Abstract
Talins are adaptor proteins that regulate focal adhesion signaling by conjugating integrins to the cytoskeleton. Talins directly bind integrins and are essential for integrin activation. We previously showed that β1 integrins are activated in metastatic prostate cancer (PCa) cells, increasing PCa metastasis to lymph nodes and bone. However, how β1 integrins are activated in PCa cells is unknown. In this study, we identified a novel mechanism of β1 integrin activation. Using knockdown experiments, we first demonstrated talin1, but not talin2, is important in β1 integrin activation. We next showed that talin1 S425 phosphorylation, but not total talin1 expression, correlates with metastatic potential of PCa cells. Expressing a non-phosphorylatable mutant, talin1S425A, in talin1-silenced PC3-MM2 and C4-2B4 PCa cells, decreased activation of β1 integrins, integrin-mediated adhesion, motility, and increased the sensitivity of the cells to anoikis. In contrast, re-expression of the phosphorylation-mimicking mutant talin1S425D led to increased β1 integrin activation and generated biologic effects opposite to talin1S425A expression. In the highly metastatic PC3-MM2 cells, expression of a non-phosphorylatable mutant, talin1S425A, in talin1-silenced PC3-MM2 cells, abolished their ability to colonize in the bone following intracardiac injection, while re-expression of phosphorylation-mimicking mutant talin1S425D restored their ability to metastasize to bone. Immunohistochemical staining demonstrated that talin S425 phosphorylation is significantly increased in human bone metastases when compared to normal tissues, primary tumors, or lymph node metastases. We further showed that p35 expression, an activator of Cdk5, and Cdk5 activity were increased in metastatic tumor cells, and that Cdk5 kinase activity is responsible for talin1 phosphorylation and subsequent β1 integrin activation. Together, our study reveals Cdk5-mediated phosphorylation of talin1 leading to β1 integrin activation is a novel mechanism that increases metastatic potential of PCa cells.
Keywords: Cdk5, integrin, metastasis, prostate cancer, talin
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed cancer in men in the western world with a total of nearly 122 000 deaths anticipated in 2013.1, 2 Patients with organ-confined PCa are usually successfully treated, but for those with distant metastases, the 5-year survival decreases to 28%.2 While numerous alterations in both tumor cells and the tumor microenvironment are required for metastasis of solid tumors,3 recent studies have implicated aberrant expression of talin in promoting such critical steps in metastasis as adhesion, migration, invasion, cellular survival and proliferation.4-6
Two distinct talin genes (talin1 and talin2, 74% sequence identity) are expressed in vertebrates, with talin1 expressed in nearly every tissue and talin2 normally expressed primarily in heart, brain, testis and muscles.7-9 Talins are located at the adhesion complex between cells and their extracellular matrix (ECM), and regulate integrin and focal adhesion signaling.10 Structurally, the “talin head” is comprised largely of a FERM domain, which is linked to a structure termed the “talin rod”, a long carboxy-terminal region.11, 12 Major insights into the principal function of talins were derived from experiments demonstrating that the talin head binds the cytoplasmic tails of β integrin subunits,13 leading to the final step in “inside-out” integrin activation.14 Talin also functions as an adaptor protein as it can also bind vinculin, FAK and actin, thereby conjugating integrins to the cytoskeleton,15 and further promoting integrin-mediated signal transduction.
Talin is phosphorylated at multiple sites.16 One of the phosphorylation sites in talin is S425, which is phosphorylated by Cdk5.17 Phosphorylation of talin on S425 was shown to affect talin protein stability and ubiquitylation,17 but potential roles of this phosphorylation in integrin binding have not been assessed previously.
Our laboratories have focused on the roles of β1 integrins in PCa metastasis. Using a conformation-specific antibody, we demonstrated that β1 integrins are constitutively activated in PCa cell lines with high metastatic potential, but not in cancer cell lines with low metastatic potential.18 Activation of β1 integrins promotes resistance to anoikis through activation of FAK/Akt-mediated survival pathway; and neutralizing β1 integrin activation using a specific anti-β1 integrin antibody (mAb 33B6) reduces PCa metastasis in vivo.18 However, the mechanisms by which β1 integrins are activated in highly metastatic PCa cell lines are unknown.
In this study, we demonstrate that talin1 S425 phosphorylation is required for β1 integrin activation in PCa cells and bone colonization of metastatic PCa cells following intracardiac injection. We further demonstrate that increased activity of Cdk5 in metastatic PCa cells is directly responsible for talin1 S425 phosphorylation. These results provide a novel mechanism by which talin1 leads to inside-out activation of β1 integrins, and further provides a mechanism by which talin1 S425 phosphorylation-mediated integrin activation promotes metastatic potential of PCa cells.
RESULTS
Talin1 S425 phosphorylation correlates with β1 integrin activation in PCa cells
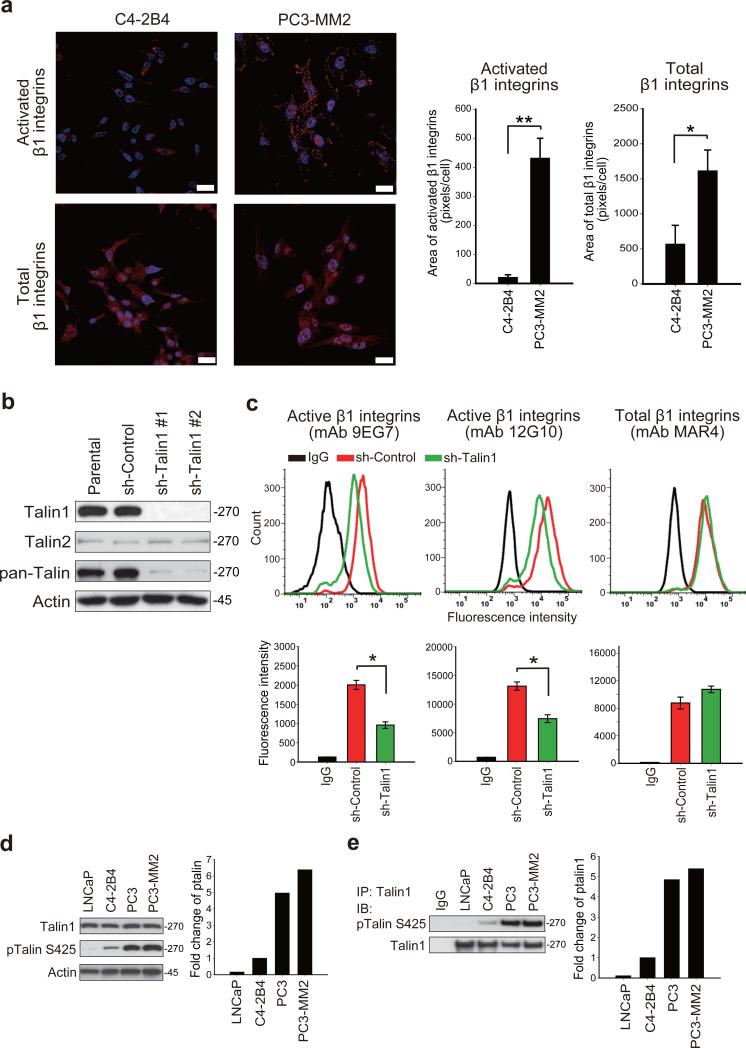
