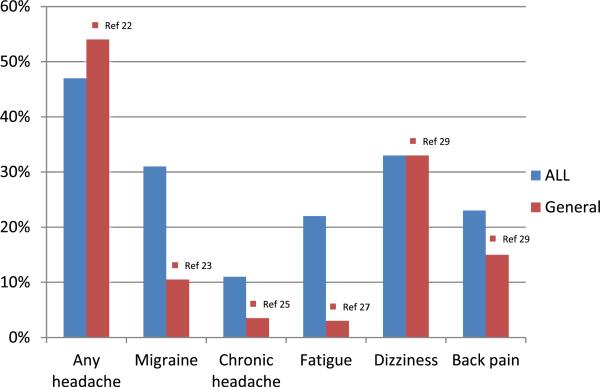
Most commonly reported neurologic symptoms were neuropathy (63 %), headaches (47 %), dizziness (33 %), migraine headaches (31 %), tension type headaches (30 %), and fatigue (22 %). Prevalence of any headache in ALL survivors is perhaps not different from that in the general population [22]. However, migraine headaches are more common in ALL survivors compared to reported prevalence of 7–9 % [22, 23, and Fig. 2]. Tension type headaches and chronic daily headaches in our study are also more common than the 10– 25 % and 3.5 % reported in adolescents [24, 25]. The prevalence of fatigue in our cohort is similar to that reported in ALL survivors and more than that reported in healthy adolescents [26, 27]. We found neuropathy to be less prevalent than that reported by Ramchandren et al. but they had only 37 ALL survivors in their cohort [28]. Dizziness in our cohort was more prevalent compared to CCSS but not much different to otherwise healthy children [29]. However, persistent dizziness of 5 % is similar to the 3.5 % reported by CCSS. Finally, back pain was not related to weight of the back pack, was more common in our cohort when compared to healthy children [29], but much less than that reported in ALL survivors (23 versus 44 %) by Bowers et al. [30]. Eligibility criteria were similar in both studies, but their cohort included 99 survivors, questionnaire was self-administered, and there was no physician contact.
Fig. 2.
Comparison of symptom prevalence in ALL survivors of this cohort with that in the general population
Impairment of bowel and bladder function in a significant minority of patients was a surprising finding of our study. This, to our knowledge, has not been reported in childhood ALL survivors. Ten or more doses of intrathecal chemotherapy increased the risk of bladder dysfunction. It is unclear if this is due to subtle myelopathy, radiculopathy, vincristine-related neuropathy and dysautonomia, or other reasons. The presence of these symptoms highlights the fact that patients may not always volunteer information unless specifically asked.
Reassuringly, only a few had significant disability. This is also reflected in the maintenance of good quality of life in a majority of participants. It seems female gender, history of leukemia relapse, and ≥10 doses of intrathecal chemotherapy may predispose to impairment of QOL. These were the risk factors for symptoms which correlated with impaired QOL.
There are some inherent weaknesses of this study. Recruitment of only 37.5 % of potential participants raises the question of selection bias. However, we believe selection bias is minimal if any as 80 % of eligible and available survivors participated and that there was no difference between participants and non-participants of the study. Single institution cohort with lack of racial diversity and control group also limits generalizability of this study. However, normal data is available on US population for many of the explored symptoms (Fig. 2). Neurologic symptoms were not recorded at the time of ALL diagnosis and this may also limit the conclusion that ALL or its treatment leads to high prevalence of these symptoms. However, we did ask the participants to recall the date of onset of each symptoms and only a handful reported these symptoms prior to ALL diagnosis except 15 of 76 who had headache. The strength of this study lies in the evaluation by a board-certified neurologist in a predefined systematic manner and that the questionnaire was administered by a trained investigator.
In conclusion, 83 % of childhood ALL survivors displayed some neurologic symptoms and signs. Severe neurologic disability is uncommon, and QOL is well maintained in majority of ALL survivors. Physicians should proactively enquire about neurologic symptoms during patient follow-up and offer amelioration when present. This study identifies ALL relapse as perhaps the most important risk factor for neurologic morbidity in childhood ALL survivors.
Acknowledgments
Funding Supported by Cancer Center Support Grant CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities.
Footnotes
Financial disclosure All authors have no financial relationships relevant to this article to disclose.
Conflict of interest There are no financial disclosures, conflicts of interest, and/or acknowledgements for the authors in regards to this manuscript.
Informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients/parents for being included in the study.
Contributor Information
Raja B. Khan, Division of Neurology, St. Jude Children's Research Hospital, Mail stop 220, Memphis, TN 38015, USA
Melissa M. Hudson, Department of Epidemiology and Cancer Control, St. Jude Children's Hospital, Memphis, TN, USA
Davonna S. Ledet, Division of Neurology, St. Jude Children's Research Hospital, Mail stop 220, Memphis, TN 38015, USA
E. Brannon Morris, Division of Neurology, St. Jude Children's Research Hospital, Mail stop 220, Memphis, TN 38015, USA.
Ching-Hon Pui, Department of Oncology, St. Jude Children's Hospital, Memphis, TN, USA.
