Abstract
Objectives
A secondary objective of this head-to-head study of lisdexamfetamine dimesylate (LDX) and atomoxetine (ATX) was to assess treatment response rates in children and adolescents with attention-deficit hyperactivity disorder (ADHD) and an inadequate response to methylphenidate (MPH). The primary efficacy and safety outcomes of the study, SPD489-317 (ClinicalTrials.gov NCT01106430), have been published previously.
Methods
In this 9-week, double-blind, active-controlled study, patients aged 6–17 years with a previous inadequate response to MPH were randomized (1:1) to dose-optimized LDX (30, 50 or 70 mg/day) or ATX (patients <70 kg: 0.5–1.2 mg/kg/day, not to exceed 1.4 mg/kg/day; patients ≥70 kg: 40, 80 or 100 mg/day). Treatment response was a secondary efficacy outcome and was predefined as a reduction from baseline in ADHD Rating Scale IV (ADHD-RS-IV) total score of at least 25, 30 or 50 %. Sustained response was predefined as a reduction from baseline in ADHD-RS-IV total score (≥25, ≥30 or ≥50 %) or a Clinical Global Impressions (CGI)–Improvement (CGI–I) score of 1 or 2 throughout weeks 4–9. CGI–Severity (CGI–S) scores were also assessed, as an indicator of remission.
Results
A total of 267 patients were enrolled (LDX, n = 133; ATX, n = 134) and 200 completed the study (LDX, n = 99; ATX, n = 101). By week 9, significantly (p < 0.01) greater proportions of patients receiving LDX than ATX met the response criteria of a reduction from baseline in ADHD-RS-IV total score of at least 25 % (90.5 vs. 76.7 %), 30 % (88.1 vs. 73.7 %) or 50 % (73.0 vs. 50.4 %). Sustained response rates were also significantly (p < 0.05) higher among LDX-treated patients (ADHD-RS-IV ≥25, 66.1 %; ADHD-RS-IV ≥30, 61.4 %; ADHD-RS-IV ≥50, 41.7 %; CGI–I, 52.0 %) than among ATX-treated individuals (ADHD-RS-IV ≥25, 51.1 %; ADHD-RS-IV ≥30, 47.4 %; ADHD-RS-IV ≥50, 23.7 %; CGI–I, 39.3 %). Finally, by week 9, 60.7 % of patients receiving LDX and 46.3 % of those receiving ATX had a CGI–S score of 1 (normal, not at all ill) or 2 (borderline mentally ill), and greater proportions of patients in the LDX group than the ATX group experienced a reduction from baseline of at least one CGI–S category.
Conclusions
Both LDX and ATX treatment were associated with high levels of treatment response in children and adolescents with ADHD and a previous inadequate response to MPH. However, within the parameters of the study, LDX was associated with significantly higher treatment response rates than ATX across all response criteria examined. In addition, higher proportions of patients in the LDX group than the ATX group had a CGI–S score of 1 or 2 by week 9, indicating remission of symptoms. Both treatments were generally well tolerated, with safety profiles consistent with those observed in previous studies.
Key Points
| This study presents treatment response rates from a head-to-head, randomized, double-blind clinical trial of lisdexamfetamine dimesylate (LDX) and atomoxetine (ATX) in the treatment of children and adolescents with attention-deficit hyperactivity disorder and a previous inadequate response to methylphenidate therapy |
| LDX treatment was consistently associated with statistically significantly higher treatment response rates than ATX across seven predefined response and sustained response criteria |
| Higher proportions of patients receiving LDX than ATX had a Clinical Global Impressions–Severity score of 1 (normal, not at all ill) or 2 (borderline mentally ill) by week 9, indicating remission of symptoms |
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder that is estimated to affect approximately 5 % of children and adolescents and 3 % of adults worldwide [1–3]. ADHD is characterized by symptoms of inattention, hyperactivity and/or impulsivity and is associated with substantial functional impairment across the lifespan [4, 5]. In addition, ADHD is associated with reduced health-related quality of life for both patients and their families [6]. The prodrug stimulant lisdexamfetamine dimesylate (LDX) is an effective treatment for ADHD in children, adolescents and adults [7–13] and is currently licensed as a first-line pharmacological therapy for ADHD in the US, Canada, Brazil and Australia. LDX is the only long-acting amfetamine formulation available in Europe, where it is licensed in several countries for the treatment of ADHD in children aged 6 years and over when the response to previous methylphenidate (MPH) therapy is considered clinically inadequate.
