Abstract
Background
We analyzed whether difference exist in the clinical manifestations and outcomes of hepatocellular carcinoma (HCC) according to the two major etiologies of HCC from a nationwide, population-based, random HCC registry.
Methods
Of the 31,521 new HCC cases registered at the Korea Central Cancer Registry between 2003 and 2005, 4,630 (14.7%) were randomly abstracted, and followed up until December 2011. Of those, 2,785 hepatitis B virus (HBV)-related and 447 hepatitis C virus (HCV)-related HCC patients were compared.
Results
The mean annual incidence rates of HBV- and HCV-related HCC incidence per 100,000 persons were 20.8 and 4.9, respectively. The annual incidence rate of HBV-related HCC peaked at 50–59 age group (46.5 per 100,000 persons), while the annual incidence rate of HCV-related HCC increased gradually to the ≥70 year age group (13.2 per 100,000 persons). Large tumors (≥5 cm) and portal vein invasion at initial diagnosis were more frequent in HBV-related HCC, while multiple tumors were more frequent in HCV-related HCC. In outcome analysis, HBV-related HCC showed poorer survival than HCV-related HCC [median survival: 1.34 vs. 2.17 years, adjusted hazard ratio (95% confidence interval): 0.88 (0.78–0.98), P = 0.03, adjusted for age, gender, Child-Pugh class, AJCC/mUICC stage, and initial treatment modality]. However, when divided according to the AJCC/mUICC stage, survival difference was observed only for those with AJCC/mUICC stage IV tumor, but not for AJCC/mUICC stage I, II or III tumors. The treatment outcome of each modality (resection, ablation, and transartherial chemoeombolization) was comparable between the two etiologies.
Conclusion
HBV-related and HCV-related HCC have clear differences in clinical manifestation, requiring different screening strategy according to etiology to optimize HCC surveillance in HBV-endemic area. However, etiology did not affect treatment outcomes and long-term survival within the same stage except for far advanced tumors.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths in the world [1]. HCC continues to increase progressively in incidence, being a major global health problem [2], [3]. The two most important risk factors for HCC are the hepatitis B virus (HBV) and hepatitis C virus (HCV) infection [4]. Worldwide, approximately 54% of cases are attributed to HBV infection (which affects 400 million people globally), while 31% are attributed to HCV infection (170 million) [5]. HCC is a major cancer in Korea [6], with a high incidence rate [2]. High incidence rate of HCC closely reflects a high prevalence rate of HBV or HCV infection [2]. Korea is an endemic area of HBV infection [7], although the prevalence of HBV is rapidly decreasing due to a nationwide universal vaccination program [8]. HBV accounts for 68∼78% of all HCC diagnosed in Korea [9]. Almost all Korean chronic hepatitis B patients are infected with HBV genotype C [10], which progresses more rapidly to HCC [8]. HCV accounts for 10∼15% of HCC diagnosed in Korea [11], and >95% of Korean chronic hepatitis C patients are infected with HCV genotype 1b or 2 [12].
Despite the histological similarities in the resulting carcinomas, there are evidences suggesting two distinct oncogenic pathways and natural histories between these major etiologies of HCC. In HBV infection, in addition to chronic inflammation, the integration of HBV DNA into the host hepatocyte DNA and the expression of viral proteins, which transactivate human oncogenes, may play a role in hepatocarcinogenesis, while in HCV infection, chronic inflammation seems to play a main role in oncogenesis [13]–[15]. Thus, cirrhosis almost always accompanies HCV-related HCC [16], but not in HBV-related HCC [17], [18]. HBV infection usually occurs in the perinatal period in an endemic area, while the immune status of the host is still immature [8]. Meanwhile, HCV infection occurs in adults with a fully matured immune system [19]. A combination of virus-specific, host genetic, environmental, and immune-related factors will affect the HCC manifestations, thus these differences in hepatocarcinogenesis may affect clinical manifestations as well as patients outcome. Indeed, several previous studies have assessed the impact of viral etiology on clinical manifestation and long-term outcome [20]–[22]. However, still controversies exist whether different HCC surveillance and management strategies according to the viral etiologies are needed. This question has not been answered, in part, because most studies were performed on a single hospital base which inevitably has a selection bias, with limited sample size and limited follow-up period. The sample sizes were 205, 359 and 127 in reports by Shiratori et al.'s [20], Tanabe et al.'s [21], and Hiotis et al.'s [22], respectively. Therefore, in this study, we used data from population-based nationwide cancer registry which has less selection bias, with large study sample and long-term follow-up period, and assessed whether true differences exist in clinical manifestations and long-term outcomes of HCC patients between the two viral etiologies.
Methods
Data source
In Korea, the largest government-endorsed, population-based cancer registry, called the Korea Central Cancer Registry (KCCR), was established in 1980. Patients diagnosed with cancer receive additional economic assistance from the National Health Insurance Service when registered at the KCCR, hence, almost all incidences of cancer (>95%) occurring in the population are included in the registry [23]. Therefore, the KCCR has the advantage that it has high case completeness as a cancer registry. However, the KCCR do not collect detailed information, such as liver function, tumor characteristics, treatment modalities, etc. To fully collect detailed clinical, tumor characteristics as well as treatment information and long-term outcomes of Korean HCC patients, the Korean Liver Cancer Study Group (KLCSG) built a randomly selected, population based HCC cohort based on KCCR registry (KLCSG random cohort).