We previously demonstrated that β1 integrins were constitutively activated in metastatic PC3 and PC3-MM2 cells relative to less metastatic LNCaP and C4-2B4 cells.18 We further examined β1 integrin activation by immunofluorescence staining using a conformation-specific antibody (mAb 12G10). Activation of β1 integrins, determined by fluorescence area, was 20-fold higher in metastatic PC3-MM2 cells as compared to low metastatic C4-2B4 cells (Figure 1a). As talin is required for inside-out integrin activation and is overexpressed in PCa,4 we determined whether talin expression contributes to constitutive β1 integrin activation. Because talins are expressed from two genes, talin1 and talin2, we first compared the effect of talin1 and talin2 in β1 integrin activation by gene silencing in PC3-MM2 cells. Silencing of talin1 did not affect the expression of talin2 (Figure 1b). While both talin1 and talin2 are expressed in PC3-MM2 cells, talin1 is far more abundant, as total talin1 and talin2, detected by the pan-talin antibody, 8D4, was significantly reduced in talin1-silenced cells (Figure 1b). In addition, talin1-silenced cells possessed a rounded morphology and impaired spreading (Supplementary Figure S1b), a phenotype not observed in talin2-silenced cells (Supplementary Figure S1a, b). Next, the levels of total and activated β1 integrins in talin1 and talin2-silenced PC3-MM2 cells were determined. Flow cytometric analysis demonstrated that silencing of talin1 (Figure 1c), but not talin2 (Supplementary Figure S1c), reduced β1 integrin activation, without affecting expression of total β1 integrins. These results demonstrate that talin1, but not talin2, is primarily responsible for β1 integrin activation in PCa cells. To determine the correlation of talin1 expression with β1 integrin activation, we examined talin1 expression in low metastatic LNCaP, C4-2B4, metastatic PC3 and PC3-MM2 cells. The level of talin1 expression was similar in each cell line (Figure 1d). These results suggest that increased talin1 expression alone is insufficient to activate β1 integrins. Since talin phosphorylation on S425 is also associated with some integrin functions such as migration,17 we next examined talin1 and talin2 phosphorylation on S425 by immunoblotting. Talin (comprising talin1 and talin2) was highly phosphorylated on S425 in metastatic PC3 and PC3-MM2 cells compared to low metastatic LNCaP and C4-2B4 cells (Figure 1d). To examine talin1 phosphorylation specifically, cell lysates were immunoprecipitated with a talin1 specific antibody, followed by immunoblotting with talin S425 phosphorylation. Talin1 was highly phosphorylated in PC3 and PC3-MM2 cells (Figure 1e), consistent with immunoblotting observed from whole cell lysates (Figure 1d). These results demonstrate that levels of talin1 S425 phosphorylation correlate with activated β1 integrins in PC3-MM2 cells.
Figure 1.
Talin1 S425 phosphorylation correlates with β1 integrin activation in metastatic PCa cells. (a) Immunofluorescence staining of activated β1 integrins (top panels) using a conformation-specific antibody (mAb 12G10) and total β1 integrins (bottom panels). Nuclei were counterstained by Hoechst 33342. Scale bar represents 25 μm. Quantitation of immunofluorescence area is shown in the right panel. *P < 0.05 and **P < 0.001. (b) Silencing of talin1 in PC3-MM2 cells by shRNA expression. A non-targeting shRNA sequence was used as a sh-control. (c) Flow cytometric analysis of total and activated β1 integrins in talin1-silenced PC3-MM2 cells (top panels). Quantitation of fluorescence intensity is shown in the bottom panels. *P < 0.005. (d) Immunoblotting of total talin1 and talin S425 phosphorylation in PCa cell lines. (e) Immunoprecipitation of talin1 followed by immunoblotting of talin S425 phosphorylation in indicated PCa cells.
Talin1 S425 phosphorylation promotes β1 integrin activation
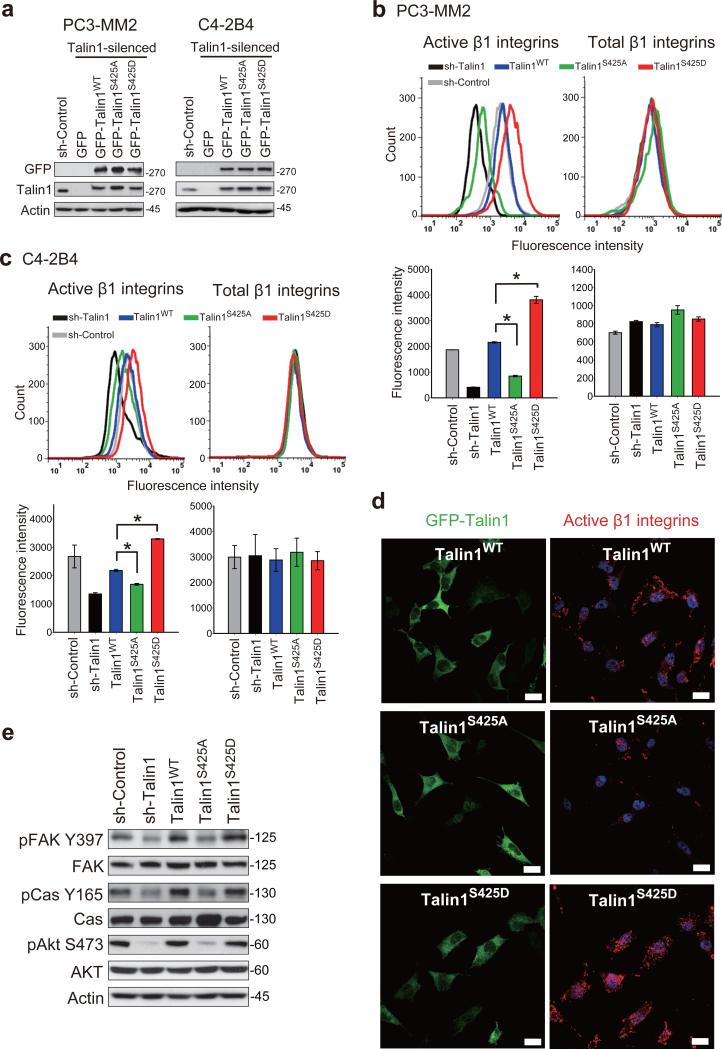
To determine if talin1 S425 phosphorylation were required for β1 integrin activation, we silenced endogenous talin1 and stably expressed either talin1 wild-type (GFP-talin1WT), a nonphosphorylatable mutant (GFP-talin1S425A), or a phosphorylation-mimicking mutant (GFP-talin1S425D) in PC3-MM2 and C4-2B4 cells as described in Materials and methods. Levels of expression of each form of talin1 were similar (Figure 2a). Flow cytometric analysis demonstrated that talin1WT expressed in talin1-silenced PC3-MM2 cells restored β1 integrin activation (Figure 2b). In contrast, expression of talin1S425A inhibited β1 integrin activation by 60%, whereas talin1S425D promoted β1 integrin activation by 76% relative to talin1WT. Similar results were observed when talin1WT and mutants were expressed in C4-2B4 cells in which endogenous talin1 was silenced (Figure 2c). We further assessed the effects of talin1 phosphorylation on β1 integrin activation by immunofluorescence staining. In agreement with the flow cytometric analysis, fewer activated β1 integrins were expressed in talin1S425A cells relative to talin1WT cells, while talin1S425D cells increased clustering of active β1 integrins (Figure 2d). Next, we examined whether talin1 phosphorylation affects downstream integrin signaling. The PC3-MM2 talin1S425A cells were decreased in expression of pFAK Y397, pp130 Cas Y165 and pAkt S473 but not total FAK, p130 Cas or Akt (Figure 2e), consistent with decreased signaling through activated β1 integrins. Similarly, in talin1-silenced cells, pFAK Y397, pp130 Cas Y165 and pAkt S473 were decreased with no decrease in expression of these proteins; whereas in cells re-transfected with talin1WT, activation of these downstream intermediates was restored. Together, these results demonstrate that talin1 S425 phosphorylation promotes β1 integrin activation and downstream signaling in metastatic PCa cells.