Instruments such as the ADHD Rating Scale IV (ADHD-RS-IV) [14], the Swanson, Nolan and Pelham version IV (SNAP-IV) scale [15, 16] and the Conners’ Parent Rating Scale-Revised (CPRS-R) [17] may be used in routine clinical practice to assess symptoms in patients with ADHD, and changes in mean scores provide evidence of treatment-related improvements in symptoms. Considerable normative data are available for these scales and they have all demonstrated good reliability and discriminant validity in children and adolescents [14, 18]. However, while informative, changes in mean rating-scale scores cannot describe the degree of symptom improvement experienced at an individual level. An alternative approach to assessing the efficacy of a medication in patients with ADHD is to conduct responder analyses in order to establish the proportion of patients who show a clinically relevant response to treatment, where clinical response is defined a priori. The value of responder analyses when assessing the benefits of pharmacological treatment options is recognized by the requirement of the European Medicines Agency (EMA) that clinical response outcomes be included in all European regulatory trials for new ADHD medications [19]. Another approach to exploring clinical benefit at the level of the individual is to examine the numbers of patients who shift to a less severe Clinical Global Impressions (CGI)–Severity (CGI–S) category as a result of treatment.
The primary efficacy and safety outcomes from a head-to-head study of LDX and atomoxetine (ATX) in the treatment of ADHD have been reported (study SPD489-317; ClinicalTrials.gov identifier: NCT01106430) [20]. This 9-week, double-blind, randomized study was conducted in children and adolescents who had previously responded inadequately to MPH. In this primary analysis, a single definition of clinical response was used—a CGI–Improvement (CGI–I) score of 1 or 2. The time to first clinical response (the primary study endpoint) was significantly shorter with LDX treatment than with ATX treatment (median, 12.0 days and 21.0 days, respectively; p = 0.001), and the proportion of patients with a CGI–I score of 1 or 2 by the end of the 9-week study was significantly higher (81.7 and 63.6 %, respectively; p = 0.001) [20]. Owing to a lack of consensus within the published literature on the most appropriate definition of clinical response, we now report the results of further prespecified responder analyses from SPD489-317. These are based on multiple ADHD-RS-IV and CGI–I criteria that have been used in previous responder analyses to assess the efficacy of LDX or ATX treatment [7, 21–27]. We also present shifts from baseline to week 9 in patients’ severity of illness based on CGI–S categories as an indication of remission.
Methods
The study procedures of this international, double-blind, randomized, parallel-group, phase IIIb trial have been fully described previously [20]. The trial was conducted in accordance with current applicable regulations, the International Conference on Harmonisation of Good Clinical Practice, and local ethical and legal requirements. Each patient’s parent or legally authorized representative provided written, informed consent, and assent was obtained from each participant (as applicable) before commencing study-related procedures. The study was conducted between June 2010 and July 2012 at 51 centres in nine countries in Europe and North America.
Study Population
Children and adolescents (aged 6–17 years) with a primary diagnosis of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) were eligible for enrolment in the study. Patient inclusion and exclusion criteria have been described previously [20]. Key inclusion criteria were an investigator-rated ADHD-RS-IV total score of 28 or higher at baseline (indicating symptoms of at least moderate severity) and an inadequate response to previous or current MPH treatment. Inadequate response included, but was not limited to, the presence of some residual ADHD symptoms, inadequate duration of action, variable symptom control, or the investigator’s judgement that the patient may benefit clinically from an alternative to MPH. Key exclusion criteria were previous exposure to amfetamine or ATX, intolerable side effects with previous MPH treatment, failure to respond to more than one previous course of one MPH medication (defined as worsened, unchanged or minimally improved symptoms), previous treatment with more than one MPH medication (with the exception of short-term dose titration with immediate-release MPH [e.g. for ≤4 weeks] with an adequate response), and good control of symptoms with acceptable tolerability on current ADHD medication. Patients with a comorbid psychiatric diagnosis with significant symptoms (except for oppositional defiant disorder) were also excluded.
Study Drug Administration
Patients underwent a 1-week washout period (if applicable) before baseline (visit 0), after which they were randomized (1:1) to receive once-daily LDX or ATX. The 9-week, double-blind, evaluation period comprised 4 weeks of dose optimization (visits 1–4) and 5 weeks of dose maintenance (visits 4–9), followed by a 1-week washout period and safety follow-up (visit 10). Dose optimization was based on achieving an ‘acceptable response’, defined as a reduction in ADHD-RS-IV total score of at least 30 % from baseline and a CGI–I score of 1 or 2, with acceptable tolerability. Study drugs were to be taken daily, at approximately 0700 h (±2 h), beginning on the day after visit 0. During the dose-optimization period, doses were increased at weekly intervals if an acceptable response with the current dose was not achieved, and one dose reduction was permitted if a patient experienced unacceptable tolerability. Patients in the LDX group initially received 30 mg/day and, if required, were titrated to 50 mg/day and then to 70 mg/day. ATX was available in 10, 18, 25, 40 and 60 mg capsules. In the ATX group, patients who weighed less than 70 kg at baseline initially received 0.5 mg/kg/day which was increased to a target dose of 1.2 mg/kg/day (not to exceed 1.4 mg/kg/day), in accordance with the dose recommended by the prescribing information for the drug [28]. Patients in the ATX group who weighed at least 70 kg at baseline initially received 40 mg/day and, if required, were titrated to 80 mg/day and then to 100 mg/day. Doses could not be changed beyond visit 3; participants unable to tolerate the study drug after this point were to be withdrawn from the study and complete an early termination visit. Patients in whom an acceptable response was achieved were to continue on their optimal dose for the remainder of the double-blind evaluation period (visits 4–9).