The KLCSG random cohort was built as follows: Between 2003 and 2005, a total of 31,521 new cases of HCC were registered at the KCCR from about 500 hospitals nationwide. First, 25–30 hospitals were randomly selected after being stratified by region and number of cases registered, then 16.5% of HCC cases from each year were randomly selected, this gave a total of 5,262 HCC cases. Three trained abstractors visited 32 hospitals throughout the country between May 2009 and May 2010, and obtained information of each case regarding the clinical and tumor characteristics and treatment modality. Among the 5,262 cases, abstraction was possible for 4,630 HCC cases (14.7%). The major reasons of abstraction failure included malignancy other than HCC (e.g., intrahepatic cholangiocarcinoma, hepatoblastoma, etc…), data duplication or unavailable data, etc. After excluding 110 cases, which the date of the diagnosis was not between 2003 and 2005, a total of 4,520 patients were enrolled in the KLCSG random cohort. The diagnosis of HCC was made clinically or histologically based on the guidelines proposed by the KLCSG and the National Cancer Center [6]. In brief, clinical diagnosis of HCC was defined by one imaging technique (spiral CT scan, dynamic MRI, or hepatic artery angiography) showing a compatible feature of HCC in patients with alpha-fetoprotein level of more than 400 ng/ml or two imaging techniques showing compatible feature of HCC in patients with alpha-fetoprotein level of less than 400 ng/ml [6].
Out of the 4,520 patients, we analyzed 3,232 patients with either HBV or HCV infection (HBV: 2,785, HCV: 447). HBV-related HCC was defined when the presence of hepatitis B surface antigen was documented with a history of chronic liver disease. HCV-related HCC was defined when the presence of HCV RNA or anti-HCV were documented with a history of chronic liver disease. Forty-one patients had dual HBV and HCV infection, and were excluded from the analysis. The study protocol was reviewed and approved by the Institutional Review Board at Samsung Medical Center. The requirement for the informed consent was exempted by the Institutional Review Board because the study was based on the retrospective analysis of existing administrative and clinical data. Patient records/information was anonymized and de-identified prior to analysis
Variables
The KLCSG random cohort registry collected information including age, gender, date of diagnosis, etiology, Child-Pugh class, tumor number, tumor size, presence of portal vein invasion and extrahepatic spread, American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) tumor-node-metastasis (TNM) stage, and applied treatment modality. At the time of data abstraction, Barcelona Clinic Liver Cancer (BCLC) staging and performance status was not collected. Hence, we re-coded the BCLC stage with Child-Pugh class, tumor size, tumor number and presence of portal vein invasion and extrahepatic spread, omitting performance status. Surgical resection, local ablation and transplantation were defined as curative therapies; and transarterial chemoembolization (TACE), transarterial chemoinfusion (TACI), systemic chemotherapy and radiation were regarded as palliative therapies. Survival data was collected from the National Statistics Service, and survival data of each patient were acquired up to December 2011.
Statistical analysis
Statistical analysis was performed using the chi-square test to compare discrete variables and the t-test for continuous variables between HBV and HCV-related HCC. The survival rate was calculated and plotted by using the Kaplan-Meier method. To assess whether etiology was associated with survival, the Cox proportional hazard model was used, and a P value of less than 0.05 was considered to be significant.
Results
Clinical characteristics
The baseline characteristics at diagnosis are compared in Table 1. Mean age at diagnosis was much younger in HBV than HCV-related HCC (53.9±9.7 vs. 65.4±8.8, P<0.01). In HBV-related HCC, a comparably significant proportion of patients were under 40 years old (7%). Sixty-five percent were age between 40 and 59, and 28% were over 60 years. While in HCV-related HCC, those diagnosed below the age of 40 years was rare (1%) and most patients (77%) were over 60 years. In HBV-related HCC, the mean age at diagnosis was much younger for men than women (53.2±9.3 vs. 57.2±10.7, P<0.01), while in HCV-related HCC, the mean age at diagnosis was marginally younger for men than women (64.9±8.6 vs. 66.9±9.4 for men vs. women, P = 0.05).
Table 1. Characteristics of study population.
