Abstract
The purpose of this study was to test for differences in brain shape among children with cleft palate only (CP) (n = 22), children with cleft lip and palate (CLP) (n = 35), and controls (n = 39) using Euclidean distance matrix analysis. Sixteen percent of interlandmark distances differed between children with CP and controls, 10% differed between children with CLP and controls, and 10% differed between children with CP and children with CLP. Major differences in brain shape associated with CL/P included posterior expansion of the occipital lobe, reorientation of the cerebellum, heightened callosal midbody, and posterior displacement of the caudate nucleus and thalamus. Differences in brain shape unique to CP and to CLP were also identified. These results expand upon previous volumetric studies on brain morphology in individuals with CL/P and provide additional evidence that the primary defect in CL/P results in both facial and brain dysmorphology.
Keywords: brain morphology, nonsyndromic orofacial clefting, geometric morphometrics
INTRODUCTION
Nonsyndromic cleft lip and/or palate (CL/P) is one of the most common congenital anomalies in the world, occurring in 1 out of 700 newborns. Although traditionally considered to be a craniofacial disorder, CL/P has been found to be associated with poor academic achievement, a low verbal IQ, and deficits in rapid verbal labeling, verbal fluency, and short-term memory1–4. These cognitive deficits have been attributed to secondary factors, including low self-esteem, depressed mood, and hearing and speech deficits5–9; however, it is possible that these deficits are instead the result of abnormal brain structure and function. Development of the face, the craniofacial skeleton, and the brain are known to be closely linked under both normal and pathological conditions10–12, and a malformation that affects the face may be associated with malformation of the developing brain.
To date, a handful of neuroanatomical studies have been completed on individuals with CL/P, all of which have identified structural brain abnormalities. Nopoulos et al. (2007)13 found total brain volume to be smaller in children with CL/P and identified abnormal tissue distribution within the brain. Specifically, the occipital lobe was enlarged while the frontal lobe, cerebellum, caudate, putamen and globus pallidus were reduced in volume. Adults with CL/P exhibited the opposite pattern – the frontal and parietal lobes were abnormally large while the temporal and occipital lobes were abnormally small14–15. Brain shape also differed among adults with cleft lip with or without cleft palate (CL/P) and controls16. Statistical shape analysis, unlike volumetric analysis, is able to evaluate whether changes in size are global or local, to quantify changes in shape, and to describe how regions are interrelated, thereby assessing the connectivity of the brain17–18. Based on the known developmental integration of face and brain, the purpose of this study was to test the hypothesis that brain shape differs among children with CLP, children with CP, and controls.
METHODS
Patient Population
This project was conducted using magnetic resonance images of the brain and cognitive data originally collected by a team of researchers at the University of Iowa led by Dr. Peg Nopoulos4,13. Children with CL/P were recruited by the University of Iowa Cleft Clinic. To be included in the study, a child with CL/P had to be diagnosed with nonsyndromic CL/P. All children with CL/P were first assessed by an experienced ENT for syndromic CL/P, and any child in whom syndromic CL/P was suspected was referred to a geneticist for additional testing. Exclusion criteria for children with CL/P included braces (which create an artifact in magnetic resonance imaging) and a known full-scale intelligence quotient (FSIQ) of less than 70. Children with an FSIQ of less than 70 were excluded to protect against including subjects with undiagnosed syndromic CL/P. Healthy controls were recruited from the community. Exclusion criteria for controls included braces and diagnosis of a major medical, neurologic, or psychiatric illness. All participants signed an informed consent approved by the University of Iowa review board and were compensated for their involvement in the study. This study was approved by the Johns Hopkins University institutional review board.