Efficacy and Safety Assessments
Efficacy was assessed using the investigator-rated CGI–S, CGI–I [29] and ADHD-RS-IV [14] instruments. The CGI–S provides an absolute measure of the global impression of the severity of a patient’s illness on a scale of 1 (normal, not at all ill) to 7 (among the most severely ill patients), and was completed at weeks 0, 4 and 9 and/or at early termination. The CGI–I captures the change in a patient’s condition compared with baseline on a scale from 1 (very much improved) to 7 (very much worse), and was completed at weeks 1–9 and/or at early termination. CGI–I results were dichotomized into ‘improved’ (score of 1 or 2) and ‘not improved’ (all other scores). The ADHD-RS-IV is based on the DSM-IV-TR symptomatology and comprises 18 items that are each rated on a 4-point Likert scale (0–3); thus, the total score ranges from 0 to 54, with higher scores reflecting more severe symptoms than lower scores. The ADHD-RS-IV was completed at screening, weeks 0–9 and/or at early termination.
Tolerability and safety measures have been previously reported, and included treatment-emergent adverse events (TEAEs), laboratory evaluations, physical examinations (including body mass), vital signs and electrocardiogram (ECG) parameters [20]. The Brief Psychiatric Rating Scale for Children (BPRS-C), the Columbia-Suicide Severity Rating Scale (C-SSRS) and the Udvalg for Kliniske Undersøgelser Side Effect Rating Scale-Clinician (UKU-SERS-Clin) were also used to monitor patient tolerability and safety plus the suitability of individuals to remain in the study [20].
Definitions of Response and Sustained Response
The change in ADHD-RS-IV total score from baseline was used to determine the proportion of patients responding to treatment according to three separate definitions: reductions from baseline of at least 25 %, at least 30 % or at least 50 %. Sustained response based on ADHD-RS-IV total score was defined as a reduction from baseline of at least 25, 30 or 50 % that was maintained throughout the dose-maintenance period (i.e. at weeks 4–9, inclusive). Sustained response based on CGI–I was defined as an improved CGI–I score (1 or 2) that was maintained throughout the same period. These additional analyses for response and sustained response were defined in the statistical analysis plan after finalisation of the study protocol but prior to unblinding of the data.
Clinical Global Impressions–Severity (CGI-S) Shifts
CGI–S assessments were carried out at baseline, week 4 and week 9/early termination as an indicator of remission. Shifts in CGI–S score from baseline to week 9 are presented as shift tables for individuals who had a valid score at both visits. Patients with missing baseline or week 9 assessments were excluded from the CGI–S shift tables.
Statistical Methods
Efficacy analyses were based on the intention-to-treat principle and were performed using the full analysis set, defined as all patients who were randomized and received at least one dose of study drug. Cochran–Mantel–Haenszel tests stratified by country were used to assess the effect of treatment on the cumulative proportion of responders at each of weeks 1–9. For these analyses, the last observation carried forward (LOCF) approach was applied to data that were missing owing to early termination or unavailability. Cochran–Mantel–Haenszel tests stratified by country were also used to assess the effect of treatment on the proportion of sustained responders. For these analyses, the approach to missing data was non-responder imputation, in which patients with at least one missing assessment (from week 4 to week 9) due to early termination or unavailability were classified as non-responders. A statistical comparison of CGI–S shifts associated with LDX and ATX treatment was not a prespecified analysis and p-values comparing the effects of each treatment on CGI–S shifts were not generated.
Results
Patient Disposition and Baseline Characteristics
As described previously [20], 267 patients (LDX, n = 133; ATX, n = 134) were randomized, 262 (LDX, n = 127; ATX, n = 135) were included in the full analysis set (75.2 % of patients were male), and 200 (74.9 %) completed the study (LDX, n = 99; ATX, n = 101). Baseline demographics and disease characteristics were similar between treatment groups [20]. Mean (standard deviation [SD]) baseline ADHD-RS-IV total scores were similar in both treatment groups (LDX, 42.6 [6.14], range 28–54; ATX, 41.9 [6.70], range 28–53), and mean (SD) CGI–S scores were also similar in both groups (LDX, 5.0 [0.80], range 3–7; ATX, 5.0 [0.73], range 4–7). The mean (SD) optimal dose (which was the dose that was dispensed at visit 4) for patients who received LDX during the dose-maintenance phase was 52.5 mg/day (16.10) and was 40.2 mg/day (20.05) for patients who received ATX.