| Characteristics | HBV | HCV | P-value |
| Number | 2,785 | 447 | |
| Age (years, mean ± S.D) | 53.9±9.7 | 65.4±8.8 | <0.01 |
| <40 | 193 (7%) | 4 (1%) | |
| 40–49 | 800 (29%) | 22 (5%) | |
| 50–59 | 1,010 (36%) | 77 (17%) | |
| 60–69 | 647 (23%) | 197 (44%) | |
| ≥70 | 135 (5%) | 147 (33%) | |
| Male, n (%) | 2,248 (81%) | 339 (76%) | 0.02 |
| Laboratory values | |||
| Albumin (g/dl) | 3.5 (3.0–3.9) | 3.4 (3.0–3.8) | <0.01 |
| Total bilirubin (mg/dl) | 1.1 (0.7–1.7) | 1.0 (0.7–1.6) | 0.33 |
| Prothrombin time (INR) | 1.15 (1.05–1.29) | 1.15 (1.05–1.28) | 0.67 |
| Child-Pugh class | 0.18 | ||
| A | 1,798 (65%) | 299 (67%) | |
| B | 734 (26%) | 119 (27%) | |
| C | 254 (9%) | 29 (7%) | |
| Diagnostic methods | 0.86 | ||
| Histologic | 458 (16%) | 75 (17%) | |
| Clinical | 2,327 (84%) | 372 (83%) | |
| Tumor number | 0.03 | ||
| Single | 1,972 (71%) | 294 (66%) | |
| Multiple | 813 (29%) | 153 (34%) | |
| Maximal tumor size (cm) | <0.01 | ||
| <2 | 387 (14%) | 79 (18%) | |
| 2–5 | 1,089 (39%) | 217 (49%) | |
| ≥5 | 1,309 (47%) | 151 (34%) | |
| Portal vein invasion | <0.01 | ||
| Yes | 739 (27%) | 73 (16%) | |
| Extrahepatic spread | 316 (11%) | 40 (9%) | 0.13 |
| AJCC/mUICC stage | 0.08 | ||
| I | 294 (11%) | 54 (12%) | |
| II | 1,171 (42%) | 206 (46%) | |
| III | 776 (28%) | 120 (27%) | |
| IV-A | 299 (11%) | 43 (10%) | |
| IV-B | 245 (9%) | 24 (5%) | |
| BCLC stage† | <0.01 | ||
| O | 202 (7%) | 39 (9%) | |
| A | 1,266 (46%) | 233 (52%) | |
| B | 255 (9%) | 62 (14%) | |
| C | 808 (29%) | 84 (19%) | |
| D | 254 (9%) | 29 (7%) | |
| Milan criteria | |||
| Within Milan (YES) | 1,250 (45%) | 239 (54%) | 0.01 |
| Treatment | 0.53 | ||
| Curative | 516 (19%) | 92 (21%) | |
| Palliative | 1,597 (57%) | 246 (55%) | |
| Experimental* | 2 (0.1%) | 1 (0.2%) | |
| None | 670 (24%) | 108 (24%) | |
| Specific modality | 0.01 | ||
| Resection | 295 (14%) | 34 (10%) | |
| Ablation | 194 (9%) | 54 (16%) | |
| Transplantation | 27 (1%) | 4 (1%) | |
| TACE | 1,439 (68%) | 231 (68%) | |
| TACI | 100 (5%) | 13 (4%) | |
| Systemic chemotherapy | 30 (1%) | 1 (0.3%) | |
| Radiation | 28 (1%) | 1 (0.3%) |
Abbreviation: HBV, hepatitis B virus; HCV, hepatitis C virus; S.D, standard deviation; INR, international normalized ratio; AJCC/mUICC, American Joint Committee on Cancer/International Union Against Cancer; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; TACI, transarterial chemoinfusion.
*These 3 patients received 166holmium injection therapy. Values are expressed as mean ± standard deviation, median (quartile), or no (%).
BCLC stage and performance status was not collected at the time of data collection. Hence, BCLC stage was re-coded (staged) by authors with Child-Pugh class, tumor size, tumor number and presence of portal vein invasion and extrahepatic spread, without performance status.
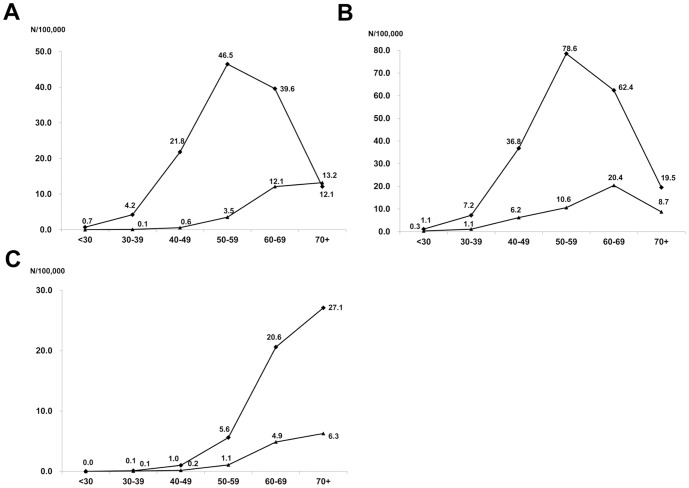
We estimated the annual incidence rate of HCC using the mid-point-population of the study period (data not shown, available at National statistics service, http://kostat.go.kr). The mean annual incidence rates of HBV- and HCV-related HCC per 100,000 persons were 20.8 and 4.9, respectively. The mean annual incidence rates of men per 100,000 were 34.3 and 9.1 for HBV- and HCV-related HCC, respectively, and were 7.9 and 2.1 for HBV- and HCV-related HCC for women. The annual incidence rate of HBV-related HCC peaked at age group 50–59, while the annual incidence rates of HCV-related HCC gradually increased until age group over 70 (Fig. 1A). In HBV-related HCC, the annual incidence rate peaked at 50–59 (78.6 per 100,000) in men, while it was 60–69 (20.4 per 100,000) in women (Fig. 1B). In HCV-related HCC, the annual incidence rate gradually increased until age over 70 in both genders (Fig. 1C).