In order to control for differences in sex and age but still maximize the sample size, children with CLP, children with CP, and controls were matched by sex and age (within 1 year). This matching process produced 18 triads, of which 11 were male and 7 were female. The remaining children with CL/P were then matched by age and sex to a control. This pairing process created 16 male CLP/control pairs, 1 female CLP/control pair, and 4 female CP/control pairs. All of the boys with CP had already been matched during the creation of the triads, and thus there were no male CP/control pairs. Ninety-six children (31 female and 65 male; ages 7 to 17 years) were included in the current study. Of these, 35 had CLP, 22 had CP, and 39 were controls. Sample descriptions are provided in Table 1. Age did not differ among children with CLP, children with CP, and controls (ANOVA F = 0.86, df = 2, p = 0.427). The sample was ethnically homogenous in order to control for racial variation in skull morphology. Seventy-nine (82%) children self-identified as Caucasian, eight (8%) as Asian American, 1 (1%) as African American, 2 (2%) as Hispanic/Latino, 1 (1%) as Native Hawaiian/Pacific Islander, 4 (4%) as biracial, and 1 (1%) did not disclose his race.
Table 1.
Demographic variables of the sample
| n | Average age (standard deviation) | Age range | FSIQ | VIQ | PIQ | |
|---|---|---|---|---|---|---|
| CONTROL | 39 | 12.5 (± 3.0) | 7.2 – 17.9 | 110 | 110 | 110 |
| Control Males | 27 | 12.5 (± 3.0) | 7.8 – 17.9 | 110 | 110 | 109 |
| Control Females | 12 | 12.5 (± 3.1) | 7.2 – 17.2 | 109 | 109 | 108 |
| CP | 22 | 11.7 (± 3.2) | 7.5 – 17.7 | 100 | 95 | 107 |
| CP Males | 11 | 11.2 (± 3.6) | 7.8 – 17.7 | 104 | 94 | 115 |
| CP Females | 11 | 12.1 (± 2.9) | 7.5 – 17.4 | 97 | 96 | 99 |
| CLP | 35 | 12.7 (± 3.1) | 7.5 – 17.2 | 104 | 101 | 106 |
| CLP Males | 27 | 12.6 (± 3.0) | 7.7 – 17.2 | 105 | 102 | 108 |
| CLP Females | 8 | 13.3 (± 3.4) | 7.5 – 16.7 | 99 | 99 | 99 |
Average age (years), full-scale IQ (FSIQ), verbal IQ (VIQ), and performance IQ (PIQ) of children with CLP, children with CP, and controls by sex. Age, FSIQ, and PIQ did not differ among children with CLP, children with CP, and controls. VIQ was significantly lower in children with CP relative to controls, but no difference in VIQ was detected between children with CLP and either children with CP or controls.
Cognitive Assessment
Cognition was assessed in every child using a battery of neuropsychological tests that measured IQ and several other cognitive domains. Neuropsychological tests were administered by cognitive specialists at the University of Iowa. A description of this assessment is available in Conrad et al. (2009). Approximately 90% of the children included in this study were included in the sample assessed in Conrad et al. (2009). Like in Conrad et al. (2009), after controlling for differences in socioeconomic status, ANCOVA revealed no difference in full-scale IQ (FSIQ) (F = 1.84, df = 2, p = 0.164) or performance IQ (PIQ) (F = 0.13, df = 2, p = 0.880) among children with CP, those with CLP, and controls (Table 1). VIQ was significantly different among the three groups (F = 3.37, df = 2, p = 0.039). Post-hoc analysis with Bonferroni correction revealed that VIQ was lower in children with CP (x̄ = 97) relative to controls (x̄ = 108) (95% CI: 0.26 – 22.62), but no difference in VIQ was detected between CLP (x̄ = 102) and controls (95% CI: −2.66 – 15.68) or children with CP (95% CI: −15.84 – 5.99).
Imaging Methods
Images were obtained with a 1.5-T Signa magnetic resonance scanner (General Electric, Milwaukee, Wisconsin) using a T1 sequencing protocol. Post-acquisition processing was completed by technicians at the University of Iowa using the software BRAINS (Brain Research: Analysis of Images, Networks, and Systems19–23).