Figure 2.
Talin1 S425 phosphorylation promotes β1 integrin activation. (a) Expression of GFP (empty vector), GFP-talin1WT and specified GFP-tagged talin1 mutants in talin1-silenced PC3-MM2 and C4-2B4 cells. (b-c) Flow cytometric analysis of activated and total β1 integrins in (b) PC3-MM2 and (c) C4-2B4 cells expressing talin1WT and mutants (top panels). Quantitation of fluorescence intensity is shown in the bottom panels. *P < 0.005. (d) Immunofluorescence staining of GFP and activated β1 integrins in PC3-MM2 cells expressing talin1WT and mutants. Nuclei were counterstained by Hoechst 33342. Scale bar represents 25 μm. (e) Immunoblotting of pFAK Y397, total FAK, pp130 Cas Y165, total p130 Cas, pAkt S473 and total Akt in PC3-MM2 cells expressing talin1WT and mutants.
Effects of talin1 S425 phosphorylation on anoikis resistance, adhesion and motility
We previously demonstrated that increased β1 integrin activation promoted anoikis resistance, and increased adhesion and motility in PC3-MM2 cells.18 To determine the effect of talin1 S425 phosphorylation on these biologic properties, we used the PC3-MM2 and C4-2B4 cells described above expressing talin1WT and mutants. Expression of talin1WT, integrin-activating and -inhibiting talin1 mutant proteins had no affect on proliferation rates of PC3-MM2 cells or C4-2B4 cells (Supplementary Figure S2a). However, in suspension (anoikis conditions), 30% of the non-phosphorylatable talin1S425A-expressing cells died after 24 h and 40% after 48 h (Figure 3a). In contrast, no cell death was observed in both talin1WT and talin1S425D cells after 24 h. After 48 h, 35% talin1WT cells died, similar to talin1S425A, but only 16% talin1S425D died (Figure 3a). To determine if effects on viability were due to changes in apoptosis, propidium iodide staining was performed. A two-fold increase in the sub-G0/G1 population was observed in talin1S425A cells compared to talin1WT cells after 24 h (Figure 3b). After 48 h, the sub-G0/G1 population in talin1WT cells and talin1S425A cells was similar, while talin1S425D cells were still resistant to anoikis (Figure 3b). As a second measure of apoptosis, PARP cleavage was examined. PARP cleavage occurred in talin1S425A cells by 24 h, but no PARP cleavage was observed in talin1S425D cells even after 48 h (Figure 3c). Thus, increased anoikis resistance was associated with talin1 S425 phosphorylation.
Figure 3.
Talin1 S425 phosphorylation promotes anoikis resistance, adhesion, migration and invasion. (a) PC3-MM2 talin1WT and mutant-expressing cells were cultured in anoikis conditions. Surviving cells were counted at indicated times. *P < 0.05. (b) PC3-MM2 talin1WT and mutant-expressing cells maintained in anoikis conditions were stained with propidium iodide at indicated times followed by flow cytometric analysis. The percentage of sub-G0/G1 cells is plotted. *P < 0.005. (c) Immunoblotting of total and cleaved PARP in lysates of PC3-MM2 talin1WT and mutant-expressing cells maintained in anoikis conditions for indicated times. (d) Adhesion of PC3-MM2 talin1WT and mutant-expressing cells on fibronectin and collagen I-coated plates. Adhesion to BSA served as a negative control. *P < 0.005 and **P < 0.0005. (e) Migration of PC3-MM2 and C4-2B4 talin1WT and mutant-expressing cells on collagen I-coated membranes in modified Boyden chambers for 8 h. *P < 0.0001. (f) Invasion of PC3-MM2 and C4-B4 talin1WT and mutant-expressing cells through Matrigel-coated modified Boyden chambers for 24 h. *P < 0.0001.
We next determined whether talin1 S425 phosphorylation promoted attachment of metastatic PCa cells to ECM. For these analyses, talin1WT and mutant-expressing cells were subjected to adhesion to fibronectin and collagen I-coated culture plates. Binding of PC3-MM2 cells expressing talin1S425A to fibronectin was decreased by 74% and to collagen I by 81% relative to talin1WT cells (Figure 3d). Binding of PC3-MM2 cells expressing talin1S425D to fibronectin was increased by 66% and to collagen I by 53% relative to talin1WT cells (Figure 3d). Very similar results were observed in adhesion assays when these mutants were expressed in C4-2B4 cells (Supplementary Figure S2b). We next determined whether talin1 phosphorylation promotes motility of PCa cells on ECM. Migration assays were performed using collagen I-coated modified Boyden chambers. The ability of PC3-MM2 cells expressing talin1S425A to migrate on collagen I was reduced 52% (62% in C4-2B4 cells expressing talin1S425A) as compared to talin1WT cells, whereas the migratory ability of talin1S425D-expressing PC3-MM2 cells was increased by 30% (55% in C4-2B4 cells expressing talin1S425D) relative to respective wild-type transfected cells (Figure 3e), again correlating with the level of β1 integrin activation. Similar results were observed in invasion assays, in which cells invaded through Matrigel-coated modified Boyden chambers. The invasive ability of PC3-MM2 cells expressing talin1S425A was reduced 95% (90% in C4-2B4 cells expressing talin1S425A) as compared to talin1WT cells, whereas the invasive ability of talin1S425D-expressing PC3-MM2 cells was increased by 40% (43% in C4-2B4 cells expressing talin1S425D) relative to respective wild-type transfected cells (Figure 3f), demonstrating that talin1 S425 phosphorylation promotes cell migratory and invasive abilities.