Figure 1. The age-specific incidence rates of hepatocellular carcinoma (HCC) by the etiology (A), by gender in HBV-related HCC (B) and by gender in HCV-related HCC (C).
The annual incidence rates of HBV-related HCC peaked in the 50–59 age group, while the annual incidence rates of HCV-related HCC kept gradually increasing until age ≥70 s. Similar trend was observed after stratified by gender, although the peak mean annual incidence rates was observed in the 50–59 in men and in the 60–69 in women in HBV-related HCC (B). Diamonds (♦) and triangles (▴) represent for HBV and HCV-related HCC in (A), men and women in (B) and (C), respectively.
Male patients comprised significantly more proportion in HBV-related HCC than HCV-related HCC, the serum albumin level was significantly lower in HCV-related HCC, however, the Child-Pugh Class at diagnosis was similar in both groups (Table 1).
Tumor characteristics and treatment pattern
Large tumor (≥5 cm, 47% vs. 34%, P<0.01) and portal vein invasion (27% vs. 16%, P<0.01) were more frequent in HBV-related HCC, however, multiple tumor was more common in HCV-related HCC (34% vs. 29%, P = 0.03). There was only a marginal difference according to AJCC/mUICC stage between HBV and HCV-related HCC (Table 1). When assessed according to the BCLC staging system (without performance status), the proportion of patients with BCLC stage C or D was higher in HBV-related HCC than in HCV-related HCC (29% vs. 19% and 9% vs. 7%), while the proportion of patients with stage 0, A or B was higher in HCV-related HCC than in HBV-related HCC (9% vs. 7%, 52% vs. 46% and 14% vs. 9%). The proportion of patients diagnosed at within the Milan criteria was significantly lower in HBV- than HCV-related HCC (45% vs. 54%, P = 0.01, Table 1). In HBV-related HCC, the proportion of patients diagnosed outside the Milan criteria was highest in age <40 years group (67.4%, 58.0%, 51.3%, 53.3%, and 57.8% for age <40, 40 s, 50 s, 60 s and ≥70 years, respectively).
When stratified according to the treatment modality (curative, palliative vs. none), there was no difference of initial treatment modality between HBV- and HCV-related HCC. Of the specific treatment modality, resection was more common in HBV-related HCC (14% vs. 10%), while local ablation therapy was more common in HCV-related HCC (16% vs. 9%).
Outcomes
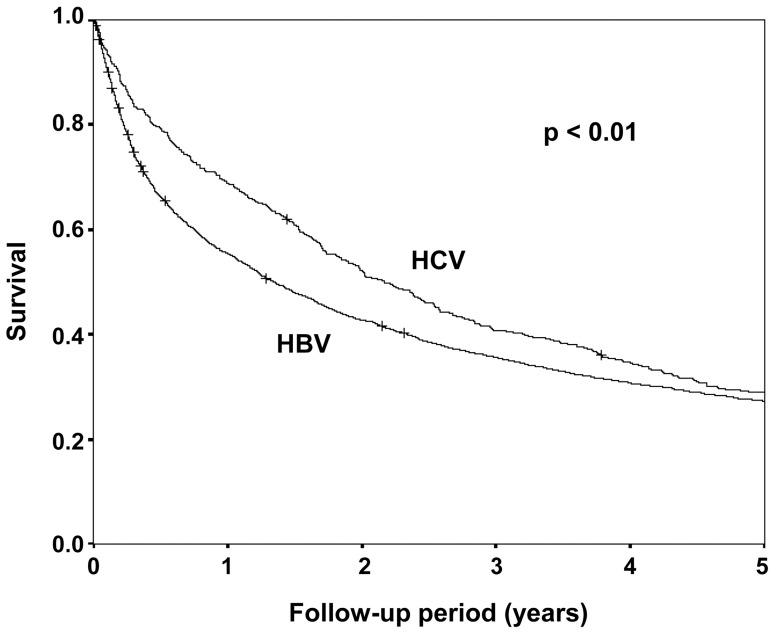
The 1, 3, and 5 year survival rate was 55%, 35%, and 27% for HBV-related HCC and was 68%, 40%, and 28% for HCV-related HCC. The median survival was significantly longer in HCV-related HCC (2.17 vs. 1.34 years, P<0.01, Fig. 2). The hazard ratio for survival was significantly lower in HCV-related HCC compared to HBV-related HCC in both un-adjusted and adjusted analysis (Table 2).
Figure 2. Long-term survival by etiology.
The median survival was significantly longer in HCV-related HCC patients than in HBV-related HCC patients. (2.17 vs. 1.34 years, P<0.01).
Table 2. Survival by etiology.
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||
| Median survival (years) | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| HBV | 1.34 | Reference | Reference | Reference | Reference | ||||
| HCV | 2.17 | 0.94 (0.84–1.05) | 0.28 | 0.88 (0.78–0.99) | 0.04 | 0.86 (0.77–0.98) | 0.02 | 0.88 (0.78–0.98) | 0.03 |
Abbreviation: HBV, hepatitis B virus; HCV, hepatitis C virus. HR, hazard ratio. Model 1 = crude hazard ratio, Model 2 = adjusted for age, gender, Model 3 = Model 2 + Child-Pugh class and AJCC/mUICC stage, Model 4 = Model 3 + initial treatment modality.