Statistical Shape Analysis
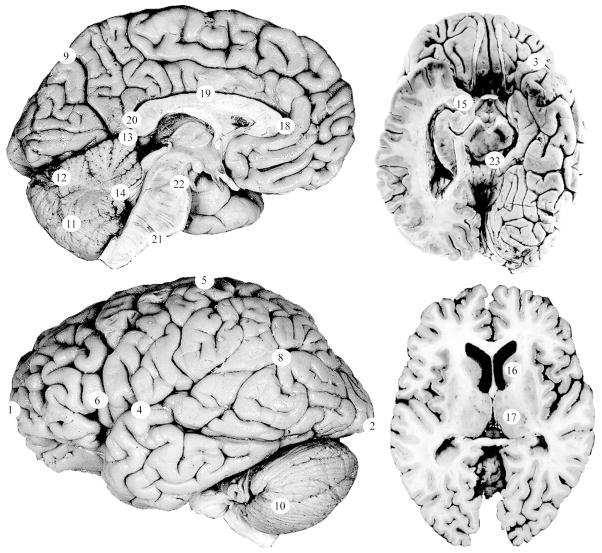
Twenty-three (23) landmarks representing cortical and subcortical structures on the midline and left side of the brain were used to assess brain shape (Table 2, Figures 1–2). Landmarks were collected blind to sex and cleft status using eTDIPS, a multidimensional volume visualization analysis software that allows landmarks to be placed on any of the three planar views or directly on a 3D reconstruction of the brain25–26. Landmarks were only collected on the left side of the brain because when Weinberg et al. (2009)16 analyzed brain shape in adults with CL/P, they used unilateral left brain landmarks. For comparative purposes, it was beneficial to employ the same technique. All of the landmarks that were used in this project were validated in an inter- and intra-observer error study. The average intraobserver error was 1.9 mm with a range of 0.72 to 5.6 mm and the average interobserver error was 1.1 mm with a range of 0.40 mm to 3.4 mm.
Table 2.
Landmarks used to assess brain shape
| Landmark Name | |
|---|---|
| 1 | Frontal pole |
| 2 | Occipital pole |
| 3 | Temporal pole |
| 4 | Central sulcus/Lateral sulcus intersection |
| 5 | Central sulcus – superior termination |
| 6 | Opercular sulcus (i.e., ascending ramus of the lateral sulcus) |
| 8 | Superior temporal sulcus – posterior inflexion |
| 9 | Parietooccipital sulcus – superior termination |
| 10 | Cerebellum – lateral pole |
| 11 | Cerebellum – midsagittal inferior |
| 12 | Cerebellum – midsagittal posterior |
| 13 | Cerebellum – midsagittal superior |
| 14 | Fourth ventricle |
| 15 | Amygdala |
| 16 | Caudate nucleus |
| 17 | Thalamus |
| 18 | Corpus callosum – genu |
| 19 | Corpus callosum – midbody |
| 20 | Corpus callosum – splenium |
| 21 | Pons – inferior |
| 22 | Pons – superior |
| 23 | Superior colliculus |
Twenty-three cortical and subcortical landmarks were used to characterize brain shape. The numbers in the left column correspond with the numbered landmarks in the figures.
Figure 1. Landmarks used to assess brain shape.
Twenty-three landmarks were used to assess brain shape. The landmark numbers correspond to the numbered list in Table 1. Detailed landmark protocols and additional images of these landmarks are available at http://www.hopkinsmedicine.org/fae/vbd.htm. Photographs were adapted with permission from the Digital Anatomist Project24.
Figure 2. Wire frame representation of landmarks.
This wire frame represents the three-dimensional spatial orientation of the twenty-three landmarks used to assess brain shape. The top wire frame is a lateral view of the brain, and the bottom wire frame is a superior view of the brain.
Landmarks were analyzed using Euclidean Distance Matrix Analysis (EDMA)27. Briefly, EDMA assesses shape differences by comparing a matrix of the linear distances that connect pairs of landmarks (i.e., interlandmark distances (ILDs)) in one sample to a matrix of the linear distances that connect the corresponding pairs of landmarks in a second sample27–28. Corresponding ILDs are compared between samples as a ratio, such that if a given ratio is equal to 1, the two samples do not differ with respect to that specific ILD. Statistical significance is assessed using a non-parametric bootstrapping algorithm that ultimately produces a confidence interval (α = 0.05) for each ILD29–30. Three pairwise comparisons were conducted in this study using EDMA: (1) CP vs. control; (2) CLP vs. control; (3) CLP vs. CP.