Talin S425 phosphorylation is mediated by p35 activation of Cdk5
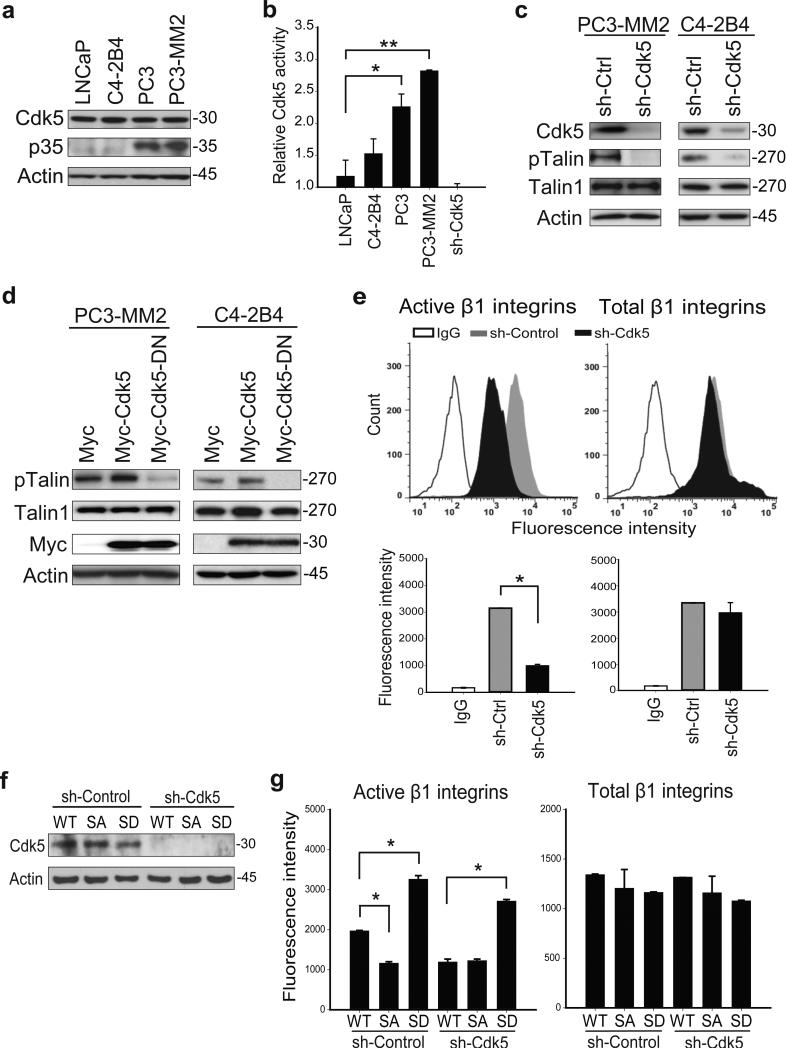
Next, we determined the mechanism by which talin1 S425 phosphorylation is increased in metastatic PCa cells. Previous work in neuronal cells demonstrated that talin phosphorylation on S425 is catalyzed by Cdk5.17 We therefore determined whether Cdk5 was responsible for talin1 S425 phosphorylation in PCa cells. For these studies, both Cdk5 expression and kinase activity were determined. Expression of Cdk5 by immunoblotting was similar in LNCaP, C4-2B4, PC3 and PC3-MM2 cells (Figure 4a). As p35 has been shown to be the principal activator of Cdk5,19, 20 and Cdk5 and p35 are both widely expressed in PCa,21 we examined the expression of p35 in high metastatic PC3, and PC3-MM2 cells, and the low metastatic LNCaP and C4-2B4 cells. Expression of p35 correlated with metastatic potential of these cells, suggesting that p35 is responsible for Cdk5 activation (Figure 4a). Cdk5 activity (as assessed by ADP production through ADP-Glo Kinase Assay kit as described in Materials and methods) increased in PC3 (2-fold relative to LNCaP) and PC3-MM2 cells (2.5-fold relative to LNCaP; Figure 4b). Inhibition of Cdk5 by the Cdk inhibitor, roscovitine, reduced talin1 phosphorylation in PC3-MM2 cells in a time-dependent manner (Supplementary Figure S3), suggesting that Cdk5 might be responsible for talin1 phosphorylation. To examine directly whether Cdk5 mediated talin1 phosphorylation, Cdk5 was silenced in PC3-MM2 and C4-2B4 cells. The results demonstrated talin1 phosphorylation was decreased by ~90% (Figure 4c). To examine whether Cdk5 kinase activity were required for talin1 phosphorylation, PCa cells were transiently transfected with a plasmid directing the expression of a dominant-negative Myc-Cdk5.17 Expression of this dominant-negative mutant inhibited talin1 phosphorylation in both PC3-MM2 and C4-2B4 cells (Figure 4d). These results demonstrate that Cdk5 activity, but not expression, promotes talin1 S425 phosphorylation in PCa cells. We next determined whether Cdk5 regulates β1 integrin activation. Silencing Cdk5 resulted in reduced β1 integrin activation in PC3-MM2 cells (Figure 4e), suggesting that Cdk5 is required for β1 integrin activation. Next, we silenced Cdk5 in PC3-MM2 cells in which talin1 was silenced and talin1WT or talin1S425D mutants were re-expressed (Figure 4f). As expected, silencing Cdk5 inhibited β1 integrin activation in talin1WT cells, but did not affect β1 integrin activation in phospho-mimicking talin1S425D cells (Figure 4g). These observations demonstrate that Cdk5 phosphorylates talin1 on S425, and its activity promotes β1 integrin activation.
Figure 4.
Cdk5 mediates talin1 S425 phosphorylation. (a) Immunoblotting of Cdk5 and p35 in PCa cells. (b) Cdk5 kinase activity in PCa cells was measured by ADP production. *P < 0.0005 and **P < 0.0001. (c) Immunoblotting of PC3-MM2 and C4-2B4 cell lysates in which knockdown of Cdk5 inhibited talin1 S425 phosphorylation. (d) Effect of Cdk5 activity on talin1 phosphorylation by overexpression of a dominant-negative Cdk5. (e) Flow cytometric analysis of activated and total β1 integrins in Cdk5-silenced PC3-MM2 cells (top panels). Quantitation of fluorescence intensity is shown in the bottom panels. *P < 0.0005. (f) Talin1WT and mutant-expressing cells were transfected with a sh-control vector or a sh-Cdk5 vector silencing Cdk5 expression, and (g) activated and total β1 integrins were measured by flow cytometric analysis. *P < 0.005.