The independent factor for survival was similar for both HBV- and HCV-related HCC. Gender, Child-Pugh Class, AJCC/mUICC stage and treatment modality was independent factors associated with survival in both HBV- and HCV-related HCC (Table 3). Age (per year) was not an independent factor for survival in HBV-related HCC, while it was an independent factor for survival in HCV-related HCC (Table 3). However, when patients were grouped in 10-year intervals, the median survival was shortest in patients under 40 (0.83 years), followed by 40–49 (1.15 years), over 70 years (1.26 years), 60–69 (1.43 years), and longest in the 50–59 age group (1.58 years) in HBV-related HCC, showing poor survival in both extreme of age group. Patients under 40 showed shorter survival than the age 50–59 group in unadjusted analysis, but when adjusted, patients under 40 showed comparable survival (Table 3). Older age groups (60–69 and ≥70 years) showed significantly shorter survival than 50–59 age group in unadjusted and adjusted analysis. In HCV-related HCC, there were only a few patients in the 40 (n = 4) and 40–49 (n = 22), so those patients were grouped as under 50 years, 50–59, 60–69 and ≥70 years. Age per year was a significant factor associated with patient survival in HCV-related HCC (Table 3). Older age groups (60s, ≥70 years) showed poorer survival than the 50–59 age group in adjusted analysis (Table 3).
Table 3. Comparison of prognostic factors between hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma.
| HBV-related HCC | HCV-related HCC | |||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 1.00 (0.99–1.01) | 0.16 | 1.00 (0.99–1.01) | 0.13 | 1.01 (1.00–1.03) | 0.01 | 1.01 (1.01–1.02) | 0.03 |
| <40 | 1.24 (1.04–1.48) | 0.01 | 1.13 (0.95–1.35) | 0.15 | 1.08 (0.64–1.81) | 0.77 | 1.32 (0.78–2.23) | 0.29 |
| 40–49 | 1.08 (0.97–1.21) | 0.13 | 0.95 (0.85–1.06) | 0.40 | ||||
| 50–59 | Reference | Reference | Reference | Reference | ||||
| 60–69 | 1.21 (1.08–1.35) | 0.01 | 1.15 (1.03–1.29) | 0.01 | 1.17 (0.87–1.59) | 0.28 | 1.41 (1.04–1.91) | 0.03 |
| ≥70 | 1.48 (1.21–1.78) | <0.01 | 1.22 (1.01–1.49) | 0.04 | 1.53 (1.12–2.08) | 0.01 | 1.46 (1.07–2.00) | 0.01 |
| Gender | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 1.27 (1.13–1.41) | <0.01 | 1.37 (1.22–1.54) | <0.01 | 1.06 (0.83–1.35) | 0.63 | 1.37 (1.06–1.78) | 0.01 |
| Child-Pugh Class | ||||||||
| A | Reference | Reference | Reference | Reference | ||||
| B | 2.20 (2.00–2.42) | <0.01 | 1.64 (1.48–1.81) | <0.01 | 2.03 (1.61–2.56) | <0.01 | 1.73 (1.36–2.20) | <0.01 |
| C | 2.82 (2.44–3.26) | <0.01 | 2.54 (2.19–2.95) | <0.01 | 2.89 (1.92–4.35) | <0.01 | 3.23 (2.08–5.00) | <0.01 |
| AJCC/mUICC stage | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 1.68 (1.37–1.94) | <0.01 | 1.72 (1.45–2.05) | <0.01 | 1.43 (0.99–2.05) | 0.05 | 1.16 (0.80–1.67) | 0.42 |
| III | 3.32 (2.78–3.96) | <0.01 | 2.76 (2.31–3.33) | <0.01 | 2.29 (1.57–3.35) | <0.01 | 1.67 (1.13–2.45) | 0.01 |
| IV | 6.07 (5.50–7.30) | <0.01 | 4.75 (3.94–5.72) | <0.01 | 4.16 (2.74–6.31) | <0.01 | 2.38 (1.54–3.68) | <0.01 |
| Treatment | ||||||||
| Curative | Reference | Reference | Reference | Reference | ||||
| Palliative | 2.83 (2.46–3.25) | <0.01 | 2.44 (2.11–2.81) | <0.01 | 2.63 (1.93–3.58) | <0.01 | 2.83 (2.04–3.91) | <0.01 |
| None | 7.78 (6.68–9.07) | <0.01 | 4.97 (4.24–5.83) | <0.01 | 5.36 (3.81–7.54) | <0.01 | 4.68 (3.26–6.71) | <0.01 |
Abbreviation: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio.
When stratified by tumor burden (AJCC/mUICC stage), survival was not different in AJCC/mUICC I, II, III tumors (Table 4). However, in AJCC/mUICC stage IV, long-term survival was significantly worse in HBV-related HCC than HCV-related HCC. When stratified by treatment, patients who received curative or palliative therapy, there was no significant difference in survival. However, survival was significantly worse for HBV-related HCC for patients who had not received therapy (Table 4). Even in AJCC/mUICC stage IV tumor, there was no significant different in survival for patients who received treatment, and the survival difference was significant for patients who had no therapy (Table 4).