Scott C. Howard, Department of Oncology, St. Jude Children's Hospital, Memphis, TN, USA
Kevin R. Krull, Department of Epidemiology and Cancer Control, St. Jude Children's Hospital, Memphis, TN, USA
Pamela S. Hinds, Department of Nursing Research and Quality Outcomes, George Washington University, Washington, DC, USA
Debbie Crom, Department of Epidemiology and Cancer Control, St. Jude Children's Hospital, Memphis, TN, USA.
Emily Browne, Department of Epidemiology and Cancer Control, St. Jude Children's Hospital, Memphis, TN, USA.
Liang Zhu, Department of Biostatistics, St. Jude Children's Hospital, Memphis, TN, USA.
Shesh Rai, Department of Biostatistics, University of Louisville, Louisville, KY, USA.
Deokumar Srivastava, Department of Biostatistics, St. Jude Children's Hospital, Memphis, TN, USA.
Kirsten K. Ness, Department of Epidemiology and Cancer Control, St. Jude Children's Hospital, Memphis, TN, USA
References
- 1.Richards S, Pui CH, Gayon P. Childhood Acute Lymphoblastic Leukemia Collaborative Group (CALLCG). Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:185–95. doi: 10.1002/pbc.24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vagace JM, de la Maya MD, Caceres-Marzal C, et al. Central nervous system chemotoxicity during treatment of pediatric acute lymphoblastic leukemia/lymphoma. Crit Rev Oncol Hematol. 2012;84:274–86. doi: 10.1016/j.critrevonc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Goldsby RE, Liu Q, Nathan PC, et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:324–31. doi: 10.1200/JCO.2009.22.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24:1223–30. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolarynol Head Neck Surg. 1990;116:424–7. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Davis K, Breitbart W, Curt G. Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–91. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders. Cephalagia. 2004;24(suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–8. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 10.Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034–9. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 11.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the International League Against Epilepsy. Epilepsia. 1989;304:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott-Lennox J, Bryant-Comstock L, Lennox R, Baker GA. Reliability, validity and responsiveness of a revised scoring system for the Liverpool Seizure Severity Scale. Epilepsy Res. 2001;44:53–63. doi: 10.1016/s0920-1211(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 13.Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8:590–6. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- 14.Roese I, Kohlmann T, Raspe H. Measuring functional capacity in backache patients in rehabilitation: a comparison of standardized questionnaires. Rehabilitation (Stuttg) 1996;35:103–8. [PubMed] [Google Scholar]
- 15.Krull KR, Khan RB, Ness KK, et al. Symptoms of attention-deficit/hyperactivity disorder in long-term survivors of childhood leukemia. Pediatr Blood Cancer. 2011;57:1191–6. doi: 10.1002/pbc.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol. 2003;28:559–67. doi: 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 18.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Besson PS, Labbe EE. Use of the modified mini-mental state examination with children. J Child Neurol. 1997;12:455–60. doi: 10.1177/088307389701200708. [DOI] [PubMed] [Google Scholar]
- 20.Kelvin EA, Hesdorffer DC, Bagiella E, et al. Prevalence of self-reported epilepsy in a multiracial and multiethnic community in New York City. Epilepsy Res. 2007;77:141–50. doi: 10.1016/j.eplepsyres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–9. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wöber-Bingöl C. Epidemiology of migraine and headache in children and adolescents. Curr Pain Headache Rep. 2013 doi: 10.1007/s11916-013-0341-z. doi:10.1007/s11916-013-0341-z. [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 24.Anttila P. Tension-type headache in childhood and adolescence. Lancet Neurol. 2006;5:268–74. doi: 10.1016/S1474-4422(06)70376-3. [DOI] [PubMed] [Google Scholar]
- 25.Lipton RB, Manack A, Ricci JA, et al. Prevalence and burden of chronic migraine in adolescents: results of the chronic daily headache in adolescents study (C-dAS). Headache. 2011;51:693–706. doi: 10.1111/j.1526-4610.2011.01885.x. [DOI] [PubMed] [Google Scholar]
- 26.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). Sleep. 2008;31:271–81. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamers F, Hickie I, Merikangas KR. Prevalence and correlates of prolonged fatigue in a U.S. sample of adolescents. Am J Psychiatry. 2013;170:502–10. doi: 10.1176/appi.ajp.2012.12040454. [DOI] [PubMed] [Google Scholar]
- 28.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14:184–9. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssens KA, Rosmalen JG, Ormel J, et al. Pubertal status predicts back pain, overtiredness, and dizziness in American and Dutch adolescents. Pediatrics. 2011;128:553–9. doi: 10.1542/peds.2010-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowers DC, Griffith T, Gargan L, et al. Back pain among long-term survivors of childhood leukemia. J Pediatr Hematol Oncol. 2012;34:624–9. doi: 10.1097/MPH.0b013e31827080de. [DOI] [PubMed] [Google Scholar]