Talin1 S425 phosphorylation promotes PCa bone metastasis
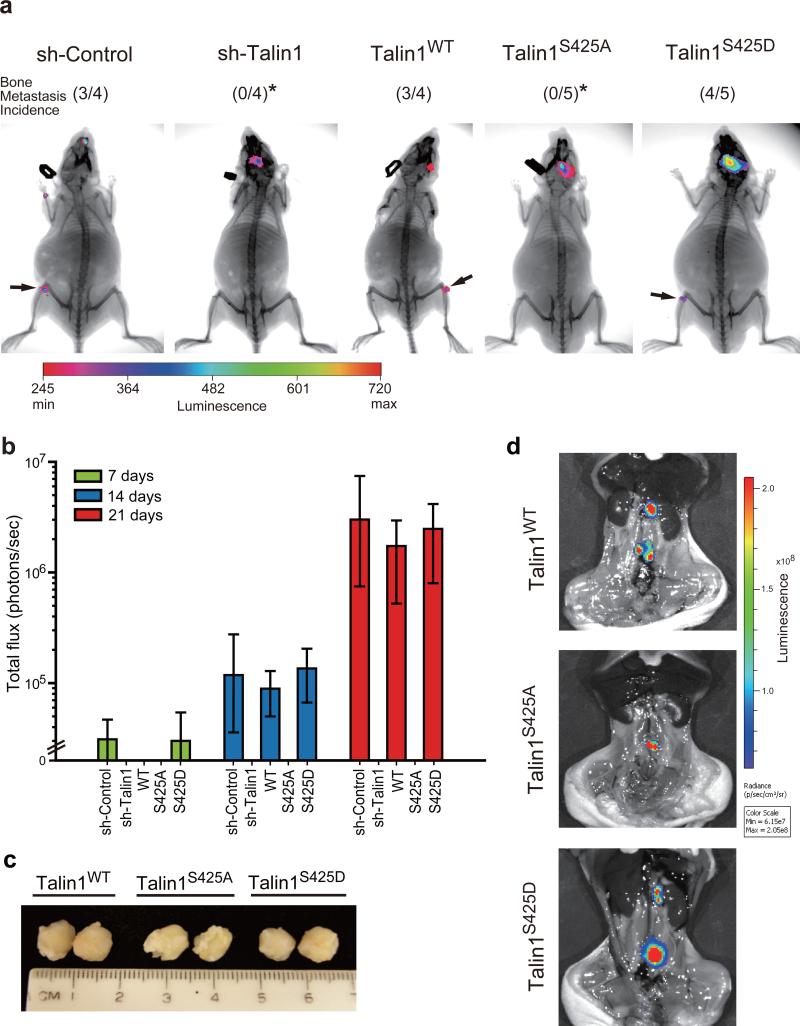
We determined whether talin S425 phosphorylation promoted metastasis in nude mouse models. To determine bone metastasis of disseminated tumor cells, intracardiac injections were performed (experimental metastasis). For these experiments, first, the sh-control, talin1-silenced, talin1 wild-type and mutated talin1-expressing PC3-MM2 cells were transduced with a plasmid directing luciferase expression. Following intracardiac injection of cells, bioluminescence imaging of mice was performed weekly. The presence of tumor in bone was examined by co-localization of X-ray and bioluminescence imaging. A representative image of mice inoculated with cells expressing each construct is shown in Figure 5a. No tumor growth was observed in any animal in the bone of talin1-silenced and talin1S425A groups. In contrast, 3 of 4 talin1WT mice and 4 of 5 talin1S425D mice demonstrated tumor growth. Tumors were noted in both legs of mice as expected in this model as well as in the heads of mice as previously observed18, 22 (for examples of bioluminescence imaging, see Supplementary Figure S4). Thus, blocking talin1 S425 phosphorylation completely inhibited bone metastasis of disseminated PC3-MM2 cells in this model. For tumors that grew, tumor size was estimated by bioluminescence imaging 7, 14 and 21 days post-intracardiac injection. Exponential growth was observed in sh-control, talin1WT and talin1S425D groups, with no difference in talin1WT and talin1S425D observed (Figure 5b).
Figure 5.
Talin1 S425 phosphorylation promotes bone colonization in vivo. (a) Luciferase-labeled PC3-MM2 sh-control, talin1-silenced, talin1WT and mutant-expressing cells (1 × 106) were injected into nude mice intracardially. After 21 days injection, bioluminescence and X-ray imaging of mice were superimposed to localize tumor growth. Arrow indicates tumor growth in femur/tibia junction. *Compared to talin1WT, P < 0.05. (b) Tumor burden in the femur/tibia of mice following intracardiac injection. Tumor growth was estimated by luciferase activity (presented as photons/sec). (c-d) PC3-MM2 talin1WT and mutant-expressing cells (5 × 105) were orthotopically injected into the prostate. (c) Sample tumor sizes of lymph node metastases. (d) Bioluminescence imaging of lymph node metastases of PC3-MM2 talin1WT and mutant-expressing cells in mice after removing the primary tumor.
We next examined whether talin1 phosphorylation affects lymph node metastasis (spontaneous metastasis) of tumor cells injected orthotopically into the prostate. Mice were sacrificed when primary tumors reached a similar size (Figure 5c), and lymph node metastases were detected by bioluminescence imaging (Figure 5d). Lymph nodes were excised and tumor was confirmed by H&E staining. Lymph node metastases developed in all groups (PC3-MM2 cells expressing talin1WT, talin1S425A and talin1S425D mutants; Figure 5d); however, as shown in Table 1, significantly fewer lymph node metastases were observed in talin1S425A mice compared to talin1WT mice. Together, our results are consistent with talin1 S425 phosphorylation promoting metastasis.
Table 1.
Effects of talin1 S425 phosphorylation on development of lymph node metastases of talin1-expressing PC3-MM2 cells injected intraprostatically
| Tumor cells | Tumor incidence | LN metastases incidence | Average # of LN metastases (range) |
|---|---|---|---|
| Talin1WT | 6/6 | 6/6 | 3 (2-4) |
| Talin1S425A | 5/5 | 5/5 | 1.8 (1-2)* |
| Talin1S425D | 5/5 | 5/5 | 2.8 (2-3) |
ANOVA and Turkey's test, talin1S425A compared to talin1WT, P < 0.01
Abbreviation: LN, lymph node.