Table 4. Adjusted difference in the survival between hepatitis B virus and hepatitis C virus related hepatocellular carcinoma by subgroup.
| Number of patients (HBV vs. HCV) | Median survival, year, (HBV vs. HCV) | Adjusted hazard ratio (95% CI) | P-value | |
| AJCC/mUICC stage I | 294 vs. 54 | 6.60 vs. 5.09 | 1.10 (0.73–1.66) | 0.63 |
| AJCC/mUICC stage II | 1,172 vs. 206 | 2.95 vs. 3.19 | 0.86 (0.71–1.03) | 0.10 |
| AJCC/mUICC stage III | 776 vs. 120 | 0.65 vs. 1.71 | 0.95 (0.76–1.19) | 0.70 |
| AJCC/mUICC stage IV | 544 vs. 67 | 0.28 vs. 0.49 | 0.65 (0.49–0.88) | 0.01 |
| Stage IV and received treatment | 311 vs. 33 | 0.48 vs. 0.95 | 0.69 (0.44–1.08) | 0.11 |
| Stage IV and received no treatment | 233 vs. 34 | 0.12 vs. 0.24 | 0.64 (0.42–0.98) | 0.04 |
| Curative treatment | 516 vs. 92 | NR vs. 6.19 | 1.09 (0.78–1.53) | 0.59 |
| Resection | 295 vs. 34 | NR vs. NR | 0.72 (0.39–1.35) | 0.31 |
| Ablation | 194 vs. 54 | 7.06 vs. 4.47 | 1.13 (0.74–1.73) | 0.55 |
| Palliative treatment | 1,597 vs. 246 | 1.48 vs. 2.12 | 0.95 (0.81–1.11) | 0.53 |
| TACE | 1,439 vs. 231 | 1.72 vs. 2.23 | 0.97 (0.83–1.14) | 0.76 |
| None | 672 vs. 109 | 0.18 vs. 0.33 | 0.75 (0.59–0.95) | 0.02 |
Abbreviation: HBV, hepatitis B virus; HCV, hepatitis C virus; CI, confidence interval; NR, not reached. In each adjusted model, hepatitis B was used as reference for hepatitis C and following variables were adjusted: age, gender, Child-Pugh class, AJCC/mUICC stage, and initial treatment modality.
Discussion
In this study, HCC patients clearly showed different clinical manifestation according to viral etiology (HBV vs. HCV). HBV-related HCC occurred at a younger age than HCV-related HCC. The annual incidence rate of HBV-related HCC peaked in the 50–59 year age group, while the annual incidence rate of HCV-related HCC gradually increased up to the over 70 year group. In HBV-related HCC, tumor was more likely to be single and larger, and accompanied by portal vein invasion. Overall, the long-term outcome was worse in HBV-related HCC than HCV-related HCC, however, the survival difference existed only for patients with advanced tumor (AJCC/mUICC stage IV). The outcome of each treatment modality (resection, ablation, and TACE) was also comparable between HBV- and HCV-related HCC.
In this study, we could estimate annual incidence rate of HCC by age groups and etiology for the first time in Korea, as the data was based on a nationwide population-based cohort, and could notice significant differences in the incidence rates of HCC by age group and viral etiology. The highest age-specific incidence rates were observed in the 50–59 age group in HBV-related HCC, while the annual incidence rates of HCV-related HCC kept gradually increasing until the ≥70 group. This phenomenon may be explained by the fact that vertical transmission is the major route of HBV acquisition in Korea [24], while HCV is usually acquired in adulthood [19]. HCC rarely developed before the age of 50 years in HCV-related HCC, likewise in countries where HBV is not endemic [13]. However, in HBV-related HCC, the age-specific incidence rate was not low in patient age less than 50 years. A clear gender difference in the age-specific incidence rate of HBV-related HCC was also observed. In men, highest age-specific incidence rates were observed in the 50–59 age group (78.6 per 100,000), while it was highest in the 60–69 age group (20.4 per 100,000) in women. In HCV-related HCC, there was no significant difference in the peak age group between men and women.
Notably, there were significant differences in the tumor characteristic at diagnosis by viral etiology. HBV-related HCC were more likely to be single, large, and accompanied by portal vein invasion. Different oncogenic mechanism by different viruses may explain the observed differences in tumor characteristics. However, it might also be attributable to the currently recommended surveillance policy for HBV and HCV infected individuals in Korea. An HCC surveillance program has been included in National Cancer Screening Program in Korea since 2003 [9]. Patients over 40 year old with risk factors such as hepatitis B, hepatitis C and cirrhosis are recommended to undergo HCC surveillance by ultrasonography and alpha-fetoprotein levels at 6-month interval. Thus, early participation of HCV-infected patients in regular surveillance program before reaching the peak age of HCC development might have a positive influence on the characteristics of tumor at the time of diagnosis. HBV-related HCC patients were diagnosed at younger age, and the proportion of patients diagnosed outside the Milan criteria was also higher in young age group in HBV-related HCC patients. Several studies reported that most of HCC patients diagnosed at young age did not receive regular HCC surveillance, and presented at advanced stage [25], [26]. As this study did not collect information regarding HCC surveillance before HCC diagnosis, careful interpretation is needed. Nevertheless, this data strongly suggest different surveillance strategy (e.g., different age cutoff for starting surveillance) according to the viral etiology is needed.