Attention-Deficit Hyperactivity Disorder Rating Scale IV Responders
For all three definitions of response based on improvements in ADHD-RS-IV, the proportion of patients who responded to treatment was statistically significantly higher in the LDX-treated group than in the ATX-treated group at each weekly visit (p < 0.05 for all response criteria) (Fig. 1). Increases in response rates over time were observed in both treatment groups, primarily during the dose optimization phase. By week 9, the proportions of patients (95 % confidence interval) with reductions from baseline in ADHD-RS-IV total score of at least 25, 30 or 50 % were 90.5 % (85.4–95.6), 88.1 % (82.4–93.7) and 73.0 % (65.3–80.8) in the LDX group, and 76.7 % (69.5–83.9), 73.7 % (66.2–81.2) and 50.4 % (41.9–58.9) in the ATX group, respectively.
Fig. 1.
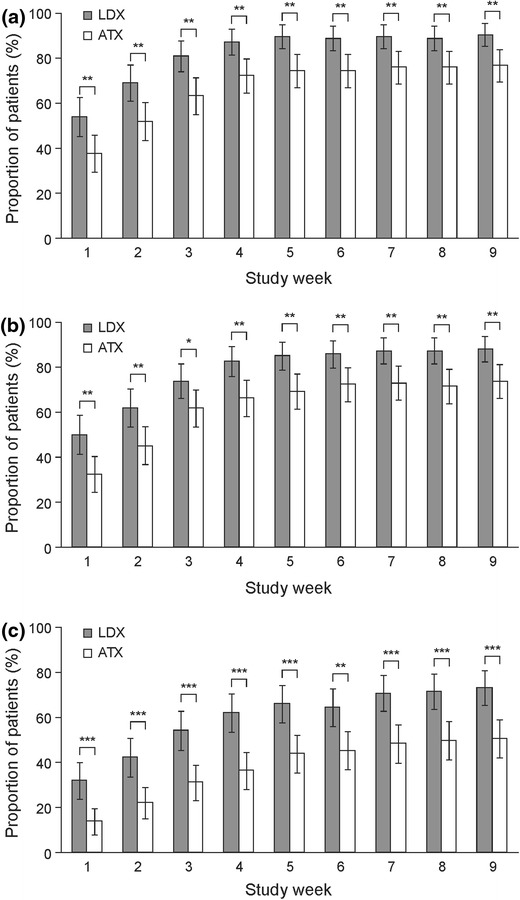
Proportion of patients classified as responders using definitions based on a (a) ≥25 %, (b) ≥30 % or (c) ≥50 % reduction in ADHD-RS-IV total score [14] from baseline (last observation carried forward). *p < 0.05, **p < 0.01, ***p < 0.001 LDX versus ATX (Cochran–Mantel–Haenszel test stratified by country). Error bars show 95 % confidence intervals. Percentages are based on the number of patients in each treatment group at the indicated study visit. ADHD-RS-IV Attention Deficit Hyperactivity Disorder Rating Scale IV, ATX atomoxetine, LDX lisdexamfetamine dimesylate
Sustained Responders
The proportion of patients who responded to treatment throughout weeks 4–9 was statistically significantly higher in the LDX group than in the ATX group for all four ADHD-RS-IV and CGI–I definitions of sustained response (p < 0.05 for all comparisons) (Fig. 2). The proportions of sustained responders decreased with increasing ADHD-RS-IV thresholds. The proportions of patients meeting the CGI–I response criteria of 1 or 2 was between the proportions experiencing a ≥30 and ≥50 % reduction in ADHD-RS-IV total score.
Fig. 2.
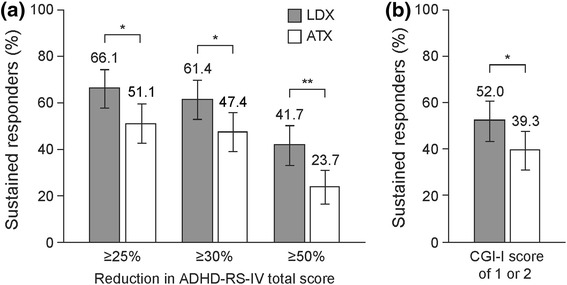
Patients classified as sustained responders (weeks 4–9) using definitions based on (a) ADHD-RS-IV total scores or (b) CGI–I scores. *p < 0.05, **p < 0.01 LDX versus ATX (Cochran–Mantel–Haenszel test stratified by country). Sustained response was defined as the indicated percentage reduction in ADHD-RS-IV total score from baseline or an improved CGI–I score (1 or 2) at all study visits in weeks 4–9. Error bars show 95 % confidence intervals. Data are based on non-responder imputation. ADHD-RS-IV Attention Deficit Hyperactivity Disorder Rating Scale IV, ATX atomoxetine, CGI–I Clinical Global Impressions–Improvement, LDX lisdexamfetamine dimesylate
CGI–S Shifts
An analysis of shifts in CGI–S scores from baseline to visit 9 is shown in Table 1, based on observed values for individuals with a valid week 9 CGI–S score (LDX, 95/127 patients [74.8 %]; ATX, 97/135 patients [71.9 %]). At week 9, no patients from either treatment group had shifted to a more severe category of illness than that observed at baseline. In the LDX group, five patients remained in their baseline CGI–S category at week 9, ten patients improved by one category and 80 patients improved by more than one category. In the ATX group, 13 patients remained in their baseline CGI–S category, 14 patients improved by one category and 70 patients improved by more than one category. At week 9, 13/20 patients in the LDX group and 9/24 patients in the ATX group had shifted from the most severe CGI–S categories of 6 (severely ill) or 7 (among the most extremely ill) to a CGI–S score of 1 (normal, not at all ill) or 2 (borderline mentally ill). A total of five patients (LDX, n = 1; ATX, n = 4) remained in the two most severe CGI–S categories at week 9.