Talin S425 phosphorylation in human PCa specimens
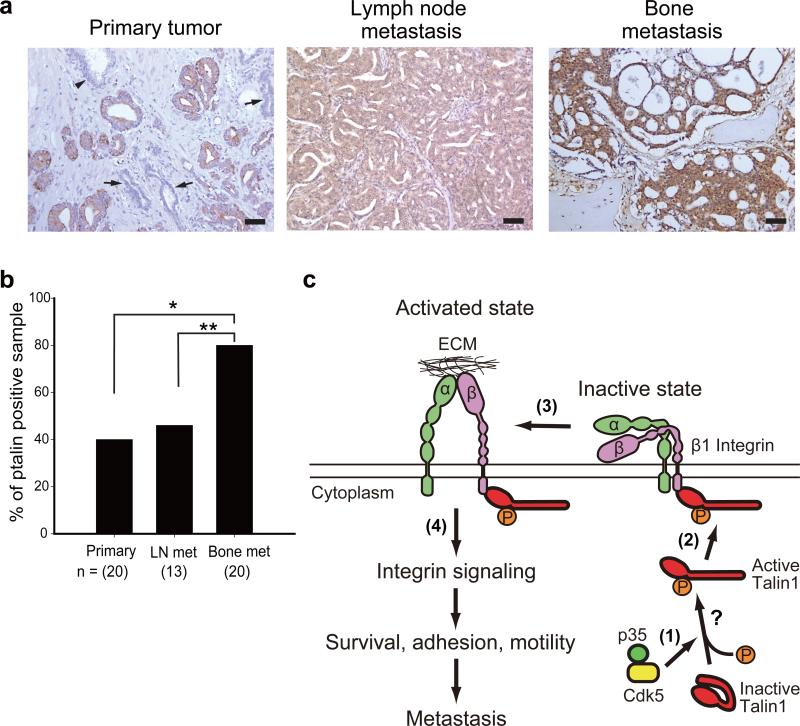
To determine clinical relevance of talin S425 phosphorylation in PCa, we performed immunohistochemical analysis on formalin-fixed human prostate tumor specimens. We examined total talin1 expression in normal glands as well as primary tumors and lymph node metastases. Results were consistent with a previous report demonstrating that talin1 expression increases in progressive statuses of PCa.4 Next we examined talin S425 phosphorylation in human primary tumors, lymph node metastases and bone metastases by immunohistochemistry. The talin S425 phospho-specific antibody used in the immunohistochemical analysis was validated using a blocking peptide (Supplementary Figure S5). Representative images of normal glands, primary tumors, lymph node metastases and bone metastases are shown in Figure 6a. Talin phosphorylation was not detected in normal or atrophied glands (Figure 6a), while 8 of 20 (40%) primary tumors and 6 of 13 (46%) lymph node metastases stained positive (Figure 6b). Phospho-talin S425 was observed in 16 of 20 (80%) bone metastases, a significant increased compared to either primary prostate tumors (P = 0.009), or to lymph node metastases (P = 0.043; Figure 6b). There was no correlation between talin phosphorylation and Gleason score. These data demonstrate increased talin S425 phosphorylation is associated with PCa bone metastasis.
Figure 6.
Talin S425 phosphorylation in stages of human PCa. (a) Immunohistochemical staining of talin S425 phosphorylation in primary tumor, lymph node metastases and bone metastases. Arrows indicate atrophied glands, and an arrow head indicates normal glands in primary tumor. Scale bar represents 100 μm. (b) Percentage of human specimens expressing talin S425 phosphorylation. *P < 0.005 and **P < 0.05. (c) Model of β1 integrin activation by talin1 S425 phosphorylation in metastatic PCa cells. (1) Overexpression of p35 increases Cdk5 activity in tumor cells of high metastatic potential, increasing phosphorylation of talin1 on S425, which induces a conformational change leading to talin1 activation. Binding of additional proteins may facilitate this conformational change. (2) Phosphorylated talin1 binds to the cytoplasmic tail of β1 integrin and (3) increases β1 integrin activation and (4) promotes integrin signaling, leading to increased survival, adhesion, motility and metastatic potential of PCa cells.
DISCUSSION
In this work, we demonstrate the importance of talin1 S425 phosphorylation for inside-out activation of β1 integrins and promotion of PCa bone metastasis. Talin1 S425 phosphorylation is significantly increased in human bone metastases relative to primary tumors or lymph node metastases. Using phosphorylation-mimicking and non-phosphorylatable talin1 mutants, we demonstrated that talin1 S425 phosphorylation plays an important role in β1 integrin activation, cell adhesion, migration, invasion, and anoikis in vitro and metastasis of PCa cells to bone in vivo. Mechanistically, we demonstrated that talin1 S425 phosphorylation is catalyzed by Cdk5, and that Cdk5 activity is increased in highly metastatic PCa cells corresponding with an increase in its activator, p35,19, 20 as compared to poorly metastatic PCa cells. Together, these results provide a novel mechanism by which p35-Cdk5-ptalin1-S425-β1 integrin activation plays a role in the metastatic progression of PCa in bone (summarized in the model in Figure 6c).
Multiple mechanisms have been implicated in talin binding to the cytoplasmic tail of β integrins. In the “closed”, autoinhibited conformation, talin1 exists as a dimer that masks its integrin and actin-binding domains.11 For either inside-out or outside-in integrin activation, talin must assume an “open” conformation.14, 23 Several mechanisms may relieve this inhibitory conformation, including binding phosphotidylinositol-4,5-bisphosphate,24, 25 calpain cleavage,26 binding kindlin and RIAM,27, 28 and constitutive Rap1 activation.29 Important to our study, the talin1 linker domain, where S425 is located, also binds the central portion of its rod domain.11 Thus, phosphorylation of talin1 on S425 is predicted to promote an “open” conformation.
Overexpression of talin occurs in several solid tumors, including PCa,4 oral squamous cell carcinoma5 and ovarian serous carcinoma,6 with overexpression correlating with higher tumor grade and metastasis.4-6 In these studies, phosphorylation of talin was not examined; therefore overexpression and phosphorylation may not be mutually exclusive mechanisms for β1 integrin activation.
We previously demonstrated that constitutive activation of β1 integrins specifically correlates with metastatic potential of established PCa cell lines, and blocking of β1 integrin activation with a monoclonal antibody (mAb 33B6) decreased metastatic growth.18 In this study, we showed that activation of β1 integrins by talin S425 phosphorylation provides a molecular mechanism of inside-out integrin activation important to PCa bone metastasis. We speculate that this process occurs through increasing the interaction of tumor cell β1 integrins with bone stroma. During PCa bone metastasis, heterodimerization of integrin subunits is altered, allowing interaction with ECM in the bone matrix.30 Several β1 integrin family proteins binding ECM proteins have been shown mediate tumor cell interaction with both the bone matrix and with stromal cells. For example, expression of α5β1 integrins facilitates adhesion and spreading of PC3 cells on fibronectin.31 Blocking α5β1 integrins by monoclonal antibodies inhibits adhesion of DU-145 PCa cell to bone marrow stromal cells.31, 32 Another β1 integrin family protein, α2β1, has been shown to increase migration and adhesion of collagen-binding LNCaP cells on collagen I, and promotes bone metastasis in vivo.33, 34 Similar to α5β1, α2β1 was found to mediate adhesion of malignant tumor-derived prostate epithelial cells to human bone marrow stroma, which can also be inhibited by antibodies targeting α2β1.35 These results suggest that interaction between tumor cell and bone matrix/stroma through β1 integrins is critical for tumor cell colonization in the bone.