The decision to undergo HCC surveillance is determined by the level of risk for HCC, which is related to HCC incidence in a pre-defined at-risk population. Although we cannot provide the exact incidence rate of HCC according to each age group and etiology, we could roughly estimate the annual incidence rate of HCC based on population and prevalence rates of HBV and HCV infection in each age group. The estimated annual incidence rates of HCC in HBV infected patients were 0.02%, 0.09%, 0.5%, 0.8%, 1.2% and 1.1% for age 20–29, 30–39, 40–49, 50–59, 60–69 and ≥70 years, respectively. The estimated annual incidence rates of HCC in HCV infected patients were 0%, 0.02%, 0.1%, 0.4%, 0.8% and 0.6% for age 20–29, 30–39, 40–49, 50–59, 60–69 and ≥70 years, respectively. Similar annual incidence rates of HCC were observed in HBV infected patients who were about 10 years younger than in HCV infected patients. Thus, HBV-infected patients may require earlier participation in an HCC surveillance program compared to that of HCV infected patients.
In this study, we observed differences of overall survival according to the viral etiology. However, when stratified by tumor stage, patients who were diagnosed at AJCC/mUICC stage I, II and III, showed similar survival in HBV- and HCV-related HCC. In AJCC/mUICC stage IV tumor, survival was significantly worse in HBV-related HCC. These findings are consistent with the study of Cantarini et al., which reported that HBV-related HCC is more aggressive than HCV-related HCC, especially for HCC diagnosed at an advanced stage [27]. Whether or not viral etiology should be considered in treatment decision is also an important issue. Indeed, several previous studies have tried to assess whether viral etiology is a significant factor determining treatment outcome, and found that viral etiology was not an independent factor for determining outcomes of HCC who underwent resection [28], [29], ablation [30], or TACE [31]. In this study, we also found that viral etiology is not an independent factor associated with survival for patients who received therapy. Even in AJCC/mUICC stage IV tumor, survival was not significantly different when patients received treatment (Table 4). Therefore, although viral etiology should be considered in estimation of patient prognosis, it may have little impact on choosing therapeutic modality.
There are some limitations in this study. Information of some important prognostic factors (e.g., performance status, serum alpha-fetoprotein levels, and use of antiviral therapy, etc.) was not collected. The BCLC stage was not collected and could not be properly assessed. There are also some remained questions whether viral etiology would affect the treatment outcome of other types of treatment, such as transplantation or sorafenib. For liver transplantation, HBV-related HCC has been reported to be an independent factor associated with better survival than HCV-related HCC [32]. For sorafenib, the median survival in the Sorafenib HCC Assessment Randomized Protocol Trial (SHARP) trial (10.7 months) was longer than the median survival of same trial performed in the Asia-Pacific Study (6.5 months) [33], [34]. Unfortunately, sorafenib had not been approved in Korea during period of the present study, so there were no patients who used sorafenib. Hence, it was impossible to assess the impact of viral etiology for the prognosis of patients treated with sorafenib. Lastly, this study only assessed first treatment modality. It is known that HCV-related tumors have higher recurrence rate after curative resection than HBV-related HCC [35], which may have affected long-term outcome.
Despite some limitations, this study provides highly reliable information from unselected nationwide cohort, which minimized selection bias, and large sample size with long term follow-up. HBV-related and HCV-related HCC have clear differences in clinical manifestation, requiring different screening strategy according to etiology to optimize HCC surveillance in HBV-endemic area. However, etiology does not affect treatment outcome and long-term survival within the same stage except for far advanced tumor.
Acknowledgments
This study was selected to use the KLCSG random cohort data in the 2012 KLCSG Research Contest. The authors thank all members of the KLCSG and the Korea Central Cancer Registry for their contribution to build the KLCSG random cohort.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The Korean Liver Cancer Study Group (KLCSG) random cohort data used for this study was obtained from the KLCSG governing committee. The right to open and distribute the raw data belongs to the KLCSG governing committee. To request the raw data, contact the KLCSG governing committee via the KLCSG office (liver@klcsg.or.kr).