RESULTS
CP vs. Control
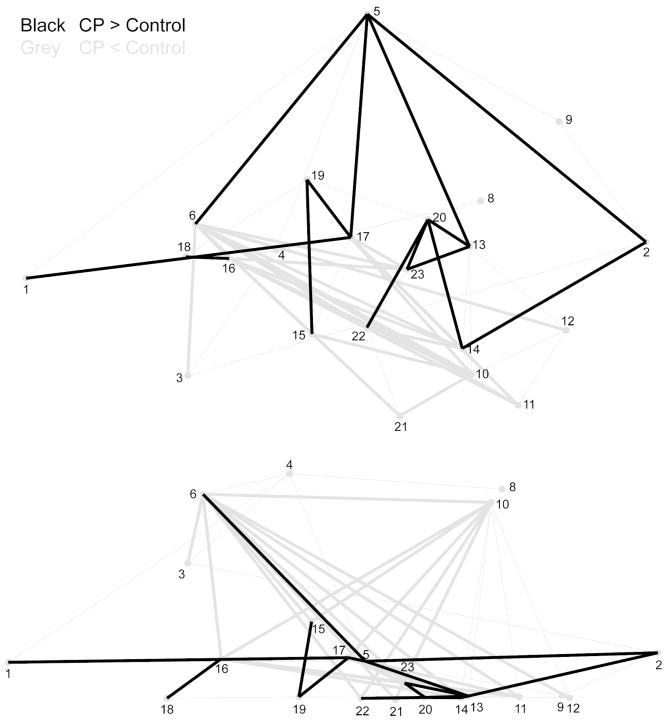
Of the 231 interlandmark distances representing brain shape in children with CP and controls, 22 (10%) were significantly smaller and 14 (6%) were significantly larger in children with CP relative to controls (Figure 3). Twenty-one ILDs (96%) were smaller by at least 2% and seven ILDs (32%) were smaller by at least 4%. In contrast, 12 ILDs (86%) were larger by at least 2% and one (7%) was larger by at least 4%. Smaller ILDs coalesced on Broca’s area (6), the caudate nucleus (16), the thalamus (17), the superior colliculus (23), the amygdala (15), the fourth ventricle (14), the lateral pole of the cerebellum (10), and the inferior cerebellar point (11). Larger ILDs coalesced on the cerebral vertex (5), the occipital pole (2), the midbody (19) and splenium (20) of the corpus callosum, the thalamus (17), the superior colliculus (23), and the superior point of the cerebellum (13). The pattern and directionality of these ILDs indicate that, relative to controls, children with CP had a heightened (5) and lengthened (1, 2) cerebrum, with a narrowed frontal lobe (6). Broca’s area (6) was shifted postero-inferiorly, suggesting that this area was reoriented and possibly reduced in children with CP. The cerebellum was reoriented such that the lateral cerebellar pole (10), the inferior cerebellum (11), and the fourth ventricle (14) were shifted anteriorly, while the superior cerebellum (13) was shifted infero-posteriorly. The callosal midbody (19) and splenium (20) were shifted superiorly, increasing the convexity of the corpus callosum. The caudate nucleus (16) and thalamus (17) were shifted postero-inferiorly, and the superior colliculus (23) was displaced anteriorly, indicating that the arrangement of the subcortical structures was altered in children with CP.
Figure 3. EDMA results of the comparison of brain shape between children with CP and controls.
Black lines represent ILDs that were significantly larger in children with CP relative to controls. Grey lines represent ILDs that were significantly smaller in children with CP relative to controls.