Our studies also examined roles of talin1 S425 phosphorylation in spontaneous lymph node metastasis following orthotopic implantation of PCa cells. In contrast to the effects of talin1 mutants on bone colonization, lymph node metastases were observed in both cells expressing wild-type and talin1S425A-expressing PC3-MM2 cells that is unable to activate β1 integrins, although the number of lymph node metastases in talin1S425A was decreased. Our results agree with a recent study of Barthel et al. who found that β1 integrins were highly activated in PCa cells derived from bone metastasis, but not in LNCaP variants selected for lymph node metastasis, from which the authors concluded β1 (and αvβ3) integrins were less important to lymph node metastasis than to bone metastasis.29
Finally, our studies examine only the role of the β1 integrin subunit in PCa progression. Several integrins, including α5β1, α2β1 and αvβ3 integrins have functional consequences for PCa metastasis. The β1 and β3 subunits have both been implicated in increased migration,29, 33-38 suggesting overlapping functions among activated integrins. The α subunits of integrins also likely play distinct but overlapping roles in PCa metastasis. Recently, the α5 subunit, but not other α subunits has been implicated in IGF-1R-mediated cell growth.39 Increased expression of α6 integrin leads to more invasive tumors in mice.40 Further studies on roles of microenvironment in integrin expression, activation, and altered heterodimerization will be required to discern roles of specific heterodimers.
In summary, we demonstrated the importance of phosphorylation of talin in β1 integrin activation. We further identified a novel mechanism for β1 integrin activation through p35-Cdk5-mediated talin1 S425 phosphorylation in metastatic PCa cells, suggesting that talin1 as well as Cdk5 may be potential targets for development of novel inhibitors of PCa bone metastasis.
MATERIALS AND METHODS
Cell lines, culture conditions and antibodies
The PC3 and PC3-MM2 cells were gifts from Dr. Isaiah J. Fidler at the M. D. Anderson Cancer Center. The PC3-MM2 is a highly metastatic cell line derived following two passages of PC3-M cells in nude mice of selecting and re-passaging lymph node metastases.41 LNCaP cells were obtained from the American Type Culture Collection. The C4-2B4 cells are a LNCaP variant derived from a mouse bone metastasis.42 All cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, checked every six months and found to be mycoplasma free, and authenticated at the M. D. Anderson Cell Identification Core Facility. The antibodies used were described in Supplementary Materials and methods.
Immunofluorescence
Cells were cultured on coverglasses, and were fixed and processed as described in Supplementary Materials and methods.
Lentivirus-mediated gene silencing
Viral pLKO.1-puro plasmids from Sigma, (St. Louis, MO) containing different shRNA sequences were used to decrease talin1 (MISSION shRNA TRC number: TRCN0000299020, TRCN0000299022), talin2 (TRCN0000436291, TRCN0000439801) and Cdk5 (TRCN0000342298) expression. The same vector containing non-targeting shRNA (Sigma, Cat. SHC016) was used as a sh-control plasmid. Lentiviral particles were generated as previously described.43 Following lentivirus infection, cells were cultured with 5 μg/ml puromycin to select stable silenced cells.
Immunoblotting and immunoprecipitation
Cells were washed twice with PBS, and were performed as described in Supplementary Materials and methods.
Flow cytometric analysis of β1 integrins
Flow cytometric analysis of β1 integrins was performed as described previously.18 Expression of activated and total β1 integrins was quantitated by geometric mean fluorescence intensity using FlowJo software (Tree Star, Ashland, OR).
Mutagenesis and transfection
Human full-length talin1 cDNA was mutated and subcloned into a pWPXL-GFP vector using In-Fusion HD cloning system (Clontech, Mountain View, CA) according to the manufacturer's instructions. Details of mutagenesis and transfection are described in the Supplementary Materials and methods.
Anoikis assays
PC3-MM2 cells expressing talin1WT and mutants were cultured in suspension in serum-free medium. Live cell number was counted by trypan blue exclusion. Cells were subjected for immunoblotting for cleaved PARP, total PARP and propidium iodide staining as described previously.18 Cells in the sub-G0/G1 were quantitated using FlowJo software.
Adhesion assays
Adhesion assays were performed as previously described with some medifications.18 After wash, attached cells on plates were incubated with 1 μM Calcein AM (BD Biosciences) for 5 min at 37°C. Fluorescence-labeled cells were lysed by 1% Triton X-100, and the fluorescence intensity was measured using an Envision plate reader (PerkinElmer, Waltham, MA).
Migration and invasion assays
Migration assays were performed using collagen I-coated polycarbonate transwell membranes in 24-well plates (8-μm pore size; Corning, Tewksbury, MA) as described previously.44 For invasion assays, Matrigel-coated filters (BD Biosciences) were used. The medium in the bottom chamber was supplemented with 0.1% fetal bovine serum as a chemoattractant. Cells were allowed to migrate for 8 h or invade for 24 h. Images of filters were acquired using a microscope with camera (Eclipse Ti-S; Nikon, Melville, NY). Cell number was counted using image analysis software (Image-Pro Plus; Media Cybernetics, Rockville, MD).
Cdk5 kinase activity assays
Cdk5 kinase activity was measured using ADP-Glo kinase assay (Promega, Madison, WI) according to the manufacturer's instructions. Additional details are provided in the Supplementary Materials and methods.
In vivo metastasis assays
PC3-MM2 sh-control, talin1-silenced with GFP, GFP-talin1WT and GFP-talin1 mutant cells were transduced with a plasmid directing luciferase expression (pLenti-PGK-Blast-V5-LUC,45 Addgene plasmid #19166) as described above. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). For intracardiac injection (experimental metastasis assays), 1 × 106 cells were injected into left ventricle of male nude mice. Bioluminescence images were acquired immediately after intracardiac injection to proper ensure distribution of cells in mice, and then were used to monitor tumor growth by a Xenogen IVIS 200 system (PerkinElmer). X-ray and matched bioluminescence images were acquired and superimposed using a Kodak In-Vivo Multispectral Imaging System FX (Carestream, Rochester, NY). For intraprostatic injection (spontaneous metastasis assays), 5 × 105 cells were injected into nude mice. Tumor growth was monitor by bioluminescence imaging. Mice with similar sized primary tumors were sacrificed, tumors were removed and lymph node metastases were identified by bioluminescence imaging.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections were de-waxed, and were performed as described in Supplementary Materials and methods.