Funding Statement
The authors have no support or funding to report.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2014) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 13 doi: []10.1022/ijc.29210. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2. Yang JD, Roberts LR (2010) Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 7: 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 4. Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korean Liver Cancer Study Group (2009) [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol 15: 391–423. [DOI] [PubMed] [Google Scholar]
- 7. Chae HB, Kim JH, Kim JK, Yim HJ (2009) Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol 15 Suppl 6: S13–24. [DOI] [PubMed] [Google Scholar]
- 8. Korean Association for the Study of the Liver (2012) KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol 18: 109–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song IH, Kim KS (2009) Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol 15 Suppl 6: S50–59. [DOI] [PubMed] [Google Scholar]
- 10. Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, et al. (2005) Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci 20: 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suh DJ, Jeong SH (2006) Current status of hepatitis C virus infection in Korea. Intervirology 49: 70–75. [DOI] [PubMed] [Google Scholar]
- 12. Sinn DH, Paik SW, Kang P, Kil JS, Park SU, et al. (2008) Disease progression and the risk factor analysis for chronic hepatitis C. Liver Int 28: 1363–1369. [DOI] [PubMed] [Google Scholar]
- 13. El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132: 2557–2576. [DOI] [PubMed] [Google Scholar]
- 14. Kim W, Oe Lim S, Kim JS, Ryu YH, Byeon JY, et al. (2003) Comparison of proteome between hepatitis B virus- and hepatitis C virus-associated hepatocellular carcinoma. Clin Cancer Res 9: 5493–5500. [PubMed] [Google Scholar]
- 15. Hai H, Tamori A, Kawada N (2014) Role of hepatitis B virus DNA integration in human hepatocarcinogenesis. World J Gastroenterol 20: 6236–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh MM, Daniel HD, Torbenson M (2010) Hepatitis C-associated hepatocellular carcinomas in non-cirrhotic livers. Mod Pathol 23: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou YM, Zhang XF, Li B, Sui CJ, Yang JM (2014) Prognosis after resection of hepatitis B virus-related hepatocellular carcinoma originating from non-cirrhotic liver. Ann Surg Oncol 21: 2406–2412. [DOI] [PubMed] [Google Scholar]
- 18. Kalayci C, Johnson PJ, Davies SE, Williams R (1991) Hepatitis B virus related hepatocellular carcinoma in the non-cirrhotic liver. J Hepatol 12: 54–59. [DOI] [PubMed] [Google Scholar]
- 19. The Korean Association for the Study of the Liver (2014) KASL clinical practice guidelines: Management of Hepatitis C. Clin Mol Hepatol 20: 89–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, et al. (1995) Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology 22: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 21. Tanabe G, Nuruki K, Baba Y, Imamura Y, Miyazono N, et al. (1999) A comparison of hepatocellular carcinoma associated with HBV or HCV infection. Hepatogastroenterology 46: 2442–2446. [PubMed] [Google Scholar]
- 22. Hiotis SP, Rahbari NN, Villanueva GA, Klegar E, Luan W, et al. (2012) Hepatitis B vs. hepatitis C infection on viral hepatitis-associated hepatocellular carcinoma. BMC Gastroenterol 12: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahn YO (2007) [Cancer registration in Korea: the present and furtherance]. J Prev Med Public Health 40: 265–272. [DOI] [PubMed] [Google Scholar]
- 24. Choi MS, Sinn DH, Kim SA, Lee YS, Choi W, et al. (2012) The clinical and laboratory characteristics of patients with chronic hepatitis B using current or past antiviral therapy in Korea: a multi-center, nation-wide, cross-sectional epidemiologic study. Gut Liver 6: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JH, Choi MS, Lee H, Kim do Y, Lee JH, et al. (2006) Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol 21: 588–594. [DOI] [PubMed] [Google Scholar]
- 26. Wang Q, Luan W, Villanueva GA, Rahbari NN, Yee HT, et al. (2012) Clinical prognostic variables in young patients (under 40 years) with hepatitis B virus-associated hepatocellular carcinoma. J Dig Dis 13: 214–218. [DOI] [PubMed] [Google Scholar]
- 27. Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, et al. (2006) Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol 101: 91–98. [DOI] [PubMed] [Google Scholar]
- 28. Kao WY, Su CW, Chau GY, Lui WY, Wu CW, et al. (2011) A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg 35: 858–867. [DOI] [PubMed] [Google Scholar]
- 29. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, et al. (2013) Comparison of clinical characteristics and survival after surgery in patients with non-B and non-C hepatocellular carcinoma and hepatitis virus-related hepatocellular carcinoma. J Cancer 4: 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen PH, Kao WY, Chiou YY, Hung HH, Su CW, et al. (2013) Comparison of prognosis by viral etiology in patients with hepatocellular carcinoma after radiofrequency ablation. Ann Hepatol 12: 263–273. [PubMed] [Google Scholar]
- 31. Takayasu K, Arii S, Ikai I, Omata M, Okita K, et al. (2006) Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 131: 461–469. [DOI] [PubMed] [Google Scholar]
- 32. Tandoi F, Ponte E, Saffioti MC, Patrono D, Mirabella S, et al. (2013) Liver transplantation for hepatocellular carcinoma within Milan Criteria in patients with Model for End-Stage Liver Disease score below 15: the impact of the etiology of cirrhosis on long-term survival. Transplant Proc 45: 2711–2714. [DOI] [PubMed] [Google Scholar]
- 33. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 34. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 35. Huang YH, Wu JC, Chen CH, Chang TT, Lee PC, et al. (2005) Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C-related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int 25: 236–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The Korean Liver Cancer Study Group (KLCSG) random cohort data used for this study was obtained from the KLCSG governing committee. The right to open and distribute the raw data belongs to the KLCSG governing committee. To request the raw data, contact the KLCSG governing committee via the KLCSG office (liver@klcsg.or.kr).