CLP vs. Control
When children with CLP were compared to controls, 16 ILDs (7%) were significantly shorter, of which 11 (69%) were shorter by at least 2% (Figure 4). Eight ILDs (3.4%) were significantly larger, of which seven (88%) were larger by at least 2% and two (25%) were larger by at least 4%. Shorter ILDs coalesced on the caudate nucleus (16), the thalamus (17), the inferior pons (21), and the inferior (11), posterior (12), and superior (13) cerebellar landmarks. Larger ILDs coalesced on the temporal (3) and occipital (2) poles and on the midbody of the corpus callosum (19). The directionality of these ILDs indicates that the occipital pole (2) was shifted supero-posteriorly, reflecting expansion and reorientation of the occipital lobe in children with CLP relative to controls. The temporal lobe (3) was also expanded laterally, while the length (12) and height (11, 13) of the cerebellum were reduced. In addition, the caudate nucleus (16), thalamus (17), and inferior pons (21) were displaced posteriorly. The midbody of the CC (19) was displaced superiorly, increasing the overall convexity of the CC. The distance between the cerebral vertex (5) and the superior temporal sulcus (8) also lengthened in children with CLP by 5%; however, as no other ILDs involving these landmarks were significant, it is unclear whether changes in this ILD reflect an increase in cerebral height, displacement of Wernicke’s area, both or neither.
Figure 4. EDMA results of the comparison of brain shape between children with CLP and controls.
Black lines represent ILDs that were significantly larger in children with CLP relative to controls. Grey lines represent ILDs that were significantly smaller in children with CLP relative to controls.
CP vs. CLP
Euclidean distance matrix analysis of children with cleft palate only versus children with cleft lip and palate identified 10 interlandmark distances (4%) that were larger in children with cleft palate only and 14 interlandmark distances (6%) that were larger in children with cleft lip and palate (Figure 5). Of the ILDs that were larger in children with CP, nine (90%) were larger by at least 2% and two (20%) were larger by at least 4%. These ILDs coalesced on the occipital pole (2), the posterior cerebellum (12), and the splenium of the corpus callosum (20). Of the ILDs that were larger in children with CLP, all were larger by at least 2% and six were larger by at least 4%. These ILDs coalesced on the temporal (3) and cerebellar (10) poles and on Broca’s area (6). Relative to children with CP, children with CLP had a reduced occipital lobe, marked by antero-superior displacement of the occipital pole (2). The temporal lobe (3) was expanded antero-laterally. The posterior extent of the cerebellum (12) was shifted anteriorly, shortening the cerebellum in the A-P direction. Broca’s area (6) was displaced laterally without concurrent displacement of the intersection of the central sulcus with the lateral sulcus (4) or superior temporal gyrus (8), indicating that the frontal lobe was widened anteriorly. Broca’s area (6) was also shifted antero-superiorly, signifying that the extent and orientation of this region differed among children with CLP and CP. The splenium of the corpus callosum (20) in children with CLP was displaced postero-inferiorly, reflecting a difference in the length and convexity of the CC. The inferior margin of the pons (21) was displaced posteriorly.
Figure 5. EDMA results of the comparison of brain shape between children with CP and children with CLP.
Black lines represent ILDs that were significantly larger in children with CP relative to children with CLP. Grey lines represent ILDs that were significantly smaller in children with CP relative to children with CLP.
Brain Shape
When the results of the three pairwise comparisons of EDMA were examined in concert, patterns of shape variation associated with CL/P in general and patterns of shape variation unique to children with CP and to children with CLP could be summarized. These results indicate that CL/P in general was associated with posterior expansion of the occipital lobe and reduction and reorientation of the cerebellum. The magnitude of expansion of the occipital lobe was greatest in children with CP. CL/P was also associated with heightening of the callosal midbody and postero-inferior displacement of the caudate nucleus and thalamus.
CP, specifically, was associated with heightening of the cerebrum, narrowing of the frontal lobe, reorientation of Broca’s area within the frontal lobe, supero-anterior displacement of the splenium of the corpus callosum, and anterior displacement of the superior colliculus. In contrast, CLP was associated with a superior shift of the occipital pole, lateral expansion of the temporal pole, and posterior displacement of the inferior pons. The posterior extent of the cerebellum was also shifted anteriorly in children with CLP, shortening the cerebellum and contributing to the overall reduction of this structure.