Statistical analyses
Data are shown as means ± s.d. One-way ANOVA and post Tukey's test were used to determine significance in metastasis assays. A chi-square test was used to evaluate immunohistochemical analysis. Other assays were determined by two-tailed Student's t-test. P-values less than 0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Joseph H. McCarty for critically reviewing the manuscript. This work was supported by the National Institutes of Health (NIH) P50 CA140388 (GEG, S-HL), a Prostate Cancer Foundation Challenge Award (GEG, S-HL), NIH RO-1 CA174798 (S-HL), DOD PC093132 (S-HL), a Cancer Prevention and Research Institute of Texas, CPRIT RP110327 (SHL) and NIH CA16672 (CCSG, M. D. Anderson Cancer Center core grant).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
REFERENCES
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer Facts & Figures. American Cancer Society; Atlanta, Georgia, USA: 2013. [Google Scholar]
- 3.Jin JK, Dayyani F, Gallick GE. Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer. 2011;128:2545–2561. doi: 10.1002/ijc.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885–1895. doi: 10.1158/0008-5472.CAN-09-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai MT, Hua CH, Tsai MH, Wan L, Lin YJ, Chen CM, et al. Talin-1 overexpression defines high risk for aggressive oral squamous cell carcinoma and promotes cancer metastasis. J Pathol. 2011;224:367–376. doi: 10.1002/path.2867. [DOI] [PubMed] [Google Scholar]
- 6.Tang H, Yao L, Tao X, Yu Y, Chen M, Zhang R, et al. miR-9 functions as a tumor suppressor in ovarian serous carcinoma by targeting TLN1. Int J Mol Med. 2013;32:381–388. doi: 10.3892/ijmm.2013.1400. [DOI] [PubMed] [Google Scholar]
- 7.Monkley SJ, Pritchard CA, Critchley DR. Analysis of the mammalian talin2 gene TLN2. Biochem Biophys Res Commun. 2001;286:880–885. doi: 10.1006/bbrc.2001.5497. [DOI] [PubMed] [Google Scholar]
- 8.Debrand E, El Jai Y, Spence L, Bate N, Praekelt U, Pritchard CA, et al. Talin 2 is a large and complex gene encoding multiple transcripts and protein isoforms. FEBS J. 2009;276:1610–1628. doi: 10.1111/j.1742-4658.2009.06893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manso AM, Li R, Monkley SJ, Cruz NM, Ong S, Lao DH, et al. Talin1 has unique expression versus talin2 in the heart and modifies the hypertrophic response to pressure overload. J Biol Chem. 2013;288:4252–4264. doi: 10.1074/jbc.M112.427484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–147. doi: 10.1016/B978-0-12-386039-2.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goult BT, Xu XP, Gingras AR, Swift M, Patel B, Bate N, et al. Structural studies on full-length talin1 reveal a compact auto-inhibited dimer: implications for talin activation. J Struct Biol. 2013;184:21–32. doi: 10.1016/j.jsb.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 14.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 15.Critchley DR, Gingras AR. Talin at a glance. J Cell Sci. 2008;121:1345–1347. doi: 10.1242/jcs.018085. [DOI] [PubMed] [Google Scholar]
- 16.Ratnikov B, Ptak C, Han J, Shabanowitz J, Hunt DF, Ginsberg MH. Talin phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2005;118:4921–4923. doi: 10.1242/jcs.02682. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Rajfur Z, Yousefi N, Chen Z, Jacobson K, Ginsberg MH. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol. 2009;11:624–630. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, et al. Targeting constitutively activated beta1 integrins inhibits prostate cancer metastasis. Mol Cancer Res. 2013;11:405–417. doi: 10.1158/1541-7786.MCR-12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai LH, Delalle I, Caviness VS, Jr., Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 20.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 21.Strock CJ, Park JI, Nakakura EK, Bova GS, Isaacs JT, Ball DW, et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66:7509–7515. doi: 10.1158/0008-5472.CAN-05-3048. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye F, Kim C, Ginsberg MH. Molecular mechanism of inside-out integrin regulation. J Thromb Haemost. 2011;9:20–25. doi: 10.1111/j.1538-7836.2011.04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martel V, Racaud-Sultan C, Dupe S, Marie C, Paulhe F, Galmiche A, et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J Biol Chem. 2001;276:21217–21227. doi: 10.1074/jbc.M102373200. [DOI] [PubMed] [Google Scholar]
- 25.Song X, Yang J, Hirbawi J, Ye S, Perera HD, Goksoy E, et al. A novel membrane-dependent on/off switch mechanism of talin FERM domain at sites of cell adhesion. Cell Res. 2012;22:1533–1545. doi: 10.1038/cr.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan B, Calderwood DA, Yaspan B, Ginsberg MH. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J Biol Chem. 2001;276:28164–28170. doi: 10.1074/jbc.M104161200. [DOI] [PubMed] [Google Scholar]
- 27.Ye F, Petrich BG, Anekal P, Lefort CT, Kasirer-Friede A, Shattil SJ, et al. The mechanism of kindlin-mediated activation of integrin alphaIIbbeta3. Curr Biol. 2013;23:2288–2295. doi: 10.1016/j.cub.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das M, Ithychanda S, Qin J, Plow EF. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim Biophys Acta. 2014;1838:579–588. doi: 10.1016/j.bbamem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthel SR, Hays D, Yazawa EM, Opperman MJ, Walley KC, Nimrichter L, et al. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 2013;73:942–952. doi: 10.1158/0008-5472.CAN-12-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, et al. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001;12:99–107. [PubMed] [Google Scholar]
- 31.Stachurska A, Elbanowski J, Kowalczynska HM. Role of alpha5beta1 and alphavbeta3 integrins in relation to adhesion and spreading dynamics of prostate cancer cells interacting with fibronectin under in vitro conditions. Cell Biol Int. 2012;36:883–892. doi: 10.1042/CBI20110522. [DOI] [PubMed] [Google Scholar]
- 32.Van der VeD, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den TwJ, Joling P. Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5beta1 integrin. Exp Cell Res. 1997;230:111–120. doi: 10.1006/excr.1996.3405. [DOI] [PubMed] [Google Scholar]
- 33.Sottnik JL, Daignault-Newton S, Zhang X, Morrissey C, Hussain MH, Keller ET, et al. Integrin alpha2beta1 (alpha2beta1) promotes prostate cancer skeletal metastasis. Clin Exp Metastasis. 2013;30:569–578. doi: 10.1007/s10585-012-9561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall CL, Dai J, van Golen KL, Keller ET, Long MW. Type I collagen receptor (alpha2beta1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res. 2006;66:8648–8654. doi: 10.1158/0008-5472.CAN-06-1544. [DOI] [PubMed] [Google Scholar]
- 35.Lang SH, Clarke NW, George NJ, Testa NG. Primary prostatic epithelial cell binding to human bone marrow stroma and the role of alpha2beta1 integrin. Clin Exp Metastasis. 1997;15:218–227. doi: 10.1023/a:1018465213641. [DOI] [PubMed] [Google Scholar]
- 36.Lin TH, Liu HH, Tsai TH, Chen CC, Hsieh TF, Lee SS, et al. CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta. 2013;1830:4917–4927. doi: 10.1016/j.bbagen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A, Languino LR. Trop-2 promotes prostate cancer metastasis by modulating beta1 integrin functions. Cancer Res. 2013;73:3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 39.Sayeed A, Fedele C, Trerotola M, Ganguly KK, Languino LR. IGF-IR promotes prostate cancer growth by stabilizing alpha5beta1 integrin protein levels. PLoS One. 2013;8:e76513. doi: 10.1371/journal.pone.0076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 41.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 42.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 43.Chang SK, Noss EH, Chen M, Gu Z, Townsend K, Grenha R, et al. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci U S A. 2011;108:8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varkaris A, Gaur S, Parikh NU, Song JH, Dayyani F, Jin JK, et al. Ligand-independent activation of MET through IGF-1/IGF-1R signaling. Int J Cancer. 2013;133:1536–1546. doi: 10.1002/ijc.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One. 2009;4:e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.