Table 1.
Observed shifts in CGI–S score from baseline to week 9 (N = 262)
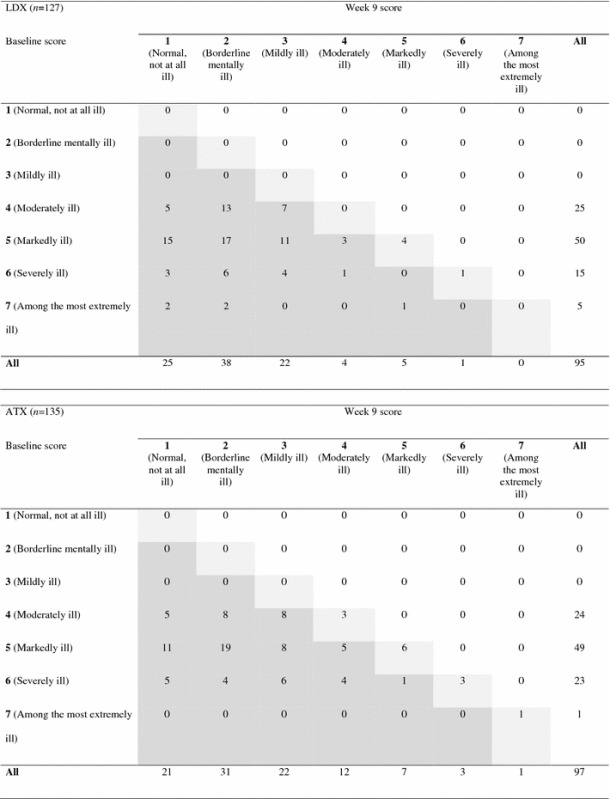
Data are based on observed values for patients with a CGI–S score at week 9. Dark grey shading indicates patients who shifted to a lower CGI–S category from baseline to week 9. Light grey shading indicates patients who remained in their baseline CGI–S category at week 9
ATX atomoxetine, CGI–S Clinical Global Impressions–Severity, LDX lisdexamfetamine dimesylate
Using the LOCF approach, 71/117 patients (60.7 %) in the LDX group and 57/123 patients (46.3 %) in the ATX group had a CGI–S score of 1 or 2 by week 9, indicating remission.
Discussion
In these analyses of data from the first head-to-head, randomized, controlled trial of LDX and ATX, LDX treatment was consistently associated with statistically significantly higher rates of clinical response than ATX treatment in children and adolescents with ADHD and an inadequate response to MPH, irrespective of the ADHD-RS-IV or CGI–I criteria used to define response (p < 0.01 for all comparisons). In addition, the proportions of patients with a sustained response, defined as those who met response criteria throughout weeks 4–9, were also statistically significantly higher among patients receiving LDX than among those receiving ATX (p < 0.05 for all comparisons). Finally, after 9 weeks of treatment, the proportion of patients who had low levels of disease severity (CGI–S score of 1 or 2) was numerically higher among individuals receiving LDX than among those receiving ATX.