DISCUSSION
The purpose of this study was to test for structural differences in brain morphology between children with CP, children with CLP and age- and sex-matched controls. The results of this study indicate that the brain is dysmorphic in children with CL/P and that specific patterns of shape variation are associated with cleft type. In a previous volumetric study on a subset of this sample, Nopoulos et al. (2007)13 found that brain volume was smaller in children with CL/P than age- and sex- matched controls, and that within the brain, tissue was abnormally distributed, such that the cerebellum, frontal lobe, and caudate nucleus were abnormally small and the occipital lobe was abnormally large. The results of this study complement and expand upon these findings. This study found that CL/P was associated with expansion of the occipital lobe, reduction and reorientation of the cerebellum, heightening of the callosal midbody, and postero-inferior displacement of the caudate nucleus and thalamus.
In accordance with Nopoulos et al. (2007)13, which identified cleft-specific differences in total brain tissue, this study identified patterns of dysmorphology specific to CL/P type. CP was associated with cerebral heightening, narrowing of the frontal lobe, reorientation of Broca’s area, and displacement of the superior colliculus and the splenium. CLP was associated with shifts in the occipital and temporal poles, displacement of the inferior pons, and shortening of the cerebellum. This study also parallels Weinberg et al. (2009)16, which found unique differences in brain shape among adults with CLP, adults with CP, and controls. Notably, however, the patterns of shape dysmorphology identified in adults with CL/P by Weinberg et al. (2009)16 and in this study on children with CL/P differ substantially. Considering that volumetric abnormalities are roughly opposite of each other in children and adults with CL/P (e.g., a smaller frontal lobe in children, but a larger frontal lobe in adults), it is not particularly surprising that most of the shape differences that were identified in children with CL/P were not the same shape differences that were identified in adults with CL/P. However, despite the differences in brain dysmorphology between children and adults with CL/P, it is important to note that all of the structures that were dysmorphic in adults with CL/P were also dysmorphic in children.
It is of note that the VIQ of the children with CP was significantly lower than that of the controls and that the VIQ of the children with CLP was intermediate between these values, although not significantly different from either group. This same trend is seen in the number of significant ILDs with 36 ILDs differing between children with CP and controls and 27 ILDs differing between children with CLP and controls. In addition, the sample used in this study was a subset of that assessed in Conrad et al. (2009)4, which documented specific deficits in verbal memory, rapid verbal labeling, and verbal fluency in children with CL/P relative to controls, providing additional support for a relationship between the brain dysmorphology identified in this study and the verbal deficits seen in this population.
The primary limitation of this study was that this sample contained both males and females and children age seven to seventeen. To date, this is the only sample of MRIs of the brain for children with CL/P in existence. The samples were matched by sex and age to minimize the effects of these variables; however, it remains unclear whether there was an effect of these variables on the results as the sample size was too small to permit analysis by sex- and cleft-type. Similarly, severity of clefting and cleft side could not be taken into account in these analyses due to sample size constraints. Larger cross-sectional studies or, ideally, longitudinal studies would help to clarify the role of sex and the time course of the morphological changes observed in children with CL/P, as well as provide finer analysis of brain morphology according to additional clefting variables.
A second limitation of this study is that it is unclear at this time the extent to which differences in skull morphology affect brain morphology in either the normal population or in individuals with CL/P. It is clear, however, that biomechanical and molecular signaling occurs between these two structures during both normal and pathological development31–33 and that pathology can affect both the skull and the brain independently as well as the interaction between these two structures10–12. Cranial base morphology, for example, differs between individuals with and without CL/P34–35. However, it is not known at this time if and how differences in cranial base morphology affect brain morphology or if the reverse is true – i.e., if it is actually brain dysmorphology driving alterations in cranial base morphology through abnormal molecular signaling pathways. This study cannot answer these questions, however, it opens the door for future research into these types of questions.
Although there is no direct clinical application of the current findings, this study provides further evidence that there is a neurological underpinning to the cognitive deficits exhibited by children with CL/P rather than a purely mechanical or social etiology as has been suggested in the past. These findings indicate that additional research should be undertaken to evaluate the neural networks underlying identified volumetric and shape abnormalities and to assess the relationship between dysmorphic brain and skull development in this population. Moreover, these findings suggest that a neurological approach may be helpful in understanding the precise pathophysiology of CL/P, which, to date, remains unclear.
CONCLUSION
In conclusion, this study identified brain dysmorphology in children with CL/P. These results indicate that the aberrant biological mechanisms responsible for this disorder play a role in both face and brain development. Furthermore, the findings here lend support to the notion that cognitive deficits associated with CL/P may be due to abnormal brain structure. Additional longitudinal studies of infants with CL/P should be conducted in order to assess this relationship. In addition, as this study was the first of its kind, further research is necessary to verify the results of this study and to determine whether they are applicable to broader demographic samples. Future studies should also be conducted that directly test for a relationship between these morphological differences and cognitive deficits and longitudinal studies should be undertaken to examine the progression of these morphological changes through childhood and adolescence.
Acknowledgments
Work was performed at the Center for Functional Anatomy and Evolution at Johns Hopkins University School of Medicine, Baltimore, Maryland and at the Department of Psychiatry at the University of Iowa Carver College of Medicine, Iowa City, IA. An oral presentation of this study was presented by Madeleine B. Chollet at the 68th Annual Meeting of the American Cleft Palate – Craniofacial Association, April 2011, San Juan, Puerto Rico.
Funding Source: This study was supported by grants F31 DE021302-01 and 5 R01 DE014399-05 from the National Institutes of Dental and Craniofacial Research. Publication of this article was funded in part by the Open Access Promotion Fund of the Johns Hopkins University Libraries.
Footnotes
Author Contributions: MBC was the principal investigator who designed the study under the mentorship of VBD and PN. PN and ALC obtained the original MRIs and cognitive data, while MBC collected the landmark data. MBC, VBD, and ALC were involved in statistical analysis. MBC wrote the first draft of manuscript, and all coauthors contributed in critical review of manuscript writing.
Declaration of Conflicting Interests: The authors have no conflicts of interest relevant to this article to disclose.
Ethical Approval: This study was approved by the University of Iowa Institutional Review Board and the Johns Hopkins Institutional Review Board. All participants signed an informed consent approved by the University of Iowa review board and were compensated for their involvement in the study.
References
- 1.Richman LC. Cognitive patterns and learning disabilities in cleft palate children with verbal deficits. J Speech Hear Res. 1980;23:447–456. doi: 10.1044/jshr.2302.447. [DOI] [PubMed] [Google Scholar]
- 2.Richman LC, Ryan SM. Do the reading disabilities of children with cleft fit into current models of developmental dyslexia? Cleft Palate Craniofac J. 2003;40:154–157. doi: 10.1597/1545-1569_2003_040_0154_dtrdoc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 3.Richman LC, Wilgenbusch T, Hall T. Spontaneous verbal labeling: visual memory and reading ability in children with cleft. Cleft Palate-Craniofacial J. 2005;42:565–569. doi: 10.1597/04-128r.1. [DOI] [PubMed] [Google Scholar]
- 4.Conrad AL, Richman L, Nopoulos P, Dailey S. Neuropsychological functioning in children with non-syndromic cleft of the lip and/or palate. Child Neuropsychol. 2009;15:471–484. doi: 10.1080/09297040802691120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estes RE, Morris HL. Relationships among intelligence, speech proficiency, and hearing sensitivity in children with cleft palates. Cleft Palate J. 1970;7:763–773. [PubMed] [Google Scholar]
- 6.Harper DC, Richman LC. Personality profiles of physically impaired adolescents. J Clin Psychol. 1978;34:636–642. doi: 10.1002/1097-4679(197807)34:3<636::aid-jclp2270340311>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Richman LC. Differing views of behavior of cleft palate children. Cleft Palate J. 1978;15:360–364. [PubMed] [Google Scholar]
- 8.Richman LC, Eliason M. Type of reading disability related to cleft type and neuropsychological patterns. Cleft Palate J. 1984;21:1–6. [PubMed] [Google Scholar]
- 9.Kapp-Simon K. Self concept of primary school age children with cleft lip, cleft palate or both. Cleft Palate J. 1986;23:24–27. [PubMed] [Google Scholar]