The present responder data, and those previously reported for this study which found that a significantly greater proportion of patients receiving LDX (81.7 %) than ATX (63.6 %) achieved a CGI–I score of 1 or 2 by visit 9 (p < 0.01) [20], are generally consistent with those observed in previous studies of LDX and ATX, despite differences in study designs and patient populations [7, 20–23, 25–27]. Several other clinical trials have found LDX to be associated with significantly higher proportions of treatment responders than placebo irrespective of the age of the patients [7, 20, 23, 25, 27]. When clinical response was defined as at least a 30 % reduction from baseline in ADHD-RS-IV total score, approximately 65 % of adult patients receiving LDX were categorized as responders after 4 weeks of treatment, compared with approximately 35 % of those receiving placebo [7]. Three LDX studies used the combined response criteria of at least a 30 % reduction from baseline in ADHD-RS-IV total score and a CGI–I score of 1 or 2. First, a US-based study in children with ADHD found that 79.3 % of patients treated with LDX responded, compared with 29.2 % of those receiving placebo [25]. Among the subgroup of study participants who had previously experienced an inadequate response to MPH treatment, response rates for LDX (78.9 %) were similar to those in the overall study population and to the responder rates observed for LDX in the present study, which was also conducted in patients with a history of inadequate response to MPH treatment [20, 25]. Second, clinical response was examined post hoc in a 12-month, open-label LDX study in adults with ADHD, categorized according to their baseline CGI–S score (4, 5 or ≥6) [23]. This study revealed numerically higher (a statistical analyses was not performed) proportions of clinical responders among individuals with more severe baseline illness than those with less severe baseline illness (CGI–S of 4, 78.9 %; CGI–S of 5, 83.5 %; CGI–S of ≥6, 88.4 %) [23]. In addition, 71.3 % of patients had at least a 50 % reduction from baseline in ADHD-RS-IV total score [23], a value which, despite differences in study design and population, is very similar to the results of the present 9-week, double-blind, paediatric study (LDX, 73.0 %). Third, 74.2 % of patients receiving LDX compared with 10.7 % of those receiving placebo met the combined response criteria in a 7-week European regulatory trial in children and adolescents with ADHD, and 78.0 and 14.4 %, respectively, had a CGI–I score of 1 or 2 at endpoint [27]. As required by the EMA, this last study included an active comparator treatment (osmotic-release oral system MPH; OROS-MPH) to validate the study design and to contextualize the results. A post hoc comparison indicated that the proportion of patients who responded to LDX was significantly (p < 0.05) larger than the proportion who responded to OROS-MPH (combined criteria, 55.9 %; CGI–I of 1 or 2, 60.6 %) [27]. Given that this study was neither designed nor powered to provide a direct comparison between treatments, these findings should be considered as preliminary. The results of ongoing parallel-group studies in adolescents (ClinicalTrials.gov identifiers: NCT01552915 and NCT01552902) will provide definitive evidence of the relative benefits of LDX and MPH.
The results of the present responder analyses are also broadly consistent with those of previous studies of ATX. Pooled data from six randomized controlled trials of 6–9 weeks in duration in children and adolescents with ADHD (N = 1,069) revealed that 60 % of patients had at least a 25 % reduction from baseline in ADHD-RS-IV total score, and 47 % had a decrease of at least 40 % [21]. Another pooled analysis combined data from three Canadian open-label studies in children and predicted that 75 % of patients would achieve at least a 25 % reduction from baseline in ADHD-RS-IV total score after 7.2 weeks of ATX treatment [22]. This finding is very similar to the results of the present study. A meta-analysis indicated that the proportions of patients with a clinically relevant response to ATX were not significantly different from the proportions responding to MPH treatment, when response was defined as a reduction of at least 40 % (53.6 vs. 54.4 %) or at least 25 % (69.0 vs. 70.0 %) from baseline in ADHD-RS-IV total score, or as achieving a CGI–S score of 1 or 2 (18.2 vs. 24.3 %) [24]. However, it should be noted that only one of the seven studies included in the meta-analysis compared ATX with a long-acting MPH formulation (OROS-MPH), and in that study the clinical response to OROS-MPH was superior to that of ATX [26].
Responder analyses allow the degree of clinically meaningful symptom improvement in individual patients to be established. Despite the recognized benefits of responder analyses, a consensus has not been reached on the most appropriate criteria to use when assessing clinical response to ADHD medication and, as described above, various response criteria have been used in studies assessing the efficacy of LDX and ATX. In the present study, increasing the degree of change in ADHD-RS-IV total score required for response resulted in a decrease in responder rates. Response rates based on a CGI–I score of 1 or 2 [20] appeared to correspond with an ADHD-RS-IV total score reduction of between 30 and 50 %. This is slightly lower than findings from a previous analysis of two LDX studies in paediatric populations, where reductions from baseline in ADHD-RS-IV total score of 80, 52 and 27 % correspond to CGI–I scores of 1 (very much improved), 2 (much improved) and 3 (minimally improved), respectively, perhaps due to differences in study design. The authors of that study suggested that on the basis of these results, a reduction in ADHD-RS-IV total score of at least 50 % should be used to define clinical response [30].
Despite a continued lack of consensus on the appropriate threshold to use when defining a clinically relevant response to treatment, it was clear in the present head-to-head study that the relative benefits of LDX compared with ATX treatment remained similar irrespective of which response criterion was used. This finding is consistent with a meta-analysis of 32 clinical trials that concluded that both short- and long-acting psychostimulants were significantly more effective than non-stimulants [31]. The present study also examined sustained response, revealing that a significantly larger proportion of patients receiving LDX than those receiving ATX met continued response criteria throughout weeks 4–9. This is the first study of either LDX or ATX to determine sustained response, an obviously desirable outcome in the clinical setting.
A criticism of responder analyses is that they do not take into account patients’ baseline disease severity. Therefore, individuals with severe baseline symptoms may meet clinical response criteria at study endpoint despite significant residual symptoms and/or impairment. To address this, some studies have assessed the proportions of patients whose symptoms reduce to below a defined ‘remission’ threshold. However, a consensus has still to be reached on the most appropriate criterion to be used to define remission. In previous studies of LDX and ATX, an ADHD-RS-IV score of 18 or less [22, 23, 25] or a CGI–S score of 2 or less [22, 32] were used to define remission. In the present study, CGI–S scores (based on LOCF) revealed that 60.7 % of patients receiving LDX and 46.3 % of those receiving ATX had a CGI–S score of 2 or less by week 9. These values are consistent with those observed in a pooled analysis of three open-label studies which concluded that the probability of children achieving a CGI–S score of 2 or less was 8 % after 4 weeks of ATX treatment and 47 % after 12 weeks of ATX treatment [22].
Safety outcomes from this study have previously been published [20]. In summary, similar proportions of patients in both treatment groups reported TEAEs (LDX, 71.9 %; ATX, 70.9 %). In addition, changes in mean vital signs and ECG parameters, and in the frequency of outliers and potentially clinically important observations, were generally similar between treatment groups [20].
The strengths of this study include its double-blind, randomized, parallel-group, dose-optimized design, and the large international patient population. In addition, the results are particularly pertinent in Europe, given the recent approval of LDX in several European countries for the treatment of children and adolescents in whom previous MPH treatment was clinically inadequate; under these circumstances, the choice of medication in most countries will be between LDX and ATX. It is unclear whether this highly selected patient population, who were required to meet multiple inclusion and exclusion criteria relating to their previous exposure and/or response to ADHD medication, would have been more likely to respond to one treatment arm than the other. However, similarities in the results of this and other studies suggest that these inclusion/exclusion criteria do not unduly favour either treatment arm. In addition, the details of the study design, as described in the paediatric investigation plan, were agreed with the EMA. A potential limitation of this study is the 9-week duration which might have limited the potential benefits of ATX. Indeed, a meta-analysis of pooled data from ATX studies (N = 601) indicated that the response to ATX continued to grow for as long as 12 weeks, although most of the improvement occurred during the first 4 weeks and any subsequent further improvement occurred in conjunction with increasing mean ATX dose [33]. There is also evidence to suggest that higher doses of ATX than used in the present study and recommended in the product’s prescribing information (up to 1.8 mg/kg) may result in higher levels of efficacy of ATX [34, 35]. In addition, there is some evidence that ATX is more effective when administered twice daily than once daily, as used in the present study [34]. Finally, although there are only three available LDX doses, a greater variety of doses are available for ATX. Therefore, the length of the dose-optimization period of the study may not have permitted all ATX doses to be fully explored, which may have affected patient outcomes in the ATX treatment group.
Conclusions
Response rates were significantly higher in the LDX treatment group than in the ATX group among children and adolescents with at least moderately symptomatic ADHD and a previous inadequate response to MPH therapy, within the parameters of the study. The superior efficacy of LDX over ATX was maintained irrespective of the criteria used to determine a clinically relevant response to treatment.
Acknowledgements
This study was supported by funding from Shire. The authors thank the patients and their parents, and the investigators who took part in the study. Esther Cardo, David R. Coghill, Ralf W. Dittmann and Peter Nagy were principal investigators in this clinical study. Colleen S. Anderson, Beatriz Caballero, Richard Civil, Ralf W. Dittmann and Paul Hodgkins contributed to the study design. Ben Adeyi was responsible for the statistical analysis. All authors were involved in the discussion and interpretation of the data, critically revised the article and approved the manuscript before submission. Dr Tamzin Gristwood and Dr Eric Southam of Oxford PharmaGenesis™ Ltd provided writing and editing assistance, collated the comments of the authors and edited the manuscript for submission, with funding from Shire. Ben Adeyi, Colleen S. Anderson and Beatriz Caballero are employees of Shire and own stock/stock options. Richard Civil is a former employee of Shire. Paul Hodgkins is a former employee of Shire and a current employee of Vertex Pharmaceuticals. The following authors have received compensation for serving as consultants or speakers, or they, or the institutions they work for, have received research support or royalties from the companies or organizations indicated: Esther Cardo (Eli Lilly, Health Spanish Ministry Research Fund, Ministry of Education Grant Research, Shire, UCB); David R. Coghill (Flynn Pharma, Janssen-Cilag, Lilly, Medice, Novartis, Otsuka, Oxford University Press, Pfizer, Schering-Plough, Shire, UCB, Vifor Pharma); Ralf W. Dittmann (Ferring, Janssen-Cilag, Lilly, Otsuka, Shire, German Research Foundation [DFG], German Ministry of Education and Research [BMBF], Ministry of Health/German Regulatory Body [BfArM], European Union [EU FP7 program], US National Institute of Mental Health (NIMH), and he is a former employee and stockholder of Eli Lilly and Co.); Peter Nagy (Tourette Syndrome Association of USA, Hungarian Ministry of Education, National Development Agency of Hungary, Otsuka, Shire).
Footnotes
ClinicalTrials.gov identifier: NCT01106430.
References
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–948. doi: 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry J Ment Sci. 2007;190:402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 3.Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry J Ment Sci. 2009;194(3):204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Attention-deficit and disruptive behavior disorders. In: Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington: American Psychiatric Press Inc., 2000, p. 85–93.