- 10.Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53:135–143. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- 11.Jeong J, Mao J, Tenzen T, et al. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Nopoulos P, Langbehn DR, Canady J, et al. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161:753–758. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- 14.Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 15.Nopoulos P, Berg S, Canady J, et al. Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genet Med. 2002;4:1–9. doi: 10.1097/00125817-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg SM, Andreasen NC, Nopoulos P. Three-dimensional morphometric analysis of brain shape in nonsyndromic orofacial clefting. J Anat. 2009;214:926–936. doi: 10.1111/j.1469-7580.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldridge K. Central nervous system phenotypes in craniosynostosis. J Anat. 2002;201:31–39. doi: 10.1046/j.1469-7580.2002.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldridge K. Patterns of differences in brain morphology in humans as compared to extant apes. J Hum Evol. 2011;60:94–105. doi: 10.1016/j.jhevol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreasen NC, Cohen G, Harris G, et al. Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen NC, Cizadlo T, Harris G, et al. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993;5:121–130. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- 21.Andreasen NC, Harris G, Cizadlo T, et al. Techniques for measuring sulcal/gyral patterns in the brain as visualized through magnetic resonance scanning: BRAINPLOT and BRAINMAP. Proc Natl Acad Sci. 1994;91:93–97. doi: 10.1073/pnas.91.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MM, Jr, Sulik KK. Perspectives on holoprosencephaly: Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J Craniofac Genet Dev Biol. 1992;12:196–244. [PubMed] [Google Scholar]
- 23.Magnotta V, Harris G, Andreasen NC, et al. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 24.Digital Anatomist Project. University of Washington; 1995. http://www9.biostr.washington.edu. [Google Scholar]
- 25.Mullick R, Venkataraman S, Warusavithana S, et al. eTDIPS: 2D/3D image processing system for volume rendering and telemedicine. Presented at the Annual Meeting of the Society for Computer Applications in Radiology (SCAR); June 1998. [Google Scholar]
- 26.Mullick R, Warusavithana SV, Shalini V, Pang P. Plug-ins: a software model for biomedical imaging and visualization research. Presented at the Biomedical Imaging Symposium: Visualizing the Future of Biology and Medicine, National Institutes of Health (NIH); June 1999. [Google Scholar]
- 27.Lele S, Richtsmeier J. An invariant approach to the statistical analysis of shapes. Boca Raton: Chapman & Hall/CRC; 2001. [Google Scholar]
- 28.Lele S. Euclidean distance matrix analysis (EDMA) of landmarks data: estimation of mean form and mean form difference. Math Geol. 1993;25:573–602. [Google Scholar]
- 29.Lele S, Richtsmeier J. Euclidean distance matrix analysis: a coordinate free approach to comparing biological shapes in landmark data. Am J Phys Anthropol. 1991;86:415–428. doi: 10.1002/ajpa.1330860307. [DOI] [PubMed] [Google Scholar]
- 30.Lele S, Richtsmeier J. Estimating confidence intervals for the comparison of forms. Am J Phys Anthropol. 1995;98:73–86. doi: 10.1002/ajpa.1330980107. [DOI] [PubMed] [Google Scholar]
- 31.Boughner JC, Wat S, Diewert VM, et al. Short-faced mice and developmental interactions between the brain and face. J Anat. 2008;213:646–662. doi: 10.1111/j.1469-7580.2008.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu D, Marcucio RS. A SHH-responsibe signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieberman DE, Hallgrimsson B, Liu W, et al. Spatial packing, cranial base angulation, and craniofacial shape variation in the mammalian skull: testing a new model using mice. J Anat. 2008;212:720–735. doi: 10.1111/j.1469-7580.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horswell BB, Gallup BV. Cranial base morphology in cleft lip and palate: a cephalometric study from 7 to 18 years of age. J Oral Maxillofac Surg. 1992;50:681–685. doi: 10.1016/0278-2391(92)90095-h. [DOI] [PubMed] [Google Scholar]
- 35.Molsted K, Kjaer I, Dahl E. Cranial base in newborns with complete cleft lip and palate: radiographic study. Cleft Palate Craniofacial J. 1995;32:199–205. doi: 10.1597/1545-1569_1995_032_0199_cbinwc_2.3.co_2. [DOI] [PubMed] [Google Scholar]