- 5.National Institute for Health and Clinical Excellence. Diagnosis and management of ADHD in children, young people and adults. National clinical practice guideline number 72. London: National Institute for Health and Clinical Excellence, 2009.
- 6.Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Dopfner M, Hollis C, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83–105. doi: 10.1007/s00787-009-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler LA, Goodman DW, Kollins SH, Weisler RH, Krishnan S, Zhang Y, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–1373. doi: 10.4088/JCP.v69n0903. [DOI] [PubMed] [Google Scholar]
- 8.Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007;62(9):970–976. doi: 10.1016/j.biopsych.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29(3):450–463. doi: 10.1016/S0149-2918(07)80083-X. [DOI] [PubMed] [Google Scholar]
- 10.Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013 doi: 10.1016/j.euroneuro.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(4):395–405. doi: 10.1016/j.jaac.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Wigal SB, Kollins SH, Childress AC, Squires L. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;3(1):17. doi: 10.1186/1753-2000-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav Brain Funct. 2010;6:34. doi: 10.1186/1744-9081-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuPaul GJ, Power T, Anastopoulos A, Reid R. The ADHD Rating Scale-IV, checklist, norms, and clinical interpretation. New York: Guildford; 1998. [Google Scholar]
- 15.Bussing R, Fernandez M, Harwood M, Wei H, Garvan CW, Eyberg SM, et al. Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms: psychometric properties and normative ratings from a school district sample. Assessment. 2008;15(3):317–328. doi: 10.1177/1073191107313888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40(2):168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–268. doi: 10.1023/A:1022602400621. [DOI] [PubMed] [Google Scholar]
- 18.Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. V: scales assessing attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(9):1015–1037. doi: 10.1097/01.CHI.0000070245.24125.B6. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Guideline on the clinical investigation of medicinal products for the treatment of attention deficit hyperactivity disorder (ADHD). London: European Medicines Agency; 2010.
- 20.Dittmann RW, Cardo E, Nagy P, Anderson CS, Bloomfield R, Caballero B, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs. 2013;27(12):1081–92. doi:10.1007/s40263-013-0104-8. [DOI] [PMC free article] [PubMed]
- 21.Newcorn JH, Sutton VK, Weiss MD, Sumner CR. Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: the integrated data exploratory analysis (IDEA) study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):511–518. doi: 10.1097/CHI.0b013e31819c55b2. [DOI] [PubMed] [Google Scholar]
- 22.Dickson RA, Maki E, Gibbins C, Gutkin SW, Turgay A, Weiss MD. Time courses of improvement and symptom remission in children treated with atomoxetine for attention-deficit/hyperactivity disorder: analysis of Canadian open-label studies. Child Adolesc Psychiatry Ment Health. 2011;5:14. doi: 10.1186/1753-2000-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsberg L, Katic A, Adeyi B, Dirks B, Babcock T, Lasser R, et al. Long-term treatment outcomes with lisdexamfetamine dimesylate for adults with attention-deficit/hyperactivity disorder stratified by baseline severity. Curr Med Res Opin. 2011;27(6):1097–1107. doi: 10.1185/03007995.2011.567256. [DOI] [PubMed] [Google Scholar]
- 24.Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, Wyk GW. Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord. 2011;15(8):674–683. doi: 10.1177/1087054710379737. [DOI] [PubMed] [Google Scholar]
- 25.Jain R, Babcock T, Burtea T, Dirks B, Adeyi B, Scheckner B, et al. Efficacy of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder previously treated with methylphenidate: a post hoc analysis. Child Adolesc Psychiatry Ment Health. 2011;5(1):35. doi: 10.1186/1753-2000-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- 27.Soutullo C, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson C, et al. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743–751. doi: 10.1007/s40263-013-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eli Lilly and Company. Straterra® (atomoxetine): prescribing information. 2014. http://pi.lilly.com/us/strattera-pi.pdf. Accessed 17 Jun 2014.
- 29.Guy D. ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH, Psychopharmacology Research Branch Division of Extramural Research Programs, 1976.
- 30.Goodman D, Faraone SV, Adler LA, Dirks B, Hamdani M, Weisler R. Interpreting ADHD Rating Scale scores: linking ADHD Rating Scale scores and CGI levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Prim Psychiatry. 2010;17(3):44–52. [Google Scholar]
- 31.Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T. 2009;34(12):678–694. [PMC free article] [PubMed] [Google Scholar]
- 32.Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159(11):1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 33.Wilens TE, Newcorn JH, Kratochvil CJ, Gao H, Thomason CK, Rogers AK, et al. Long-term atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Pediatr. 2006;149(1):112–119. doi: 10.1016/j.jpeds.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 34.Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176. doi: 10.1186/1471-244X-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammerness P, McCarthy K, Mancuso E, Gendron C, Geller D. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: a review. Neuropsychiatr Dis Treat. 2009;5:215–226. doi: 10.2147/ndt.s3896. [DOI] [PMC free article] [PubMed] [Google Scholar]


